Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization of Materials
2.2. Acute MSNs Exposure Does Not Lead to Toxic Effects in BEAS-2B Cells
2.3. MSNs Can Induce Slight Genotoxic Damage with Short-Term Exposure While This Effect Is Not Observed in Long-Term Exposed Cells
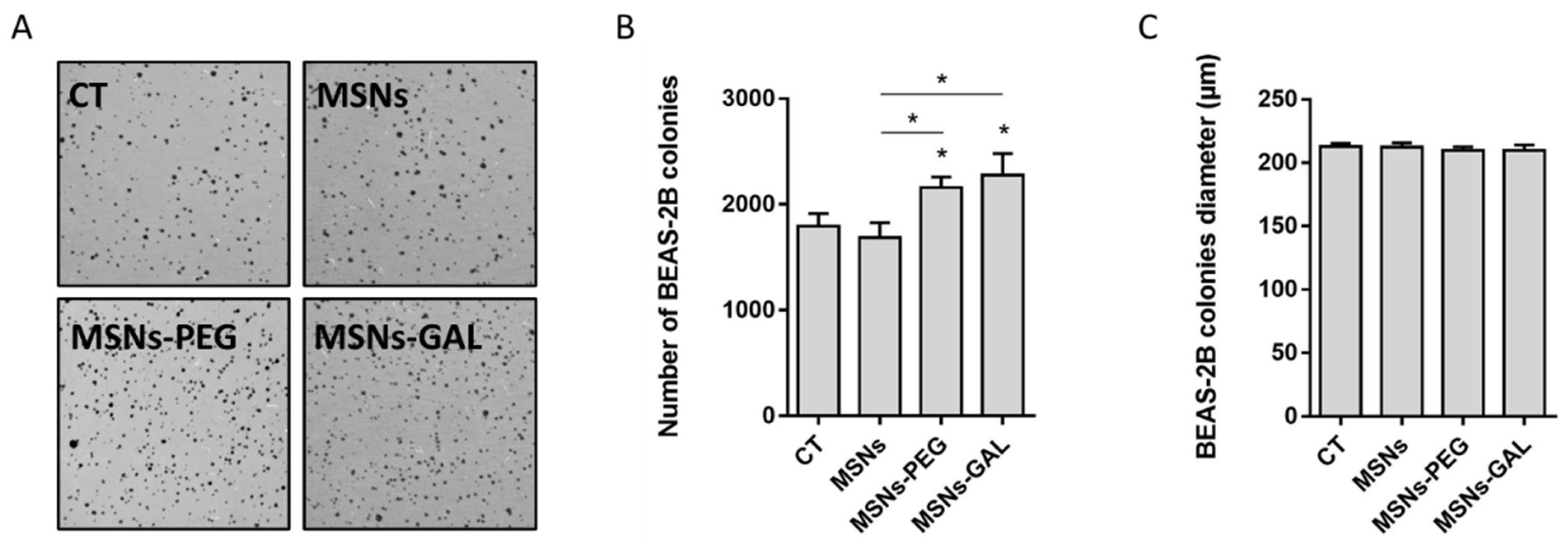
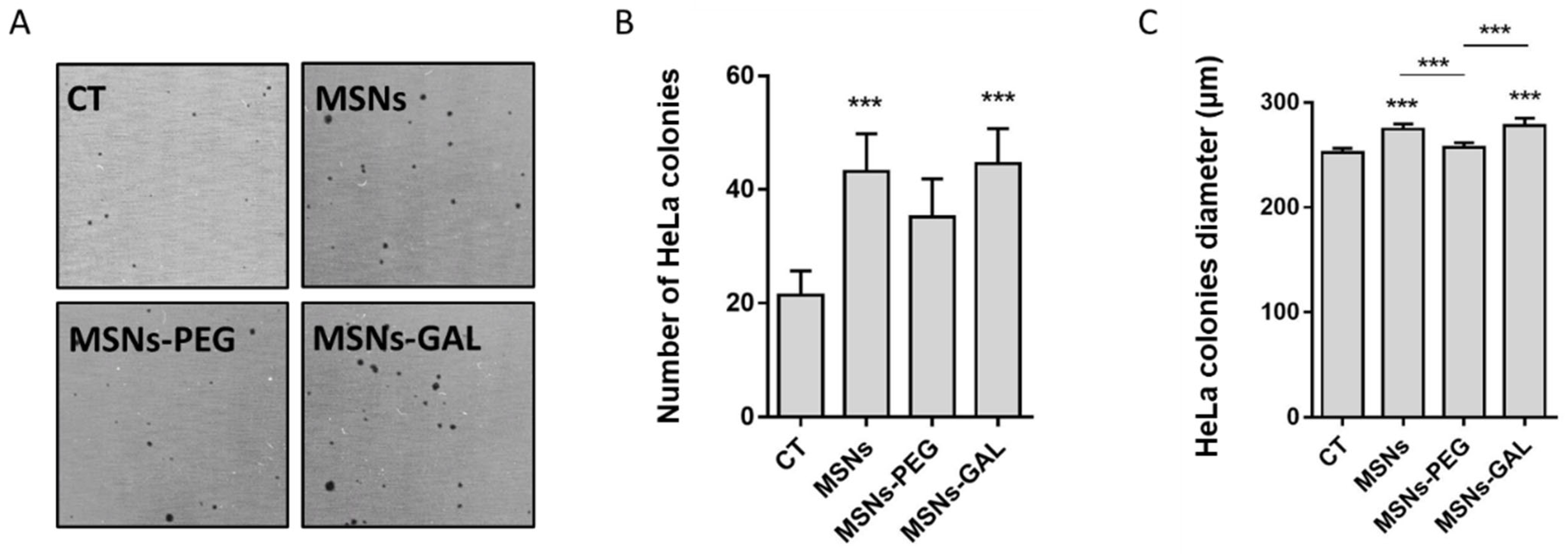
2.4. Gated MSNs Show a Distinctive Transforming Potential after Chronic Exposure
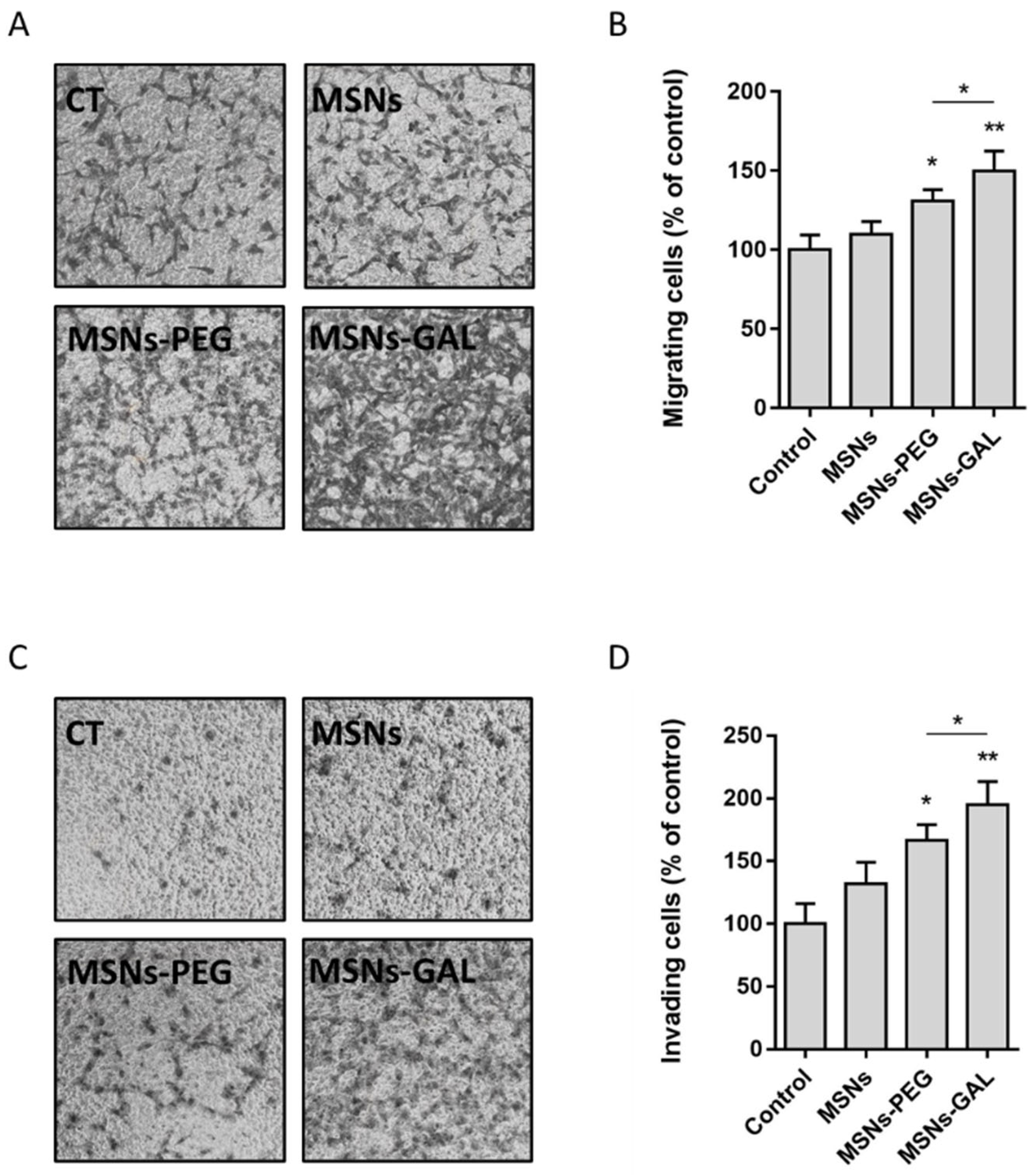
2.5. Chronically Exposed Cells Undergo Secretome Changes Leading to Cell Growth Promotion
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. General Techniques
4.3. Synthesis of MSNs
4.4. Synthesis of MSNs-PEG
4.5. Synthesis of MSNs-GAL
4.6. Cell Culture Conditions
4.7. MSNs Dispersion and In Vitro Long-Term Exposure
4.8. Cytotoxicity Assay
4.9. The Comet Assay
4.10. The Soft-Agar Assay
4.11. Migration and Invasion Assay
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Li, P.; Wang, C.; Liu, N.; Xing, D. Molecularly or atomically precise nanostructures for bio-applications: How far have we come? Mater. Horiz. 2023, 4, 100116. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Mánez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2019, 30, 1902634. [Google Scholar] [CrossRef]
- García-Fernández, A.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F. New Advances in In Vivo Applications of Gated Mesoporous Silica as Drug Delivery Nanocarriers. Small 2020, 16, 1902242. [Google Scholar] [CrossRef] [PubMed]
- Mackowiack, S.A.; Schmidt, A.; Weiss, V.; Schirnding, C.V.; Bein, T.; Bräuchle, C. Targeted Drug Delivery in Cancer Cells with Red Light Photoactivated Mesoporous Silica Nanoparticles. Nano Lett. 2013, 13, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lao, Y.H.; Mintz, R.L.; Chen, Z.; Shao, D.; Hu, H.; Wang, H.X.; Tao, Y.; Leong, K.W. A multifunctional mesoporous silica-gold nanocluster hybrid platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay. Nanoscale 2019, 11, 2631–2636. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef]
- De Luis, B.; Llopis-Lorente, A.; Sancenón, F.; Martínez-Máñez, R. Engineering chemical communication between micro/nanosystems. Chem. Soc. Rev. 2021, 50, 8829–8856. [Google Scholar] [CrossRef]
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.Á.; Pemán, J.; Prieto Rodríguez, M.; Picornell, C.; Aznar, E.; Martínez-Máñez, R. Selective and Sensitive Probe Based in Oligonucleotide-Capped Nanoporous Alumina for the Rapid Screening of Infection Produced by Candida albicans. ACS Sens. 2019, 4, 1291–1298. [Google Scholar] [CrossRef]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired diselenide-bridged mesoporous silica nanoparticles for dual-responsive protein delivery. Adv. Mater. 2018, 28, e1801198. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Park, J.H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Brief History, Preparation Method, and Biological Application of Mesoporous Silica Molecular Sieves: A Narrative Review. Molecules 2023, 28, 2013. [Google Scholar] [CrossRef]
- Niu, M.; Zhong, H.; Shao, H.; Hong, D.; Ma, T.; Xu, K. Shape-Dependent Genotoxicity of Mesoporous Silica Nanoparticles and Cellular Mechanisms. J. Nanosci. Nanotechnol. 2016, 16, 2313–2318. [Google Scholar] [CrossRef]
- Chou, C.-C.; Chen, W.; Hung, Y.; Mo, C.-Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, X.; Pan, W.; Liu, B.; Yu, L.; Wang, H.; Li, N.; Tang, B. Ratiometric Fluorescent Quanti fi cation of the Size-Dependent Cellular Toxicity of Silica Nanoparticles. Anal. Chem. 2019, 91, 6088–6096. [Google Scholar] [CrossRef]
- Zhou, F.; Liao, F.; Chen, L.; Liu, Y.; Wang, W.; Feng, S. The size-dependent genotoxicity and oxidative stress of silica nanoparticles on endothelial cells. Environ. Sci. Pollut. Res. 2019, 26, 1911–1920. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, S.; Hu, X.; Sun, D.; Yang, J.; Yang, C.; Wu, W.; Li, Y.; Gu, X.; Li, M.; et al. Toxicity and mechanism of mesoporous silica nanoparticles in eyes. Nanoscale 2020, 12, 13637–13653. [Google Scholar] [CrossRef]
- Deng, Y.-D.; Zhang, X.-D.; Yang, X.-S.; Huang, Z.-L.; Wei, X.; Yang, X.-F.; Liao, W.-Z. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J. Hazard. Mater. 2021, 409, 124502. [Google Scholar] [CrossRef] [PubMed]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.; Rehman, A.U.; Haq, I.; Al-Dossary, A.A.; Elaissari, A.; Ahmed, N. Exploiting recent trends for the synthesis and surface functionalization of mesoporous silica nanoparticles towards biomedical applications. Int. J. Pharm. X 2022, 4, 100116. [Google Scholar] [CrossRef] [PubMed]
- Westmeier, D.; Stauber, R.H.; Docter, D. The concept of bio-corona in modulating the toxicity of engineered nanomaterials (ENM). Toxicol. Appl. Pharmacol. 2016, 299, 53–57. [Google Scholar] [CrossRef]
- De Santo, M.; Giovinazzo, A.; Fava, M.; Mazzotta, E.; De Napoli, I.E.; Greco, M.; Comandé, A.; Nigro, A.; Argurio, P.; Perrotta, I.; et al. Engineered mesoporous silica-based nanoparticles as smart chemotherapy nanodevice for bortezomib administration. Mater. Chem. Front. 2023, 7, 216–229. [Google Scholar] [CrossRef]
- Nigro, A.; Frattaruolo, L.; Fava, M.; De Napoli, I.; Greco, M.; Comandè, A.; De Santo, M.; Pellegrino, M.; Ricci, E.; Giordano, F.; et al. Bortezomib-Loaded Mesoporous Silica Nanoparticles Selectively Alter Metabolism and Induce Death in Multiple Myeloma Cells. Cancers 2020, 12, 2709. [Google Scholar] [CrossRef]
- Yu, T.; Greish, K.; McGill, L.D.; Ray, A.; Ghandehari, H. Influence of Geometry, Porosity, and Surface Characteristics of Silica Nanoparticles on Acute Toxicity: Their Vasculature Effect and Tolerance Threshold. ACS Nano 2012, 6, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 2020, 324, 471–481. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical application of mesoporous silica nanoparticles as delivery systems: A biological safety perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control. Release 2019, 304, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Giménez, C.; De La Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Mondragón, L.; Bernardos, A.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Soto, J.; Costero, A.; Manguan-García, C.; Perona, R.; et al. Targeted Cargo Delivery in Senescent Cells Using Capped Mesoporous Silica Nanoparticles. Angew. Commun. 2012, 51, 10556–10560. [Google Scholar] [CrossRef]
- Patil-Sen, Y. Advances in nano-biomaterials and their applications in biomedicine. Emerg. Top. Life Sci. 2021, 5, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Lien Sergent, J.; Paget, V.; Chevillard, S. Toxicity and Genotoxicity of Nano-SiO2 on Human Epithelial Intestinal HT-29 Cell Line. Ann. Occup. Hyg. 2012, 56, 622–630. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Xu, H.; Zheng, S.; Su, M.; Wang, J. Genotoxicity of mesoporous silica nanoparticles in human embryonic kidney 293 cells. Drug Test. Anal. 2015, 7, 787–796. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Zoljalali, N.; Hanafi-Bojd, M.Y.; Nikoofal-Sahlabadi, S.; Malaekeh-Nikouei, B. Effect of mesoporous silica nanoparticles on cell viability and markers of oxidative stress. Toxicol. Mech. Methods 2015, 25, 433–439. [Google Scholar]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.R.; Dusinska, M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagen. 2015, 56, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Dusinska, M.; Mariussen, E.; Rundén-Pran, E.; Hudecova, A.M.; Elje, E.; Kazimirova, A.; El Yamani, N.; Dommershausen, N.; Tharmann, J.; Fieblinger, D.; et al. In Vitro Approaches for Assessing the Genotoxicity of Nanomaterials. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1894, pp. 83–122. [Google Scholar] [CrossRef]
- Barguilla, I.; Maguer-Satta, V.; Guyot, B.; Pastor, S.; Marcos, R.; Hernández, A. In Vitro Approaches to Determine the Potential Carcinogenic Risk of Environmental Pollutants. Int. J. Mol. Sci. 2023, 24, 7851. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhao, X.; Fan, D. Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis. Bio-Protocol 2017, 7, e2351. [Google Scholar] [CrossRef]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The Soft Agar Colony Formation Assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef]
- Yadav, K.; Ali, S.A.; Mohanty, A.K.; Muthusamy, E.; Subaharan, K.; Kaul, G. MSN, MWCNT and ZnO nanoparticle-induced CHO-K1 cell polarisation is linked to cytoskeleton ablation. J. Nanobiotechnol. 2021, 19, 45. [Google Scholar] [CrossRef]
- Rubio, L.; Bach, J.; Marcos, R.; Hernández, A. Synergistic role of nanoceria on the ability of tobacco smoke to induce carcinogenic hallmarks in lung epithelial cells. Nanomedicine 2017, 12, 2623–2635. [Google Scholar] [CrossRef]
- Kondoh, N.; Shuda, M.; Arai, M.; Oikawa, T.; Yamamoto, M. Activation of anchorage-independent growth of HT1080 human fibrosarcoma cells by dexamethasone. Vitr. Cell. Dev. Biol. Anim. 2002, 38, 111–117. [Google Scholar] [CrossRef]
- Nakamura, M.; Kanda, S.; Kawamura, M.; Igawa, T.; Kanetake, H.; Saito, Y. Effects of low concentration of vinblastine on the anchorage-independent growth and in vitro invasion of human renal carcinoma cell lines. Cancer Lett. 1993, 69, 85–91. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 449183. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Despeaux, E.; Stueckle, T.A.; Chi, A.; Castranova, V.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Role of mesothelin in carbon nanotube-induced carcinogenic transformation of human bronchial epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L538–L549. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, B.; Yang, Y.; Xin, Y.; Xie, Y.-C.; Guo, L.-H.; Mantell, L.L. Carbon Nanomaterials Stimulate HMGB1 Release from Macrophages and Induce Cell Migration and Invasion. Toxicol. Sci. 2019, 172, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, D.; Yang, J.; Zhang, D. Doxorubicin promotes breast cancer cell migration and invasion via DCAF13. FEBS Open Bio 2022, 12, 221–230. [Google Scholar] [CrossRef]
- Liu, C.-L.; Chen, M.-J.; Lin, J.-C.; Lin, C.-H.; Huang, W.-C.; Cheng, S.-P.; Chen, S.-N.; Chang, Y.-C. Doxorubicin Promotes Migration and Invasion of Breast Cancer Cells through the Upregulation of the RhoA/MLC Pathway. J. Breast Cancer 2019, 22, 185. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Lindén, M. Biodistribution and Excretion of Intravenously Injected Mesoporous Silica Nanoparticles: Implications for Drug Delivery Efficiency and Safety. Enzymes 2018, 43, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Gispert, J.D.; Menchón, C.; Baldoví, H.G.; Buaki-Sogo, M.; Rocha, M.; Abad, S.; Victor, V.M.; García, H.; Herance, J.R. Novel methodology for labelling mesoporous silica nanoparticles using the 18F isotope and their in vivo biodistribution by positron emission tomography. J. Nanopart. Res. 2015, 17, 131. [Google Scholar] [CrossRef]
- Guo, C.; Hu, J.; Kao, L.; Pan, D.; Luo, K.; Li, N.; Gu, Z. Pepetide Dendron-Functionalized Mesoporous Silica Nanoparticle-Based Nanohybrid: Biocompatibility and Its Potential as Imaging Probe. ACS Biomater. Sci. Eng. 2016, 2, 860–870. [Google Scholar] [CrossRef]
- Annangi, B.; Rubio, L.; Alaraby, M.; Bach, J.; Marcos, R.; Hernández, A. Acute and long-term in vitro effects of zinc oxide nanoparticles. Arch. Toxicol. 2016, 90, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.; Sampayo-Reyes, A.; Marcos, R.; Hernández, A. Ogg1 genetic background determines the genotoxic potential of environmentally relevant arsenic exposures. Arch. Toxicol. 2013, 88, 585–596. [Google Scholar] [CrossRef] [PubMed]

| MSNs | MSNs-PEG | MSNs-GAL | |
|---|---|---|---|
| Size (nm) (TEM) | 81.7 ± 11.2 | 82.4 ± 10.5 | 80.9 ± 14.0 |
| Size (nm) (DLS) | 148 | 245 | 300 |
| PDI (width of the distribution) (DLS) | 0.242 | 0.307 | 0.215 |
| ζ Potential (mV) | −31.7 ± 5.6 | −23.1 ± 6.2 | −12.3 ± 4.37 |
| Organic content (%) | – | 23.1 | 18.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barguilla, I.; Candela-Noguera, V.; Oliver, P.; Annangi, B.; Díez, P.; Aznar, E.; Martínez-Máñez, R.; Marcos, R.; Hernández, A.; Marcos, M.D. Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles. Int. J. Mol. Sci. 2023, 24, 16158. https://doi.org/10.3390/ijms242216158
Barguilla I, Candela-Noguera V, Oliver P, Annangi B, Díez P, Aznar E, Martínez-Máñez R, Marcos R, Hernández A, Marcos MD. Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles. International Journal of Molecular Sciences. 2023; 24(22):16158. https://doi.org/10.3390/ijms242216158
Chicago/Turabian StyleBarguilla, Irene, Vicente Candela-Noguera, Patrick Oliver, Balasubramanyam Annangi, Paula Díez, Elena Aznar, Ramón Martínez-Máñez, Ricard Marcos, Alba Hernández, and María Dolores Marcos. 2023. "Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles" International Journal of Molecular Sciences 24, no. 22: 16158. https://doi.org/10.3390/ijms242216158
APA StyleBarguilla, I., Candela-Noguera, V., Oliver, P., Annangi, B., Díez, P., Aznar, E., Martínez-Máñez, R., Marcos, R., Hernández, A., & Marcos, M. D. (2023). Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles. International Journal of Molecular Sciences, 24(22), 16158. https://doi.org/10.3390/ijms242216158






