Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction
Abstract
1. Introduction
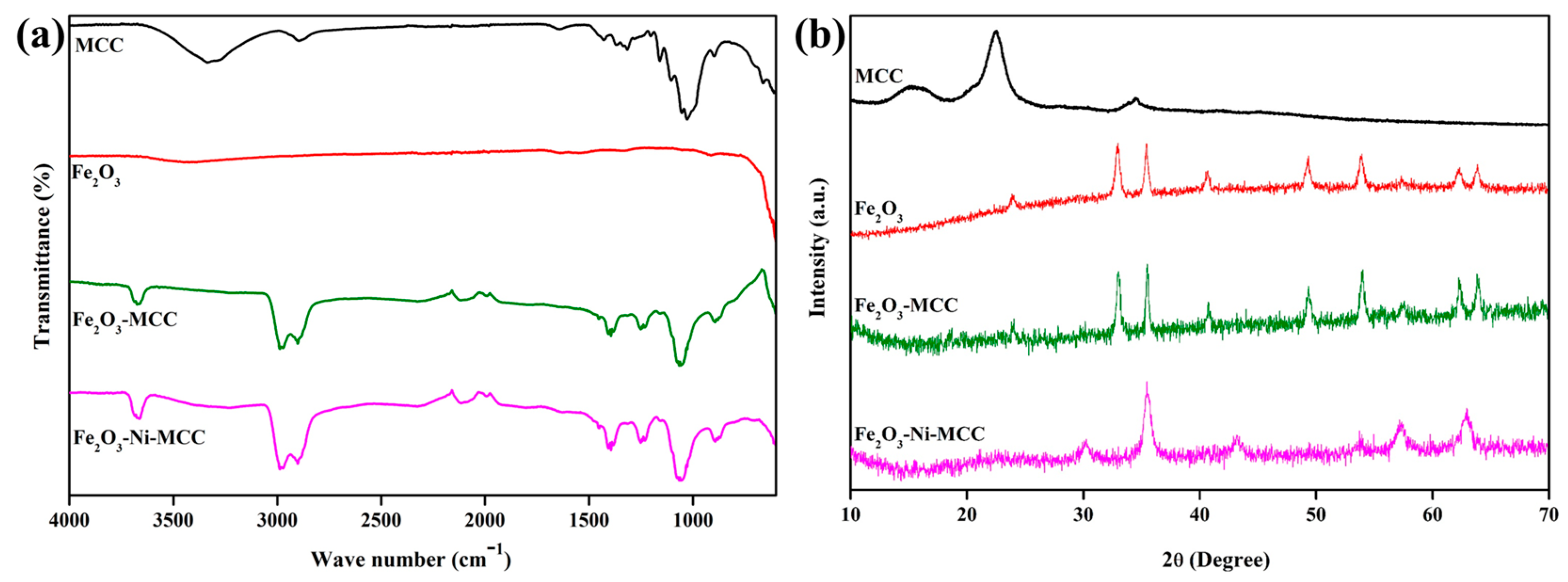
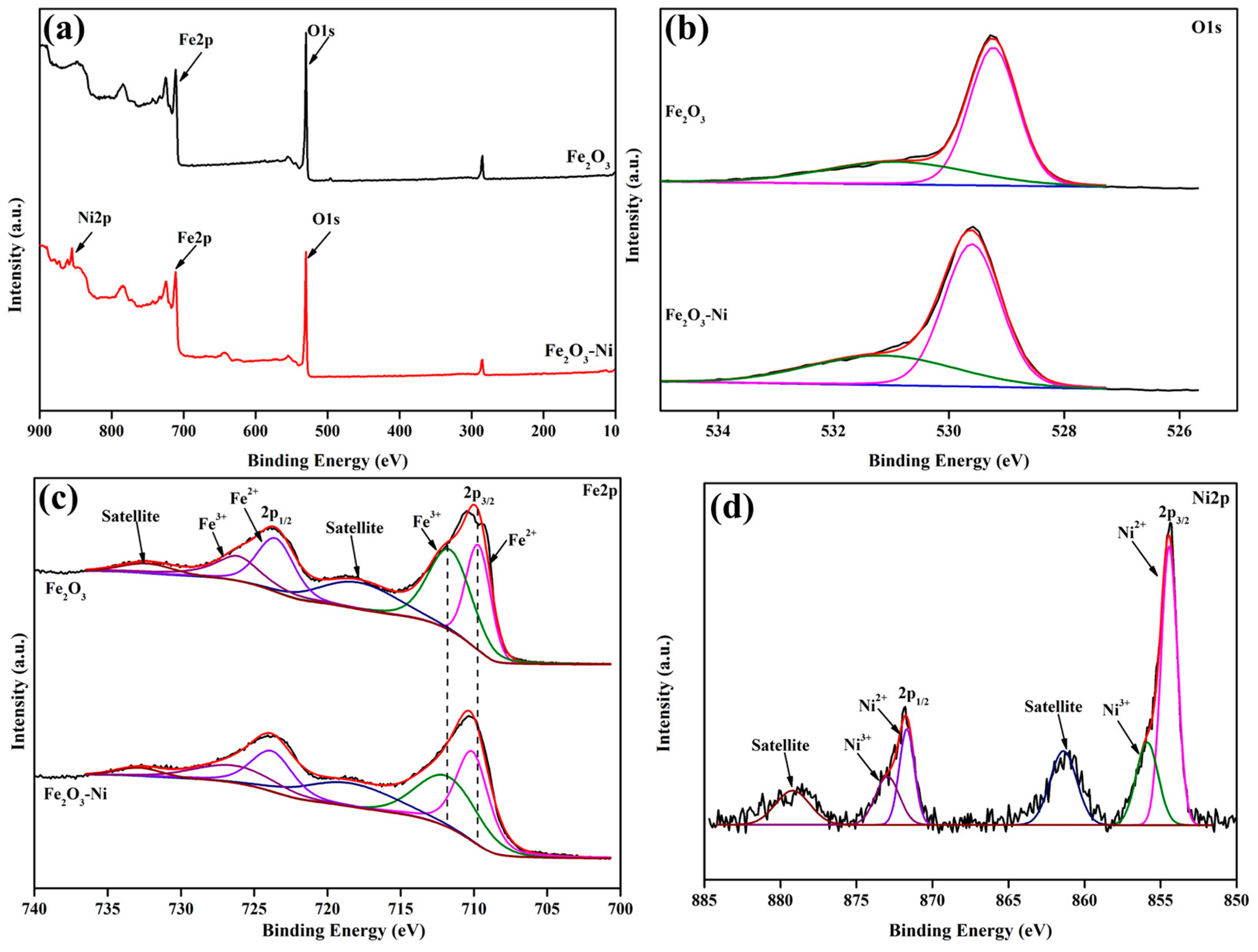
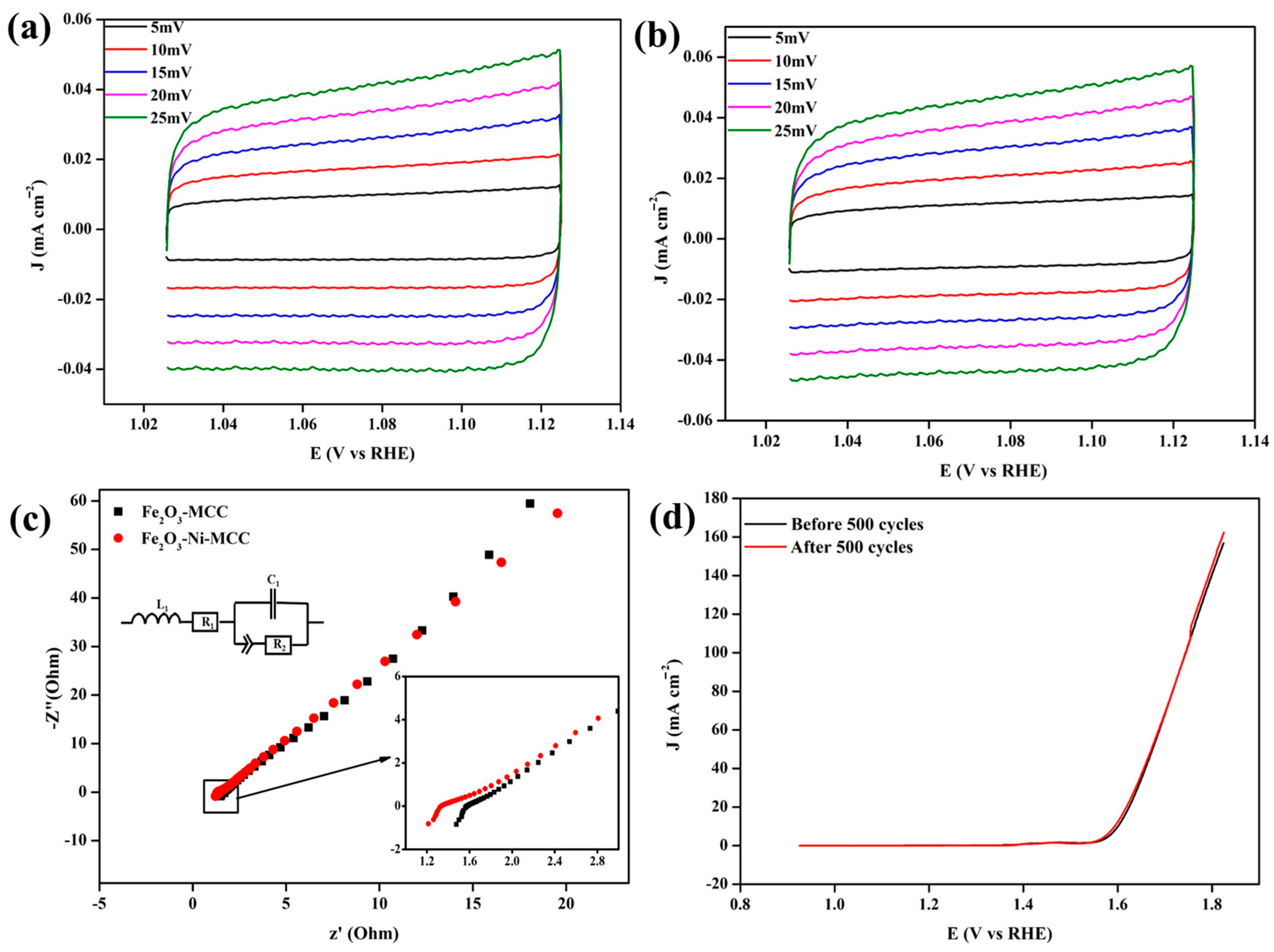
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2023, 6, e12441. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Wang, B.; Liu, S. High-Entropy Catalyst—A Novel Platform for Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2207536. [Google Scholar] [CrossRef]
- Hu, J.; Al-Salihy, A.; Zhang, B.; Li, S.; Xu, P. Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting. Int. J. Mol. Sci. 2022, 23, 15405. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, P.M.; Sarawade, P.B.; Kumar, P.; Vinu, A.; Kulkarni, A.P.; Lokhande, C.D.; Dhawale, D.S. Nanostructured Metal Phosphide Based Catalysts for Electrochemical Water Splitting: A Review. Small 2022, 18, 2107572. [Google Scholar] [CrossRef]
- Wang, Q.; He, R.; Yang, F.; Tian, X.; Sui, H.; Feng, L. An overview of heteroatom doped cobalt phosphide for efficient electrochemical water splitting. Chem. Eng. J. 2023, 456, 141056. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.-Y.; Chen, X.-N.; Huang, M.-J.; Cai, S.-H.; Han, J.-Y.; Li, J.-S. Self-supported bimetallic phosphides with artificial heterointerfaces for enhanced electrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120914. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Luo, L.; Du, Y.; Cheng, X.; Wu, Q. Nitrogen-doped Fe2O3/NiTe2 as an excellent bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2023, 639, 416–423. [Google Scholar] [CrossRef]
- Shah, S.A.; Shen, X.; Yuan, A.; Ji, Z.; Yue, X.; Zhu, G.; Zhou, H.; Xu, K.; Zhu, J.; Chen, Y. One step in-situ synthesis of Ni3S2/Fe2O3/N-doped carbon composites on Ni foam as an efficient electrocatalyst for overall water splitting. Appl. Surf. Sci. 2020, 527, 146918. [Google Scholar] [CrossRef]
- Panda, A.; Cho, H.-K.; Kim, H. A Green Synthesis of CoFe2O4 Decorated ZIF-8 Composite for Electrochemical Oxygen Evolution. Int. J. Mol. Sci. 2023, 24, 9585. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Reddy Nallabala, N.K.; Nguyen, T.N.; Nguyen Dang, N.; Shim, J. Onion-Ring-like Carbon and Nitrogen from ZIF-8 on TiO2/Fe2O3 Nanostructure for Overall Electrochemical Water Splitting. J. Phys. Chem. Lett. 2021, 12, 5909–5918. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, J.; Batool, S.; Zafar, M.N.; Hanif, A.; Zahidullah; Nazar, M.F.; Ul-Hamid, A.; Jabeen, U.; Dahshan, A.; et al. Design and fabrication of Fe2O3/FeP heterostructure for oxygen evolution reaction electrocatalysis. J. Alloys Compd. 2022, 894, 162409. [Google Scholar] [CrossRef]
- Karuppasamy, L.; Gurusamy, L.; Ananan, S.; Barton, S.C.; Liu, C.-H.; Wu, J.J. Metal-organic frameworks derived interfacing Fe2O3/ZnCo2O4 multimetal oxides as a bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2023, 449, 142242. [Google Scholar] [CrossRef]
- Kim, J.; Heo, J.N.; Do, J.Y.; Chava, R.K.; Kang, M. Electrochemical Synergies of Heterostructured Fe2O3-MnO Catalyst for Oxygen Evolution Reaction in Alkaline Water Splitting. Nanomaterials 2019, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, Y.; Mo, S.; Guo, D.; Liu, L. Fast construction of (Fe2O3)x@Ni-MOF heterostructure nanosheets as highly active catalyst for water oxidation. J. Alloys Compd. 2022, 892, 162149. [Google Scholar] [CrossRef]
- Cui, T.; Zhai, X.; Guo, L.; Chi, J.-Q.; Zhang, Y.; Zhu, J.; Sun, X.; Wang, L. Controllable synthesis of a self-assembled ultralow Ru, Ni-doped Fe2O3 lily as a bifunctional electrocatalyst for large-current-density alkaline seawater electrolysis. Chin. J. Catal. 2022, 43, 2202–2211. [Google Scholar] [CrossRef]
- Fereja, S.L.; Li, P.; Zhang, Z.; Guo, J.; Fang, Z.; Li, Z.; Chen, W. Construction of NiCo2S4/Fe2O3 hybrid nanostructure as a highly efficient electrocatalyst for the oxygen evolution reaction. Electrochim. Acta 2022, 405, 139793. [Google Scholar] [CrossRef]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, 2001642. [Google Scholar] [CrossRef]
- Li, H.; Cai, C.; Wang, Q.; Chen, S.; Fu, J.; Liu, B.; Hu, Q.; Hu, K.; Li, H.; Hu, J.; et al. High-performance alkaline water splitting by Ni nanoparticle-decorated Mo-Ni microrods: Enhanced ion adsorption by the local electric field. Chem. Eng. J. 2022, 435, 134860. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Ji, W.-B.; Hu, B.-C.; Liang, H.-W.; Xu, X.-X.; Yu, Z.-L.; Li, B.-Y.; Yu, S.-H. Partially oxidized Ni nanoparticles supported on Ni-N co-doped carbon nanofibers as bifunctional electrocatalysts for overall water splitting. Nano Energy 2018, 51, 286–293. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Yu, J.; Xiong, G.; Niu, H.; Li, Y.; Sun, D.; Zhang, X.; Liu, H.; Zhou, W. Underfocus Laser Induced Ni Nanoparticles Embedded Metallic MoN Microrods as Patterned Electrode for Efficient Overall Water Splitting. Adv. Sci. 2022, 9, 2105869. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Li, J.-F.; Lu, Z.-W.; Zhang, J.; He, C.-T. Biomass-Derived Carbon-Based Multicomponent Integration Catalysts for Electrochemical Water Splitting. ChemSusChem 2023, 16, e202300214. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; He, D.; Monestel, H.G.R.; Mu, S. Scalable cellulose-sponsored functionalized carbon nanorods induced by cobalt for efficient overall water splitting. Carbon 2018, 137, 274–281. [Google Scholar] [CrossRef]
- Li, R.; Kamali, A.R. Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder. Gels 2023, 9, 701. [Google Scholar] [CrossRef]
- Kasprzak, D.; Stępniak, I.; Galiński, M. Electrodes and hydrogel electrolytes based on cellulose: Fabrication and characterization as EDLC components. J. Solid State Electrochem. 2018, 22, 3035–3047. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Z.; Li, W.; Chen, C.; Yang, J.; Liu, J.; Gong, F.; Liao, J.; Wu, M. Cellulose-Hydrogel-Derived Self-Activated Carbon/SnO2 Nanocomposites for High-Performance Lithium Storage. ACS Appl. Energy Mater. 2019, 2, 5171–5182. [Google Scholar] [CrossRef]
- Gwóźdź, M.; Brzęczek-Szafran, A. Carbon-Based Electrocatalyst Design with Phytic Acid—A Versatile Biomass-Derived Modifier of Functional Materials. Int. J. Mol. Sci. 2022, 23, 11282. [Google Scholar] [CrossRef]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Recent development of transition metal doped carbon materials derived from biomass for hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 32436–32454. [Google Scholar] [CrossRef]
- Salleh, N.A.; Kheawhom, S.; Mohamad, A.A. Chitosan as biopolymer binder for graphene in supercapacitor electrode. Results Phys. 2021, 25, 104244. [Google Scholar] [CrossRef]
- Jeong, S.S.; Böckenfeld, N.; Balducci, A.; Winter, M.; Passerini, S. Natural cellulose as binder for lithium battery electrodes. J. Power Sources 2012, 199, 331–335. [Google Scholar] [CrossRef]
- Sonker, A.K.; Xiong, S.; Aggarwal, R.; Olsson, M.; Spule, A.; Hosseini, S.; Sonkar, S.K.; Matic, A.; Westman, G. Exfoliated MoS2 Nanosheet/Cellulose Nanocrystal Flexible Composite Films as Electrodes for Zinc Batteries. ACS Appl. Nano Mater. 2023, 6, 8270–8278. [Google Scholar] [CrossRef]
- Böckenfeld, N.; Jeong, S.S.; Winter, M.; Passerini, S.; Balducci, A. Natural, cheap and environmentally friendly binder for supercapacitors. J. Power Sources 2013, 221, 14–20. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef]
- Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227. [Google Scholar] [CrossRef]
- Andres, B.; Dahlström, C.; Blomquist, N.; Norgren, M.; Olin, H. Cellulose binders for electric double-layer capacitor electrodes: The influence of cellulose quality on electrical properties. Mater. Des. 2018, 141, 342–349. [Google Scholar] [CrossRef]
- Teng, C.P.; Tan, M.Y.; Toh, J.P.; Lim, Q.F.; Wang, X.; Ponsford, D.; Lin, E.M.; Thitsartarn, W.; Tee, S.Y. Advances in Cellulose-Based Composites for Energy Applications. Materials 2023, 16, 3856. [Google Scholar] [CrossRef]
- Palanisamy, G.; Sadhasivam, T.; Park, W.-S.; Bae, S.T.; Roh, S.-H.; Jung, H.-Y. Tuning the Ion Selectivity and Chemical Stability of a Biocellulose Membrane by PFSA Ionomer Reinforcement for Vanadium Redox Flow Battery Applications. ACS Sustain. Chem. Eng. 2020, 8, 2040–2051. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Huang, X.; Xie, F.; Xiong, X. Surface-modified microcrystalline cellulose for reinforcement of chitosan film. Carbohydr. Polym. 2018, 201, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.Z.; Chowdhury, Z.Z.; Hamid, S.B.; Ali, M.E. Statistical Optimization for Acid Hydrolysis of Microcrystalline Cellulose and Its Physiochemical Characterization by Using Metal Ion Catalyst. Materials 2014, 7, 6982–6999. [Google Scholar] [CrossRef] [PubMed]
- Supeno, S.; Daik, R.; El-Sheikh, S.M. The Synthesis of a Macro-initiator from Cellulose in a Zinc-Based Ionic Liquid. BioResources 2014, 9, 1267–1275. [Google Scholar] [CrossRef][Green Version]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Belmont, CA, USA, 2014. [Google Scholar]
- Sosiati, H.; Rizky, A.M.; Latief, A.L.M.; Adi, R.K.; Hamdan, S. The mechanical and physical properties of microcrystalline cellulose (MCC)/sisal/PMMA hybrid composites for dental applications. Mater. Res. Express 2023, 10, 035301. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Hu, Y.-R.; Mei, C.-G.; Ma, M.-G. Cellulose/vaterite nanocomposites: Sonochemical synthesis, characterization, and their application in protein adsorption. Mater. Sci. Eng. C 2019, 96, 426–435. [Google Scholar] [CrossRef]
- Duarte, P.; Pereira, S.; Cunha, I.; Pimentel, A.; Dionísio, M.; Fortunato, E.; Martins, R.; Pereira, L. Cellulose-Based Solid Electrolyte Membranes through Microwave Assisted Regeneration and Application in Electrochromic Displays. Front. Mater. 2020, 7, 269. [Google Scholar] [CrossRef]
- Zhu, P.; Feng, L.; Ding, Z.; Bai, X. Preparation of Spherical Cellulose Nanocrystals from Microcrystalline Cellulose by Mixed Acid Hydrolysis with Different Pretreatment Routes. Int. J. Mol. Sci. 2022, 23, 10764. [Google Scholar] [CrossRef]
- Abbasi, S.; Hekmat, F.; Shahrokhian, S. Beyond hierarchical mixed nickel-cobalt hydroxide and ferric oxide formation onto the green carbons for energy storage applications. J. Colloid Interface Sci. 2021, 593, 182–195. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Huo, W.; Li, S.-S.; Fang, J.-H.; Li, L.; Zhang, B.-Y.; Zhang, F.; Zhang, Y.-X.; Li, S.-W. Crystal phase determined Fe active sites on Fe2O3 (γ- and α-Fe2O3) yolk-shell microspheres and their phase dependent electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2020, 533, 147368. [Google Scholar] [CrossRef]
- Tahir, M.N.; Herzberger, J.; Natalio, F.; Köhler, O.; Branscheid, R.; Mugnaioli, E.; Ksenofontov, V.; Panthöfer, M.; Kolb, U.; Frey, H.; et al. Hierachical Ni@Fe2O3 superparticles through epitaxial growth of γ-Fe2O3 nanorods on in situ formed Ni nanoplates. Nanoscale 2016, 8, 9548–9555. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, Y.; Guo, Q.; Liu, J.; Wang, Y.; Yang, M.; Xia, H. Controllable Synthesis of TiO2@Fe2O3 Core-Shell Nanotube Arrays with Double-Wall Coating as Superb Lithium-Ion Battery Anodes. Sci. Rep. 2017, 7, 40927. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Ren, J.; Yao, H.-C.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Han, L.-F.; Li, Z.-J. Synergistic photocatalysis of Cr(VI) reduction and 4-Chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J. Hazard. Mater. 2016, 311, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.-S.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Highly Efficient Photoelectrochemical Water Splitting: Surface Modification of Cobalt-Phosphate-Loaded Co3O4/Fe2O3 p–n Heterojunction Nanorod Arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Lu, X.-F.; Chen, X.-Y.; Zhou, W.; Tong, Y.-X.; Li, G.-R. α-Fe2O3@PANI Core–Shell Nanowire Arrays as Negative Electrodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar] [CrossRef]
- Hengne, A.M.; Samal, A.K.; Enakonda, L.R.; Harb, M.; Gevers, L.E.; Anjum, D.H.; Hedhili, M.N.; Saih, Y.; Huang, K.-W.; Basset, J.-M. Ni–Sn-Supported ZrO2 Catalysts Modified by Indium for Selective CO2 Hydrogenation to Methanol. ACS Omega 2018, 3, 3688–3701. [Google Scholar] [CrossRef]
- Gao, X.; Du, X.; Liu, D.; Gao, H.; Wang, P.; Yang, J. Core-shell gold-nickel nanostructures as highly selective and stable nonenzymatic glucose sensor for fermentation process. Sci. Rep. 2020, 10, 1365. [Google Scholar] [CrossRef]
- Samanta, A.; Jana, S. Ni-, Co-, and Mn-Doped Fe2O3 Nano-Parallelepipeds for Oxygen Evolution. ACS Appl. Nano Mater. 2021, 4, 5131–5140. [Google Scholar] [CrossRef]
- Samanta, A.; Das, S.; Jana, S. Doping of Ni in α-Fe2O3 Nanoclews to Boost Oxygen Evolution Electrocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 12117–12124. [Google Scholar] [CrossRef]
- Tong, Y.L.; Chi, B.Q.; Qi, D.L.; Zhang, W. Rational construction of free-standing P-doped Fe2O3 nanowire arrays as highly effective electrocatalyst for overall water splitting. RSC Adv. 2021, 11, 1233–1240. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, X.; Ding, G.; Guo, Z.; Xue, Y.; Li, G.; Yu, R. Constructing oxygen vacancy-enriched Fe2O3@NiO heterojunctions for highly efficient electrocatalytic alkaline water splitting. CrystEngComm 2022, 24, 199–207. [Google Scholar] [CrossRef]
- Chao, S.; Xia, Q.; Wang, G.; Zhang, X. Fe2O3 nanoparticles immobilized on N and S codoped C as an efficient multifunctional catalyst for oxygen reduction reaction and overall water electrolysis. Int. J. Hydrog. Energy 2019, 44, 4707–4715. [Google Scholar] [CrossRef]
- Alduhaish, O.; Ubaidullah, M.; Al-Enizi, A.M.; Alhokbany, N.; Alshehri, S.M.; Ahmed, J. Facile Synthesis of Mesoporous α-Fe2O3@g-C3N4-NCs for Efficient Bifunctional Electro-catalytic Activity (OER/ORR). Sci. Rep. 2019, 9, 14139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Sun, Y.; Chen, Y.; Chen, H.; Han, S.; Lin, H. Fe2O3 nanocatalysts on N-doped carbon nanomaterial for highly efficient electrochemical hydrogen evolution in alkaline. J. Power Sources 2019, 426, 74–83. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lin, Z.; Liang, Y.; Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 2021, 4, 725–730. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, S.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@ C@ Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Lv, C.; Zhong, L.; Liu, H.; Fang, Z.; Yan, C.; Chen, M.; Kong, Y.; Lee, C.; Liu, D.; Li, S.; et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 2021, 4, 868–876. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangarasu, S.; Baby, N.; Bhosale, M.; Lee, J.; Jeong, C.; Oh, T.-H. Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. Int. J. Mol. Sci. 2023, 24, 16282. https://doi.org/10.3390/ijms242216282
Thangarasu S, Baby N, Bhosale M, Lee J, Jeong C, Oh T-H. Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. International Journal of Molecular Sciences. 2023; 24(22):16282. https://doi.org/10.3390/ijms242216282
Chicago/Turabian StyleThangarasu, Sadhasivam, Nimisha Baby, Mrunal Bhosale, Jaeman Lee, Changseong Jeong, and Tae-Hwan Oh. 2023. "Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction" International Journal of Molecular Sciences 24, no. 22: 16282. https://doi.org/10.3390/ijms242216282
APA StyleThangarasu, S., Baby, N., Bhosale, M., Lee, J., Jeong, C., & Oh, T.-H. (2023). Fe2O3/Ni Nanocomposite Electrocatalyst on Cellulose for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. International Journal of Molecular Sciences, 24(22), 16282. https://doi.org/10.3390/ijms242216282





