Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin–Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model
Abstract
:1. Introduction
2. Results
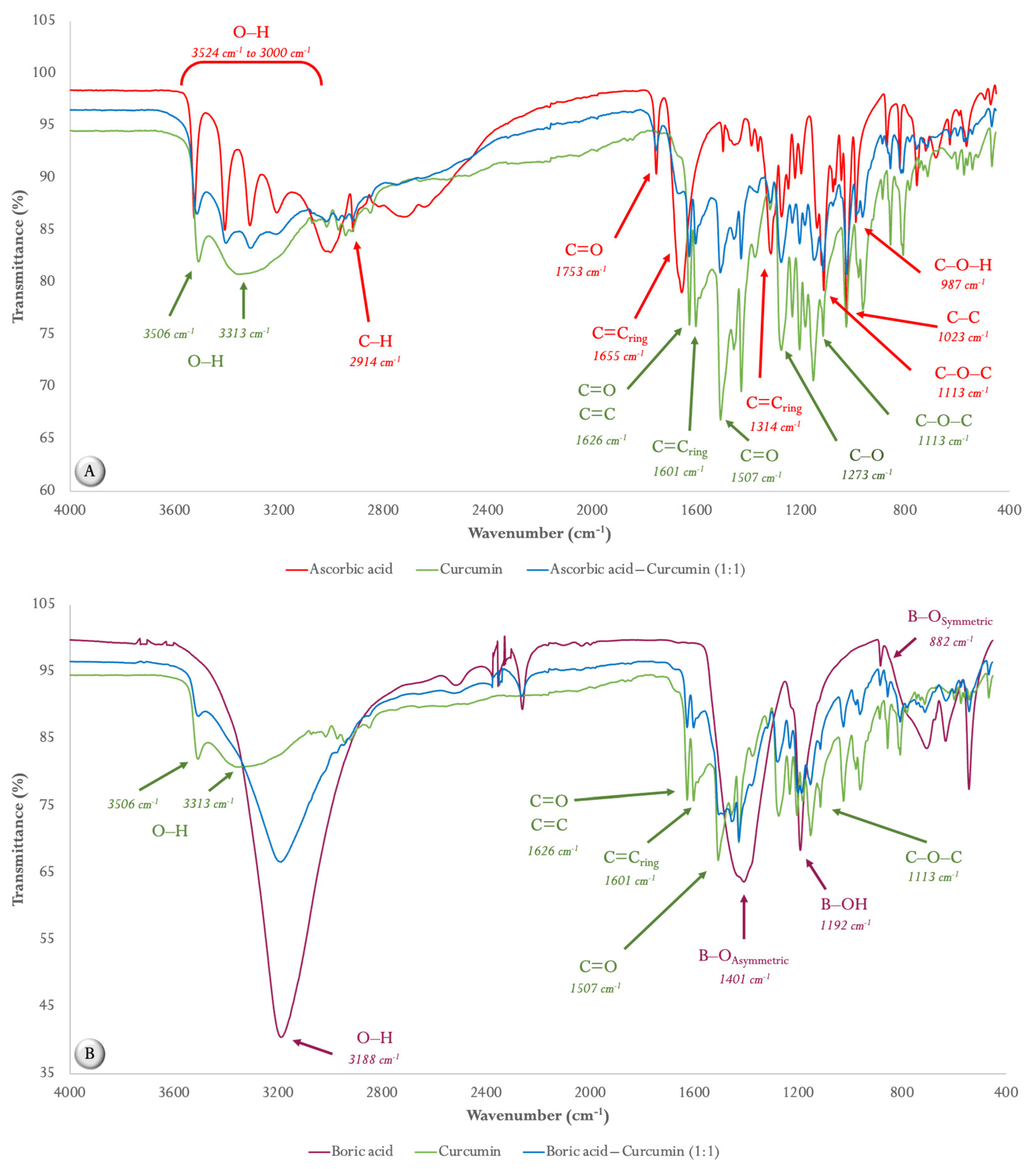
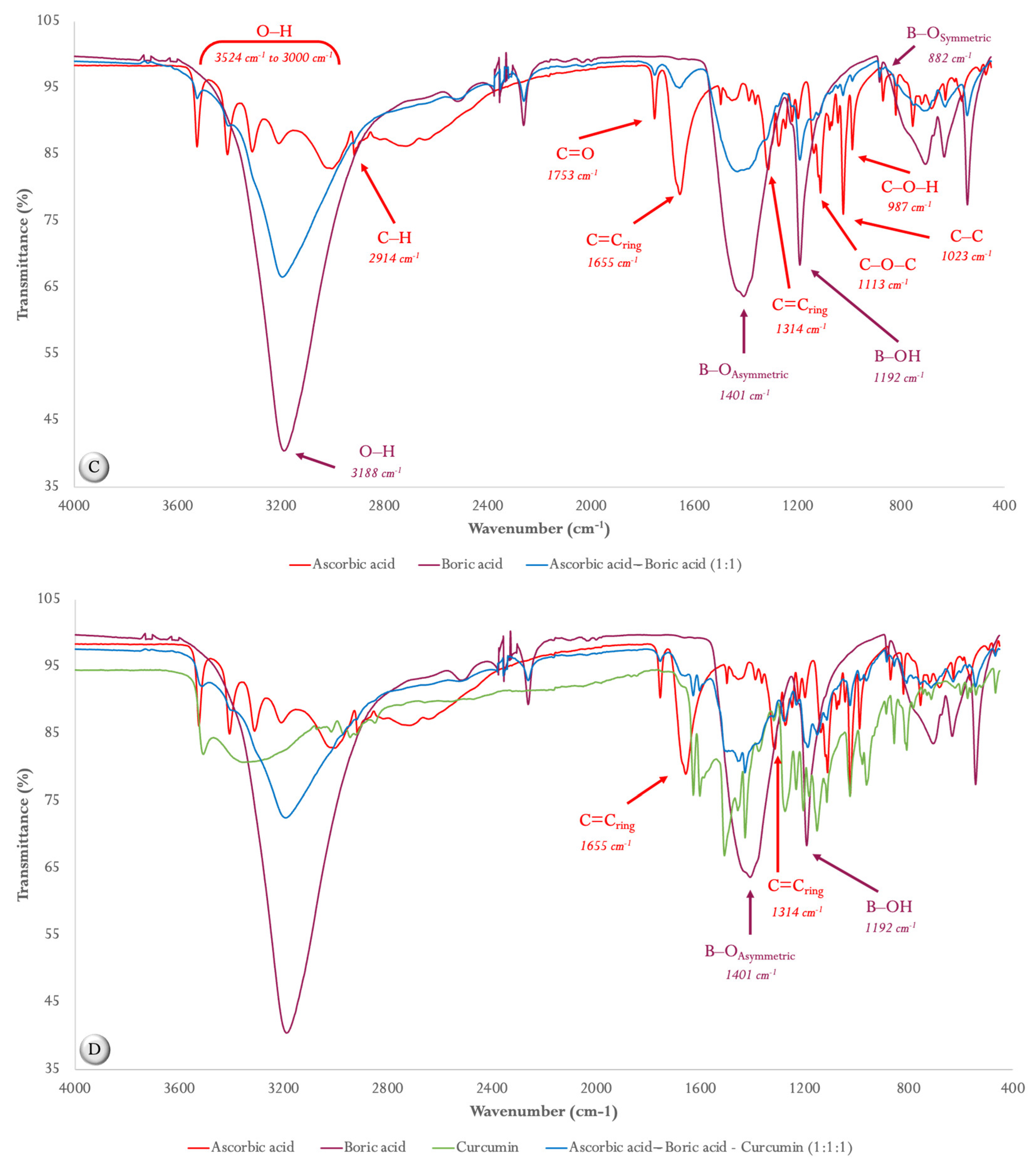
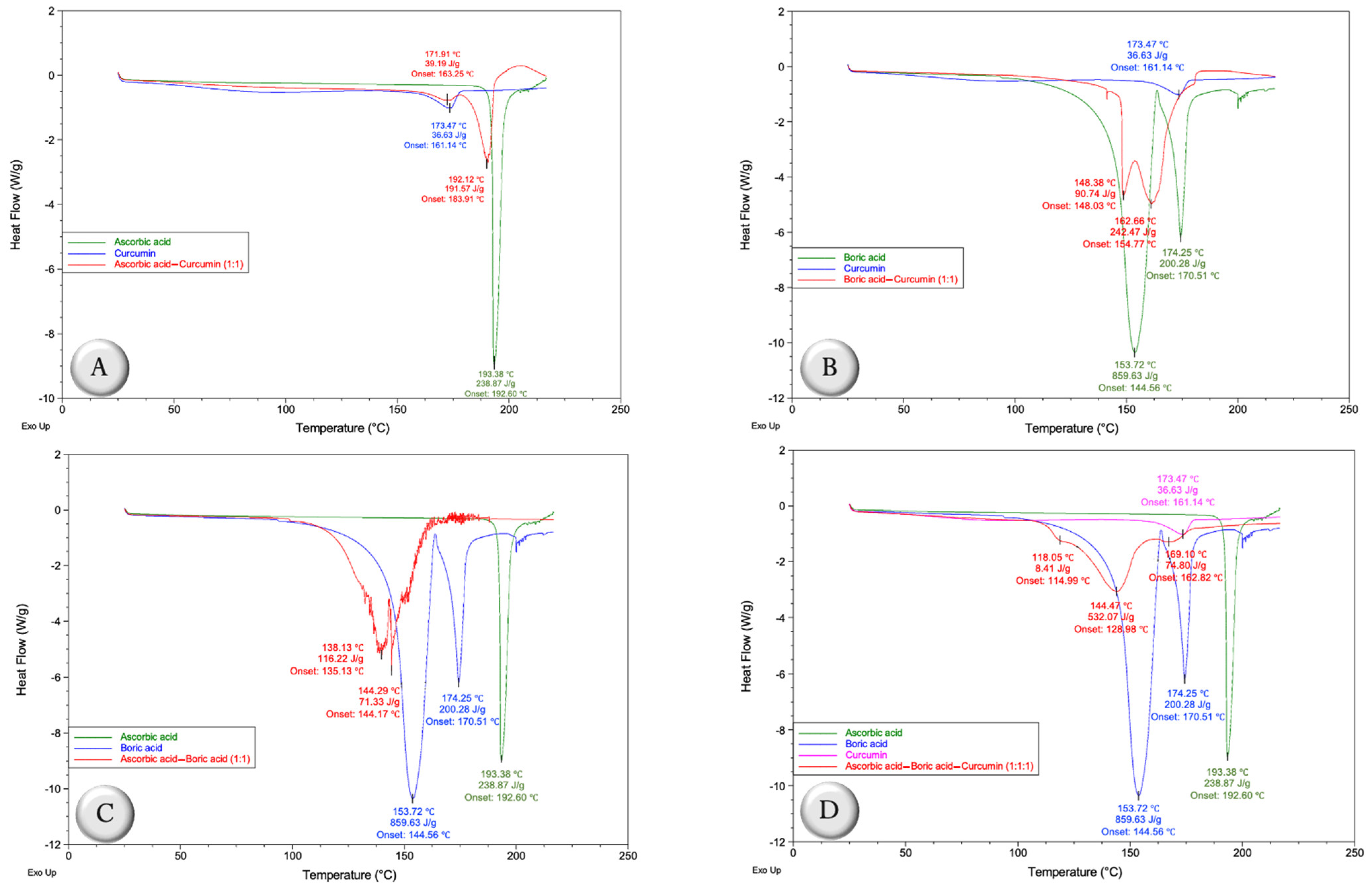
2.1. Compatibility Studies
2.1.1. ATR FT-MIR Spectroscopy
2.1.2. Differential Scanning Calorimetry (DSC)
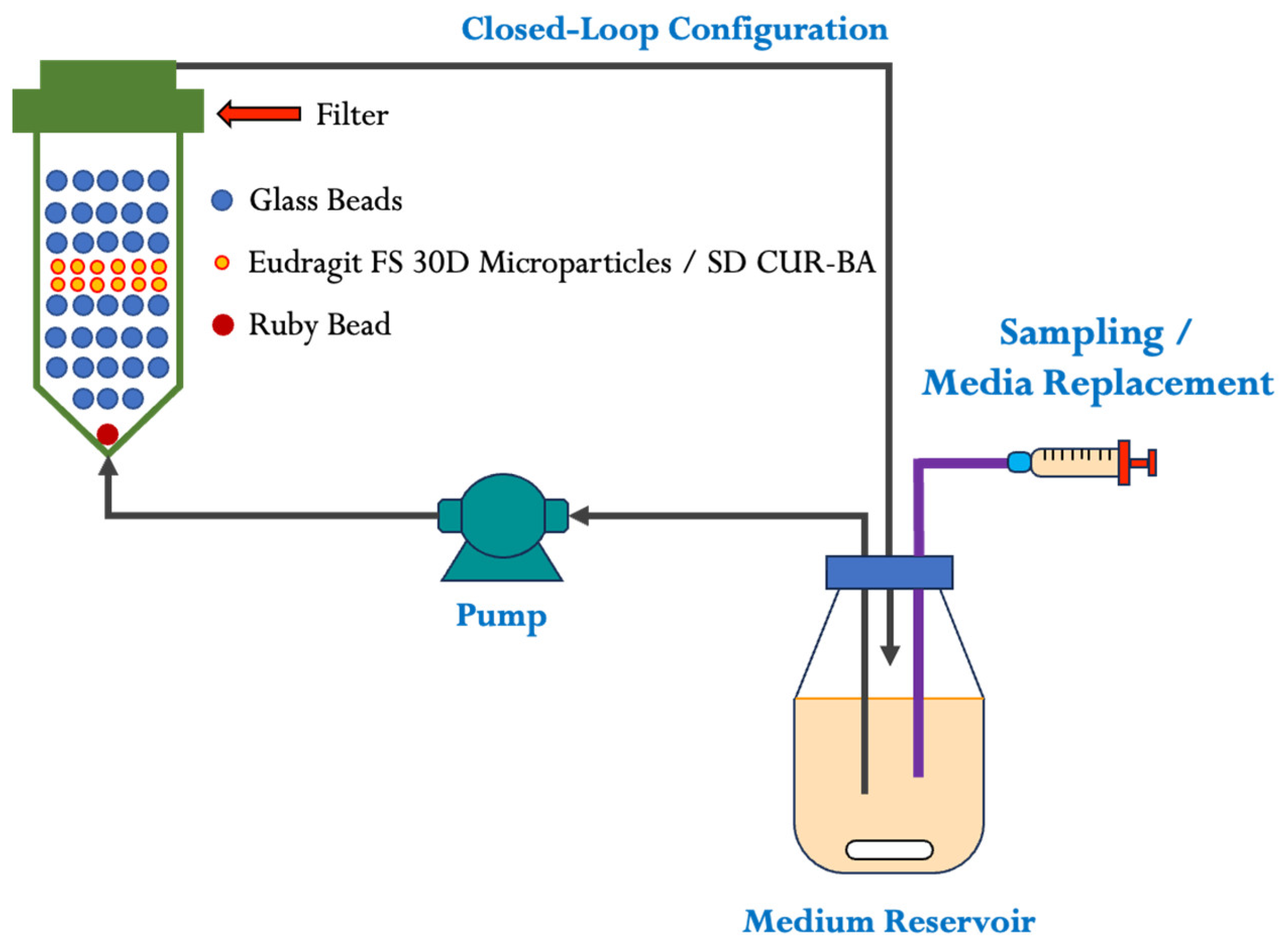
2.2. Characterization of Eudragit FS 30D Microparticles and Release Studies
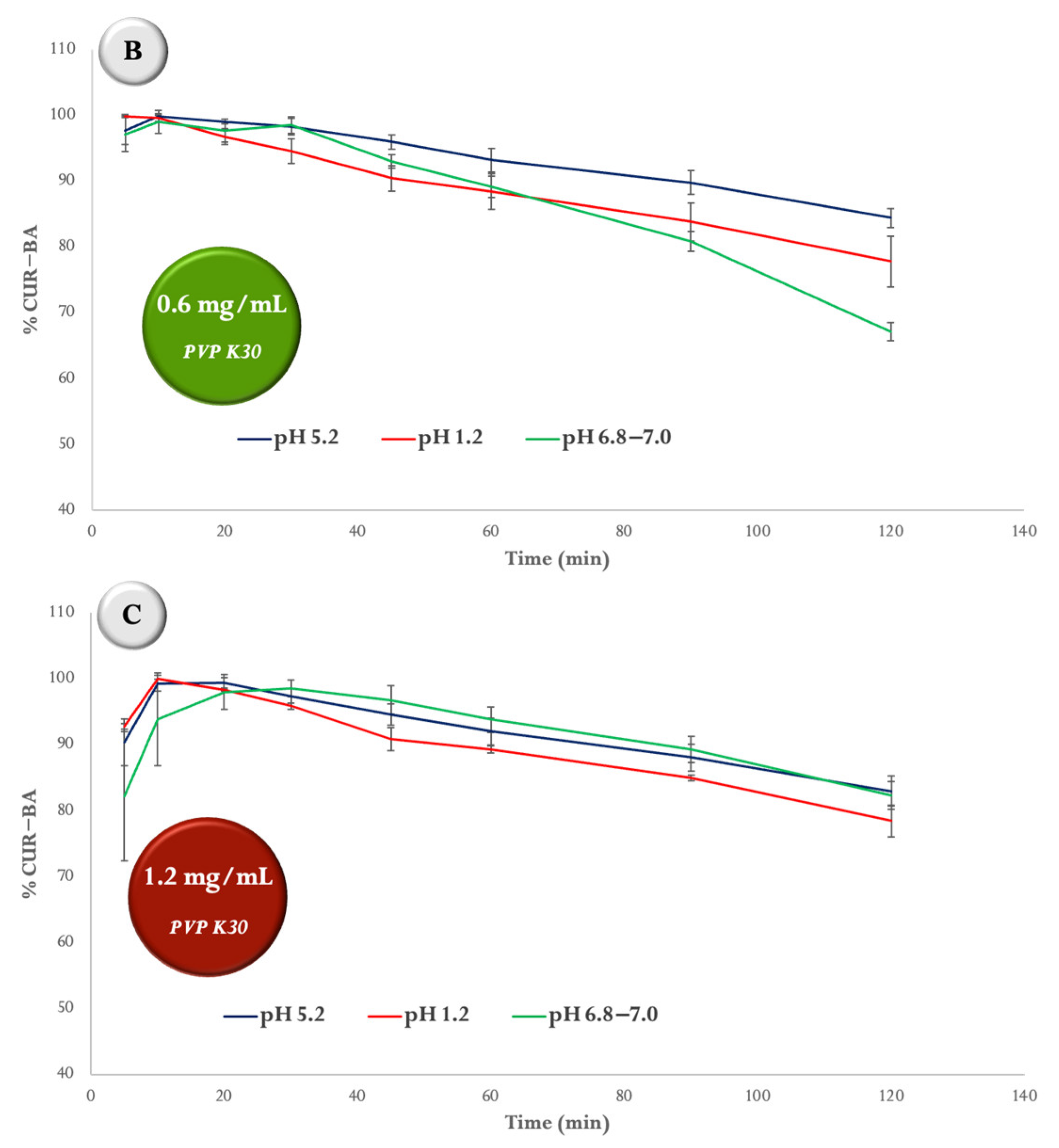
2.3. Degradation Studies of SD CUR–BA at Different pH
2.4. In Vivo Studies
3. Discussion
4. Materials and Methods
4.1. Compatibility Studies for Formulation Selection
4.1.1. Preparation of Mixtures
4.1.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy of the Middle Infrared Region (ATR-FTIR-MIR)
4.1.3. Characterization of the Mixtures via Differential Scanning Calorimetry (DSC)
4.2. Preparation of the Solid Dispersion of Curcumin–Boric Acid (SD CUR–BA)
4.3. Obtention of Eudragit FS 30D Microparticles of the Curcumin–Boric Acid Solid Dispersion
4.4. Eudragit FS 30D Microparticle Release Studies
4.5. Degradation Studies of SD CUR–BA at Different pH
4.6. In Vivo Experiments
4.6.1. Experimental Groups and Diets
4.6.2. Salmonella Strain and Culture Conditions
4.6.3. Experimental Design
4.6.4. Salmonella Recovery
4.6.5. Determination of FITC-d in Serum Samples
4.7. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamed, E.A.; Abdelaty, M.F.; Sorour, H.K.; Elmasry, D.; Abdelmagid, M.A.; Saleh, M.A.M.; AbdelRahman, M.A.A. A pilot study on the effect of thyme microemulsion compared with antibiotic as treatment of Salmonella enteritidis in broiler. Vet. Med. Int. 2022, 2022, 3647523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, X.; Ed-Dra, A.; Zhou, X.; Jia, C.; Müller, A.; Liu, Y.; Kehrenberg, C.; Yue, M. Genome-based assessment of antimicrobial resistance and virulence potential of isolates of non-pullorum/gallinarum Salmonella Serovars recovered from dead poultry in China. Microbiol. Spectr. 2022, 10, e00965-22. [Google Scholar] [CrossRef] [PubMed]
- Aljumaah, M.R.; Suliman, G.M.; Abdullatif, A.A.; Abudabos, A.M. Effects of phytobiotic feed additives on growth traits, blood biochemistry, and meat characteristics of broiler chickens exposed to Salmonella typhimurium. Poult. Sci. 2020, 99, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.S.L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Tofazzal Hossain, M.; Ahmed, R.; Hasan, M.M.; Islam, R.; Hossen, M.I.; Shaha, S.N.; Islam, M.R. Role of different growth enhancers as alternative to in-feed antibiotics in poultry industry. Front. Vet. Sci. 2022, 8, 794588. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Méndez-Albores, A.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G.; López-Arellano, R. Comparison of PrestoBlue® and plating method to evaluate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gastrointestinal model. J. Appl. Microbiol. 2018, 124, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Latorre, J.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Merino-Guzman, R.; Méndez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; et al. Evaluation of a solid dispersion of curcumin with polyvinylpyrrolidone and boric acid against Salmonella enteritidis infection and intestinal permeability in broiler chickens: A pilot study. Front. Microbiol. 2018, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Pratama, A.R.; Yudiarti, T.; Wahyuni, H.I.; Widiastuti, E.; Sartono, T.A. Effect of acidified turmeric and/or black pepper on growth performance and meat quality of broiler chickens. Int. J. Vet. Sci. Med. 2020, 8, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef] [PubMed]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, C.A. Meta-analysis of the influence of phytobiotic (pepper) supplementation in broiler chicken performance. Trop. Anim. Health Prod. 2020, 52, 17–30. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef]
- Ojha, B.K.; Singh, P.K.; Shrivastava, N. Enzymes in the animal feed industry. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–109. [Google Scholar]
- Phillips, C.J.C.; Hosseintabar-Ghasemabad, B.; Gorlov, I.F.; Slozhenkina, M.I.; Mosolov, A.A.; Seidavi, A. Immunomodulatory Effects of Natural Feed Additives for Meat Chickens. Life 2023, 13, 1287. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Ahmed, H.A.; Selim, S. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J. Anim. Sci. 2014, 27, 847–854. [Google Scholar]
- Laudadio, V.; Nasiri-Dehbaneh, M.; Bilal, R.M.; Qotbi, A.; Javandel, F.; Ebrahimi, A.; Seidavi, A.; Slozhenkina, M.; Gorlov, I.; Dunne, P.G. Effects of different levels of dietary black cumin (Nigella sativa L.) and fenugreek (Trigonella foenum-graecum L.) and their combination on productive traits, selected blood constituents, microbiota and immunity of broilers. Anim. Biotechnol. 2022, 33, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, I.; Yildirim, E.; Kurum, A.; Bolat, D.; Cinar, M.; Basalan, M.; Yigit, A. The effect of dietary garlic (Allium sativum), black cumin (Nigella sativa) and their combination on performance, intestine morphometry, serum biochemistry and antioxidant status of broiler chickens. Braz. J. Poult. Sci. 2020, 22. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Latorre, J.D.; Merino-Guzman, R.; Morales Rodríguez, M.; Ausland, C.; Hernandez-Velasco, X.; Ortiz Holguin, O.; Delgado, R.; Hargis, B.M. Whole-genome sequence and interaction analysis in the production of six enzymes from the three Bacillus strains present in a commercial direct-fed microbial (NorumTM) using a bliss independence test. Front. Vet. Sci. 2022, 9, 784387. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Sharma, J.; Jain, S.; Aggrawal, K.L. Incompatibility studies by high performance thin-layer chromatography: In case of curcumin. Asian J. Pharm. 2013, 7. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov. Ther. 2015, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Druzina, A.A.; Grammatikova, N.E.; Zhidkova, O.B.; Nekrasova, N.A.; Dudarova, N.V.; Kosenko, I.D.; Grin, M.A.; Bregadze, V.I. Synthesis and antibacterial activity studies of the conjugates of curcumin with closo-dodecaborate and cobalt bis (dicarbollide) boron clusters. Molecules 2022, 27, 2920. [Google Scholar] [CrossRef]
- Scorei, I.R.; Biţă, A.; Mogoşanu, G.D. Boron enhances the antiviral activity of the curcumin against SARS-CoV-2. Rom. J. Morphol. Embryol. 2020, 61, 967. [Google Scholar] [CrossRef]
- Köse, D.A.; Zümreoglu-Karan, B. Complexation of boric acid with vitamin C. New J. Chem. 2009, 33, 1874–1881. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Latorre, J.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Mendez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; Tellez-Isaias, G. Evaluation of Ascorbic Acid or Curcumin Formulated in a Solid Dispersion on Salmonella enteritidis Infection and Intestinal Integrity in Broiler Chickens. Pathogens 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.U.; Socha, M.; Sejil, C.; Gibaud, S. Spray-dried microparticles of glutathione and S-nitrosoglutathione based on Eudragit® FS 30D polymer. In Annales Pharmaceutiques Francaises; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 95–104. [Google Scholar]
- Letmanski, T.; Kodym, A.; Weber, P.; Lewandowski, M.; Wisniewski, A.; Baj, T. Eudragit FS 30D as a potential polymer for use in the technology of preparing matrix tablets contain metronidazole—An experimental and mathematical modeling study. Curr. Issues Pharm. Med. Sci. 2015, 28, 97–104. [Google Scholar] [CrossRef]
- Kshirsagar, S.J.; Bhalekar, M.R.; Umap, R.R. In vitro-in vivo comparison of two pH sensitive Eudragit polymers for colon specific drug delivery. J. Pharm. Sci. Res. 2009, 1, 61. [Google Scholar]
- Ravindran, V. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 2013, 22, 628–636. [Google Scholar] [CrossRef]
- Svihus, B. Function of the digestive system. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Classen, H.L.; Apajalahti, J.; Svihus, B.; Choct, M. The role of the crop in poultry production. Worlds Poult. Sci. J. 2016, 72, 459–472. [Google Scholar] [CrossRef]
- Janocha, A.; Milczarek, A.; Kosmalski, M.; Gajownik-Mućka, P.; Radzikowski, D. Effect of Feed Additives Supplementation on the Growth Performance, Gastrointestinal Tract Characteristics, and Carcass Composition in Turkey Hens. Animals 2022, 12, 3464. [Google Scholar] [CrossRef]
- Hinton, A.; Corrier, D.E.; Spates, G.E.; Norman, J.O.; Ziprin, R.L.; Beier, R.C.; DeLoach, J.R. Biological Control of Salmonella typhimurium in Young Chickens. Avian Dis. 1990, 34, 626. [Google Scholar] [CrossRef]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef]
- Esquisabel, A.; Pastor, M.; Talavera, A.; Cedré, B.; Fernández, S.; Sifontes, S.; Aranguren, Y.; Falero, G.; García, L.; Solís, R.L. A new oral vaccine candidate based on the microencapsulation by spray-drying of inactivated Vibrio cholerae. Vaccine 2011, 29, 5758–5764. [Google Scholar]
- Kumavat, S.D.; Chaudhari, Y.S.; Borole, P.; Mishra, P.; Shenghani, K.; Duvvuri, P. Degradation studies of curcumin. Int. J. Pharm. Rev. Res. 2013, 3, 50–55. [Google Scholar]
- Yang, Q.-Q.; Cai, W.-Q.; Wang, Z.-X.; Li, Y.; Zhang, Y.; Lin, X.; Su, B.-L.; Corke, H.; Zhang, B.-B. Structural characteristics, binding behaviors, and stability of ternary nanocomplexes of lecithin, polyvinylpyrrolidone, and curcumin. LWT 2023, 175, 114489. [Google Scholar] [CrossRef]
- Jelić, D.; Liavitskaya, T.; Vyazovkin, S. Thermal stability of indomethacin increases with the amount of polyvinylpyrrolidone in solid dispersion. Thermochim. Acta 2019, 676, 172–176. [Google Scholar] [CrossRef]
- Przekwas, J.; Wiktorczyk, N.; Budzyńska, A.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Ascorbic acid changes growth of food-borne pathogens in the early stage of biofilm formation. Microorganisms 2020, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rong, X.; Liu, M.; Liang, Z.; Geng, Y.; Wang, X.; Zhang, J.; Ji, C.; Zhao, L.; Ma, Q. Intestinal mucosal immunity-mediated modulation of the gut microbiome by oral delivery of enterococcus faecium against Salmonella enteritidis pathogenesis in a laying hen model. Front. Immunol. 2022, 13, 853954. [Google Scholar] [CrossRef]
- Guo, F.; Geng, Y.; Abbas, W.; Zhen, W.; Wang, S.; Huang, Y.; Guo, Y.; Ma, Q.; Wang, Z. Vitamin D3 nutritional status affects gut health of Salmonella-challenged laying hens. Front. Nutr. 2022, 9, 888580. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Teng, P.-Y.; Kim, W.K.; Applegate, T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): An indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021, 100, 101202. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994.
- Cobb-Vantress, Inc. Broiler Performance & Nutrition Supplement; Cobb 500: Siloam Springs, AR, USA, 2018. [Google Scholar]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef]
- Vicuña, E.A.; Kuttappan, V.A.; Tellez, G.; Hernandez-Velasco, X.; Seeber-Galarza, R.; Latorre, J.D.; Faulkner, O.B.; Wolfenden, A.D.; Hargis, B.M.; Bielke, L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015, 94, 1353–1359. [Google Scholar] [CrossRef]
- Vicuña, E.A.; Kuttappan, V.A.; Galarza-Seeber, R.; Latorre, J.D.; Faulkner, O.B.; Hargis, B.M.; Tellez, G.; Bielke, L.R. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poult. Sci. 2015, 94, 2075–2080. [Google Scholar] [CrossRef]
- Baxter, M.F.A.; Merino-Guzman, R.; Latorre, J.D.; Mahaffey, B.D.; Yang, Y.; Teague, K.D.; Graham, L.E.; Wolfenden, A.D.; Hernandez-Velasco, X.; Bielke, L.R. Optimizing Fluorescein Isothiocyanate Dextran Measurement As a Biomarker in a 24-h Feed Restriction Model to Induce Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2017, 4, 56. [Google Scholar] [CrossRef]
- SAS Institute. SAS User Guide No Title 2002; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]


| Treatment | Crop Log10 cfu/g | CT Log10 cfu/g |
|---|---|---|
| 3 d of treatment | ||
| CTRL (−) | 0.00 ± 0.00 b | 0.00 ± 0.00 c |
| CTRL (+) | 3.23 ± 0.14 a | 5.76 ± 0.27 a |
| AA | 2.72 ± 0.20 a | 5.55 ± 0.19 ab |
| SD CUR–BA MP | 2.96 ± 0.25 a | 5.21 ± 0.18 ab |
| AA/SD CUR–BA MP | 3.09 ± 0.16 a | 5.16 ± 0.18 b |
| 10 d of treatment | ||
| CTRL (−) | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| CTRL (+) | 3.04 ± 0.27 a | 6.00 ± 0.20 a |
| AA | 2.31 ± 0.21 b | 5.92 ± 0.38 a |
| SD CUR–BA MP | 2.80 ± 0.42 ab | 5.13 ± 0.34 b |
| AA/SD CUR–BA MP | 2.37 ± 0.17 ab | 4.97 ± 0.27 b |
| Treatment | BW D0 (g) | BW D10 (g) | BWG (g) | FITC-d (ng/mL) |
|---|---|---|---|---|
| CTRL (−) | 61.13 ± 0.71 ab | 184.00 ± 5.00 ab | 122.17 ± 5.50 ab | 12.76 ± 7.01 c |
| CTRL (+) | 62.07 ± 0.73 a | 171.41 ± 4.23 b | 108.65 ± 4.60 b | 163.38 ± 27.01 a |
| AA | 58.30 ± 0.82 bc | 181.89 ± 5.41 ab | 123.89 ± 5.70 ab | 86.33 ± 12.27 b |
| SD CUR–BA MP | 60.69 ± 0.82 ab | 193.87 ± 5.48 a | 133.40 ± 5.73 a | 58.72 ± 9.58 bc |
| AA/SD CUR–BA MP | 57.33 ± 0.74 c | 195.33 ± 6.14 a | 137.22 ± 6.41 a | 71.48 ± 12.12 bc |
| Ingredient | g/kg |
|---|---|
| Corn | 574.5 |
| Soybean meal | 346.6 |
| Poultry fat 1 | 34.5 |
| Dicalcium phosphate | 18.6 |
| Calcium carbonate | 9.9 |
| Salt | 3.8 |
| DL-Methionine | 3.3 |
| L-Lysine HCL | 3.1 |
| Threonine | 1.2 |
| Choline chloride 60% | 2.0 |
| Vitamin premix 2 | 1.0 |
| Mineral premix 3 | 1.0 |
| Antioxidant 4 | 0.5 |
| Calculated analysis | |
| Metabolizable energy (MJ/kg) | 12.7 |
| Crude protein (g/kg) | 221.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Patlan, D.; Solis-Cruz, B.; Latorre, J.D.; Maguey-Gonzalez, J.A.; Castellanos-Huerta, I.; Beyssac, E.; Garrait, G.; Vázquez-Durán, A.; López-Arellano, R.; Méndez-Albores, A.; et al. Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin–Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model. Int. J. Mol. Sci. 2023, 24, 16186. https://doi.org/10.3390/ijms242216186
Hernandez-Patlan D, Solis-Cruz B, Latorre JD, Maguey-Gonzalez JA, Castellanos-Huerta I, Beyssac E, Garrait G, Vázquez-Durán A, López-Arellano R, Méndez-Albores A, et al. Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin–Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model. International Journal of Molecular Sciences. 2023; 24(22):16186. https://doi.org/10.3390/ijms242216186
Chicago/Turabian StyleHernandez-Patlan, Daniel, Bruno Solis-Cruz, Juan D. Latorre, Jesus A. Maguey-Gonzalez, Inkar Castellanos-Huerta, Eric Beyssac, Ghislain Garrait, Alma Vázquez-Durán, Raquel López-Arellano, Abraham Méndez-Albores, and et al. 2023. "Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin–Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model" International Journal of Molecular Sciences 24, no. 22: 16186. https://doi.org/10.3390/ijms242216186
APA StyleHernandez-Patlan, D., Solis-Cruz, B., Latorre, J. D., Maguey-Gonzalez, J. A., Castellanos-Huerta, I., Beyssac, E., Garrait, G., Vázquez-Durán, A., López-Arellano, R., Méndez-Albores, A., Hargis, B. M., & Tellez-Isaias, G. (2023). Evaluation of the Antimicrobial Activity of a Formulation Containing Ascorbic Acid and Eudragit FS 30D Microparticles for the Controlled Release of a Curcumin–Boric Acid Solid Dispersion in Turkey Poults Infected with Salmonella enteritidis: A Therapeutic Model. International Journal of Molecular Sciences, 24(22), 16186. https://doi.org/10.3390/ijms242216186







