The AKI-to-CKD Transition: The Role of Uremic Toxins
Abstract
1. Introduction
2. Acute and Chronic Kidney Diseases and Uremic Toxins: Definitions
2.1. AKI as a Risk Factor for CKD
2.1.1. Definition and Epidemiology of AKI
2.1.2. Changes in Kidney Function after an Episode of AKI
2.2. Uremic Toxins
3. Uremic Toxins in the AKI-to-CKD Transition
3.1. Gut Microbiota Dysbiosis and AKI-to-CKD Transition
3.2. The Tubulo-Interstitial Compartment
3.2.1. Maladaptive Repair and the EMT
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.2. Hypoxia
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.3. Organelle Stress
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.4. Metabolic Reprogramming
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.5. Epigenetic Alterations and Cell Cycle Arrest
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.6. The TGF-β Signaling Pathway
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.7. Inflammation
In the AKI-to-CKD Transition
The Involvement of UTs
3.2.8. Iron Death Pathways
In the AKI-to-CKD Transition
The Involvement of UTs
3.3. Endothelial Dysfunction
3.3.1. In the AKI-to-CKD Transition
3.3.2. Role of UTs in Endothelial Dysfunction during the AKI-to-CKD Transition
| UTs | Mechanisms Underlying the AKI-to-CKD Transition | Endothelium | ||
|---|---|---|---|---|
| Models | Main Results | References | ||
| IS | Oxidative stress | In vitro | - ↑ ICAM-1, ↑ MCP-1, ↑ NF-κB, ↑ ROS, ↑ E-selectin, ↑ IL-1β - NADPH oxidase inhibitors decreased IS-induced oxidative stress - ↓ Hypoxia-induced migration and tube formation - ↓ Vasorelaxation | [84,248,249,263,265,266,267] |
| In vivo | - ↓ eNOs - AST-120 ↑ neovascularization | [263,264] | ||
| pCS | Oxidative stress Inflammation | In vivo | - ↑ ROS | [85] |
| In vivo | - ↑ Leukocyte adhesion, ↑ vascular permeability | [268] | ||
3.4. Glomerular Injury
3.4.1. Glomerular Injury in the AKI-to-CKD Transition
3.4.2. The Role of UTs in Glomerular Injury during the AKI-to-CKD Transition
4. Therapies and Future Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sawhney, S.; Bell, S.; Black, C.; Christiansen, C.F.; Heide-Jørgensen, U.; Jensen, S.K.; Ronksley, P.E.; Tan, Z.; Tonelli, M.; Walker, H.; et al. Harmonization of Epidemiology of Acute Kidney Injury and Acute Kidney Disease Produces Comparable Findings across Four Geographic Populations. Kidney Int. 2022, 101, 1271–1281. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ordoñez, J.D.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Go, A.S. The Risk of Acute Renal Failure in Patients with Chronic Kidney Disease. Kidney Int. 2008, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; Hsu, C. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.; Orieux, A.; Clouzeau, B.; Rigothier, C.; Combe, C.; Gruson, D.; Boyer, A. The Incidence of Chronic Kidney Disease Three Years after Non-Severe Acute Kidney Injury in Critically Ill Patients: A Single-Center Cohort Study. J. Clin. Med. 2019, 8, 2215. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. Causes of Death after a Hospitalization with AKI. J. Am. Soc. Nephrol. 2018, 29, 1001–1010. [Google Scholar] [CrossRef]
- Hansen, M.K.; Gammelager, H.; Jacobsen, C.-J.; Hjortdal, V.E.; Layton, J.B.; Rasmussen, B.S.; Andreasen, J.J.; Johnsen, S.P.; Christiansen, C.F. Acute Kidney Injury and Long-Term Risk of Cardiovascular Events After Cardiac Surgery: A Population-Based Cohort Study. J. Cardiothorac. Vasc. Anesth. 2015, 29, 617–625. [Google Scholar] [CrossRef]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; De Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients with Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef]
- Wu, V.-C.; Wu, C.-H.; Huang, T.-M.; Wang, C.-Y.; Lai, C.-F.; Shiao, C.-C.; Chang, C.-H.; Lin, S.-L.; Chen, Y.-Y.; Chen, Y.-M.; et al. Long-Term Risk of Coronary Events after AKI. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Kang, M.W.; Yoo, H.-W.; Han, K.; Kim, Y.; Lee, J.P.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; et al. Postdischarge Long-Term Cardiovascular Outcomes of Intensive Care Unit Survivors Who Developed Dialysis-Requiring Acute Kidney Injury after Cardiac Surgery. J. Crit. Care 2019, 50, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Hsu, R.K.; Yang, J.; Ordonez, J.D.; Zheng, S.; Go, A.S. Elevated BP after AKI. J. Am. Soc. Nephrol. 2016, 27, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hsu, C.; Yang, J.; Tan, T.C.; Zheng, S.; Ordonez, J.D.; Liu, K.D. Acute Kidney Injury and Risk of Heart Failure and Atherosclerotic Events. Clin. J. Am. Soc. Nephrol. 2018, 13, 833–841. [Google Scholar] [CrossRef]
- Guzzi, F.; Cirillo, L.; Roperto, R.M.; Romagnani, P.; Lazzeri, E. Molecular Mechanisms of the Acute Kidney Injury to Chronic Kidney Disease Transition: An Updated View. Int. J. Mol. Sci. 2019, 20, 4941. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An Increasingly Recognized Risk Factor for CKD Development and Progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.Y.; Palevsky, P.M. The Link between Acute Kidney Injury and Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 149–154. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Bennis, Y.; Titeca-Beauport, D.; Caillard, P.; Cluet, Y.; Kamel, S.; Choukroun, G.; Maizel, J.; Liabeuf, S.; Bodeau, S. Two Rapid, Accurate Liquid Chromatography Tandem Mass Spectrometry Methods for the Quantification of Seven Uremic Toxins: An Application for Describing Their Accumulation Kinetic Profile in a Context of Acute Kidney Injury. J. Chromatogr. B 2020, 1152, 122234. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Claes, K.; Bammens, B.; De Loor, H.; Viaene, L.; Verbeke, K.; Kuypers, D.; Vanrenterghem, Y.; Evenepoel, P. P-Cresol and Cardiovascular Risk in Mild-to-Moderate Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1182–1189. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Hsu, R.K.; McCulloch, C.E.; Dudley, R.A.; Lo, L.J.; Hsu, C. Temporal Changes in Incidence of Dialysis-Requiring AKI. J. Am. Soc. Nephrol. 2013, 24, 37–42. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global Epidemiology and Outcomes of Acute Kidney Injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Kumela, K.; Belay, M.; Kebede, B.; Wobie, Y. Mortality and Predictors of Acute Kidney Injury in Adults: A Hospital-Based Prospective Observational Study. Sci. Rep. 2021, 11, 15672. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 Initiative for Acute Kidney Injury (Zero Preventable Deaths by 2025): A Human Rights Case for Nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Mehta, R.L.; Burdmann, E.A.; Cerdá, J.; Feehally, J.; Finkelstein, F.; García-García, G.; Godin, M.; Jha, V.; Lameire, N.H.; Levin, N.W.; et al. Recognition and Management of Acute Kidney Injury in the International Society of Nephrology 0by25 Global Snapshot: A Multinational Cross-Sectional Study. Lancet 2016, 387, 2017–2025. [Google Scholar] [CrossRef]
- Finlay, S.; Bray, B.; Lewington, A.; Hunter-Rowe, C.; Banerjee, A.; Atkinson, J.; Jones, M. Identification of Risk Factors Associated with Acute Kidney Injury in Patients Admitted to Acute Medical Units. Clin. Med. 2013, 13, 233–238. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Xi, X.-M.; Jia, H.-M.; Zheng, X.; Wang, M.-P.; Li, W.; Li, W.-X. Risk Factors, Clinical Features and Outcome of New-Onset Acute Kidney Injury among Critically Ill Patients: A Database Analysis Based on Prospective Cohort Study. BMC Nephrol. 2021, 22, 289. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Santos, W.J.; Zanetta, D.M.; Pires, A.C.; Lobo, S.M.; Lima, E.Q.; Burdmann, E.A. Patients with ischaemic, mixed and nephrotoxic acute tubular necrosis in the intensive care unit—A homogeneous population? Crit. Care 2006, 10, R68. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.; Kraft, W.; Farber, J. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Anders, H.-J. Crystal Nephropathies: Mechanisms of Crystal-Induced Kidney Injury. Nat. Rev. Nephrol. 2017, 13, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Turgut, F.; Awad, A.S.; Abdel-Rahman, E.M. Acute Kidney Injury: Medical Causes and Pathogenesis. J. Clin. Med. 2023, 12, 375. [Google Scholar] [CrossRef]
- Gaut, J.P.; Liapis, H. Acute Kidney Injury Pathology and Pathophysiology: A Retrospective Review. Clin. Kidney J. 2021, 14, 526–536. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute Kidney Injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Eadon, M.T.; Schwantes-An, T.-H.; Phillips, C.L.; Roberts, A.R.; Greene, C.V.; Hallab, A.; Hart, K.J.; Lipp, S.N.; Perez-Ledezma, C.; Omar, K.O.; et al. Kidney Histopathology and Prediction of Kidney Failure: A Retrospective Cohort Study. Am. J. Kidney Dis. 2020, 76, 350–360. [Google Scholar] [CrossRef]
- Gunawardena, S.; Dayaratne, M.; Wijesinghe, H.; Wijewickrama, E. A Systematic Review of Renal Pathology in Chronic Kidney Disease of Uncertain Etiology. Kidney Int. Rep. 2021, 6, 1711–1728. [Google Scholar] [CrossRef]
- Selvarajah, M.; Weeratunga, P.; Sivayoganthan, S.; Rathnatunga, N.; Rajapakse, S. Clinicopathological Correlates of Chronic Kidney Disease of Unknown Etiology in Sri Lanka. Indian. J. Nephrol. 2016, 26, 357. [Google Scholar] [CrossRef]
- Schelling, J.R. Tubular Atrophy in the Pathogenesis of Chronic Kidney Disease Progression. Pediatr. Nephrol. 2016, 31, 693–706. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney Fibrosis: From Mechanisms to Therapeutic Medicines. Signal. Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.J.; Barreto, S.; Gentil, T.; Assis, L.S.; Soeiro, E.M.; Castro, I.D.; Laranja, S.M. Risk Factors for the Progression of Chronic Kidney Disease after Acute Kidney Injury. Bras. J. Nefrol. 2017, 39, 239–245. [Google Scholar] [CrossRef]
- Xue, J.L.; Daniels, F.; Star, R.A.; Kimmel, P.L.; Eggers, P.W.; Molitoris, B.A.; Himmelfarb, J.; Collins, A.J. Incidence and Mortality of Acute Renal Failure in Medicare Beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 2006, 17, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Hsu, J.Y.; Lora, C.M.; Ricardo, A.C.; Anderson, A.H.; Bazzano, L.; Cuevas, M.M.; Hsu, C.; Kusek, J.W.; Renteria, A.; et al. CKD Progression and Mortality among Hispanics and Non-Hispanics. J. Am. Soc. Nephrol. 2016, 27, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.; Kao, W.H.L.; Xie, D.; Astor, B.C.; Li, M.; Hsu, C.; Feldman, H.I.; Parekh, R.S.; Kusek, J.W.; Greene, T.H.; et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2183–2196. [Google Scholar] [CrossRef]
- Hannan, M.; Ansari, S.; Meza, N.; Anderson, A.H.; Srivastava, A.; Waikar, S.; Charleston, J.; Weir, M.R.; Taliercio, J.; Horwitz, E.; et al. Risk Factors for CKD Progression: Overview of Findings from the CRIC Study. Clin. J. Am. Soc. Nephrolog. 2021, 16, 648–659. [Google Scholar] [CrossRef]
- Taal, M.W.; Brenner, B.M. Predicting Initiation and Progression of Chronic Kidney Disease: Developing Renal Risk Scores. Kidney Int. 2006, 70, 1694–1705. [Google Scholar] [CrossRef]
- Vaara, S.T.; Bhatraju, P.K.; Stanski, N.L.; McMahon, B.A.; Liu, K.; Joannidis, M.; Bagshaw, S.M. Subphenotypes in Acute Kidney Injury: A Narrative Review. Crit. Care 2022, 26, 251. [Google Scholar] [CrossRef]
- Amdur, R.L.; Chawla, L.S.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. Outcomes Following Diagnosis of Acute Renal Failure in U.S. Veterans: Focus on Acute Tubular Necrosis. Kidney Int. 2009, 76, 1089–1097. [Google Scholar] [CrossRef]
- Vikse, B.E.; Irgens, L.M.; Leivestad, T.; Skjærven, R.; Iversen, B.M. Preeclampsia and the Risk of End-Stage Renal Disease. N. Engl. J. Med. 2008, 359, 800–809. [Google Scholar] [CrossRef]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-Term Risk of Adverse Outcomes after Acute Kidney Injury: A Systematic Review and Meta-Analysis of Cohort Studies Using Consensus Definitions of Exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic Kidney Disease after Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Nelson, D.; Clothier, B.; Schult, T.; Nugent, S.; Greer, N.; Slinin, Y.; Ensrud, K.E. The Magnitude of Acute Serum Creatinine Increase After Cardiac Surgery and the Risk of Chronic Kidney Disease, Progression of Kidney Disease, and Death. Arch. Intern. Med. 2011, 171, 226. [Google Scholar] [CrossRef]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin. J. Am. Soc. Nephrol. 2011, 6, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, D.E.; Coca, S.G.; Kalantar-Zadeh, K. Does AKI Truly Lead to CKD? J. Am. Soc. Nephrol. 2012, 23, 979–984. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, L.; Melchinger, I.; Thiessen-Philbrook, H.; Moledina, D.G.; Coca, S.G.; Hsu, C.; Go, A.S.; Liu, K.D.; Siew, E.D.; et al. Longitudinal Biomarkers and Kidney Disease Progression after Acute Kidney Injury. JCI Insight 2023, 8, e167731. [Google Scholar] [CrossRef]
- Ko, G.J.; Grigoryev, D.N.; Linfert, D.; Jang, H.R.; Watkins, T.; Cheadle, C.; Racusen, L.; Rabb, H. Transcriptional Analysis of Kidneys during Repair from AKI Reveals Possible Roles for NGAL and KIM-1 as Biomarkers of AKI-to-CKD Transition. Am. J. Physiol. Ren. Physiol. 2010, 298, F1472–F1483. [Google Scholar] [CrossRef]
- Glassock, R.J. Uremic Toxins: What Are They? An Integrated Overview of Pathobiology and Classification. J. Ren. Nutr. 2008, 18, 2–6. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between Two Sulfate-Conjugated Uremic Toxins, p -Cresyl Sulfate and Indoxyl Sulfate, during Binding with Human Serum Albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bush, K.T.; Nigam, S.K. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in Vivo Handling of Uremic Toxins and Solutes. Sci. Rep. 2017, 7, 4939. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Yamamoto, T.; Matsuo, H.; Tan, J.K.; Ooyama, K.; Sakiyama, M.; Miyata, H.; Yamanashi, Y.; Toyoda, Y.; Higashino, T.; et al. Identification of ABCG2 as an Exporter of Uremic Toxin Indoxyl Sulfate in Mice and as a Crucial Factor Influencing CKD Progression. Sci. Rep. 2018, 8, 11147. [Google Scholar] [CrossRef] [PubMed]
- European Uremic Toxin Work Group (EUTox) —Uremic Solutes Database. List of Uremic Solutes. Available online: https://database.uremic-toxins.org/soluteList.php (accessed on 8 August 2023).
- Addi, T.; Poitevin, S.; McKay, N.; El Mecherfi, K.E.; Kheroua, O.; Jourde-Chiche, N.; De Macedo, A.; Gondouin, B.; Cerini, C.; Brunet, P.; et al. Mechanisms of Tissue Factor Induction by the Uremic Toxin Indole-3 Acetic Acid through Aryl Hydrocarbon Receptor/Nuclear Factor-Kappa B Signaling Pathway in Human Endothelial Cells. Arch. Toxicol. 2019, 93, 121–136. [Google Scholar] [CrossRef]
- Dou, L.; Poitevin, S.; Sallée, M.; Addi, T.; Gondouin, B.; McKay, N.; Denison, M.S.; Jourde-Chiche, N.; Duval-Sabatier, A.; Cerini, C.; et al. Aryl Hydrocarbon Receptor Is Activated in Patients and Mice with Chronic Kidney Disease. Kidney Int. 2018, 93, 986–999. [Google Scholar] [CrossRef]
- Adesso, S.; Magnus, T.; Cuzzocrea, S.; Campolo, M.; Rissiek, B.; Paciello, O.; Autore, G.; Pinto, A.; Marzocco, S. Indoxyl Sulfate Affects Glial Function Increasing Oxidative Stress and Neuroinflammation in Chronic Kidney Disease: Interaction between Astrocytes and Microglia. Front. Pharmacol. 2017, 8, 370. [Google Scholar] [CrossRef]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef]
- Ichii, O.; Otsuka-Kanazawa, S.; Nakamura, T.; Ueno, M.; Kon, Y.; Chen, W.; Rosenberg, A.Z.; Kopp, J.B. Podocyte Injury Caused by Indoxyl Sulfate, a Uremic Toxin and Aryl-Hydrocarbon Receptor Ligand. PLoS ONE 2014, 9, e108448. [Google Scholar] [CrossRef]
- Satoh, M.; Hayashi, H.; Watanabe, M.; Ueda, K.; Yamato, H.; Yoshioka, T.; Motojima, M. Uremic Toxins Overload Accelerates Renal Damage in a Rat Model of Chronic Renal Failure. Nephron Exp. Nephrol. 2004, 95, e111–e118. [Google Scholar] [CrossRef]
- Han, H.; Zhu, J.; Zhu, Z.; Ni, J.; Du, R.; Dai, Y.; Chen, Y.; Wu, Z.; Lu, L.; Zhang, R. p-Cresyl Sulfate Aggravates Cardiac Dysfunction Associated with Chronic Kidney Disease by Enhancing Apoptosis of Cardiomyocytes. J. Am. Heart Assoc. 2015, 4, e001852. [Google Scholar] [CrossRef]
- Hénaut, L.; Grissi, M.; Brazier, F.; Assem, M.; Poirot-Leclercq, S.; Lenglet, G.; Boudot, C.; Avondo, C.; Boullier, A.; Choukroun, G.; et al. Cellular and Molecular Mechanisms Associated with Ischemic Stroke Severity in Female Mice with Chronic Kidney Disease. Sci. Rep. 2019, 9, 6432. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, M.; Hermanowicz, J.M.; Tankiewicz-Kwedlo, A.; Kalaska, B.; Kaminski, T.W.; Nosek, K.; Wisniewska, R.J.; Pawlak, D. Neurobehavioral Effects of Uremic Toxin–Indoxyl Sulfate in the Rat Model. Sci. Rep. 2020, 10, 9483. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free P-Cresylsulphate Is a Predictor of Mortality in Patients at Different Stages of Chronic Kidney Disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The Cardiovascular Effect of the Uremic Solute Indole-3 Acetic Acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Cherng, Y.-G.; Lin, C.-S.; Shih, C.-C.; Hsu, Y.-H.; Yeh, C.-C.; Hu, C.-J.; Chen, T.-L.; Liao, C.-C. Stroke Risk and Outcomes in Patients with Chronic Kidney Disease or End-Stage Renal Disease: Two Nationwide Studies. PLoS ONE 2018, 13, e0191155. [Google Scholar] [CrossRef]
- Tan, X.; Zou, J.; Xiang, F.; Zhang, P.; Shen, B.; Wang, Y.; Ding, X.; Cao, X. P-Cresyl Sulfate Predicts Ischemic Stroke among Patients on Hemodialysis: A Prospective Cohort Study. Dis. Markers 2022, 2022, 1358419. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, P.-H.; Liang, S.-S.; Mubanga, M.; Yang, Y.-H.; Hsu, Y.-L.; Kuo, M.-C.; Hwang, S.-J.; Kuo, P.-L. Protein-Bound Uremic Toxins Are Associated with Cognitive Function among Patients Undergoing Maintenance Hemodialysis. Sci. Rep. 2019, 9, 20388. [Google Scholar] [CrossRef]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.-D.; Vanholder, R.; Choukroun, G.; Massy, Z.A. Uremic Toxicity and Sclerostin in Chronic Kidney Disease Patients. Néphrologie Thérapeutique 2014, 10, 463–470. [Google Scholar] [CrossRef]
- Wang, W.; Hao, G.; Pan, Y.; Ma, S.; Yang, T.; Shi, P.; Zhu, Q.; Xie, Y.; Ma, S.; Zhang, Q.; et al. Serum Indoxyl Sulfate Is Associated with Mortality in Hospital-Acquired Acute Kidney Injury: A Prospective Cohort Study. BMC Nephrol. 2019, 20, 57. [Google Scholar] [CrossRef]
- Veldeman, L.; Vanmassenhove, J.; Van Biesen, W.; Massy, Z.A.; Liabeuf, S.; Glorieux, G.; Vanholder, R. Evolution of Protein-Bound Uremic Toxins Indoxyl Sulphate and p-Cresyl Sulphate in Acute Kidney Injury. Int. Urol. Nephrol. 2019, 51, 293–302. [Google Scholar] [CrossRef]
- Mabuchi, H.; Nakahashi, H. A Major Inhibitor of Phenytoin Binding to Serum Protein in Uremia. Nephron 1988, 48, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The Uremic Solutes P-Cresol and Indoxyl Sulfate Inhibit Endothelial Proliferation and Wound Repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The Uremic Solute Indoxyl Sulfate Induces Oxidative Stress in Endothelial Cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Massy, Z.A.; Henaut, L.; Boudot, C.; Cagnard, J.; March, C.; Kamel, S.; Drueke, T.B.; Six, I. Para-Cresyl Sulfate Acutely Impairs Vascular Reactivity and Induces Vascular Remodeling: Para-Cresyl Sulfate and Vascular Dysfunction. J. Cell. Physiol. 2015, 230, 2927–2935. [Google Scholar] [CrossRef]
- Caillard, P.; Bennis, Y.; Six, I.; Bodeau, S.; Kamel, S.; Choukroun, G.; Maizel, J.; Titeca-Beauport, D. The Role of Gut-Derived, Protein-Bound Uremic Toxins in the Cardiovascular Complications of Acute Kidney Injury. Toxins 2022, 14, 336. [Google Scholar] [CrossRef]
- Popkov, V.A.; Zharikova, A.A.; Demchenko, E.A.; Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Gut Microbiota as a Source of Uremic Toxins. Int. J. Mol. Sci. 2022, 23, 483. [Google Scholar] [CrossRef]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.-G.; Oh, S.W.; Cho, W.Y.; Im, S.-H.; Jo, S.K. Intestinal Microbiota Control Acute Kidney Injury Severity by Immune Modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Noel, S.; Martina-Lingua, M.N.; Bandapalle, S.; Pluznick, J.; Hamad, A.R.A.; Peterson, D.A.; Rabb, H. Intestinal Microbiota-Kidney Cross Talk in Acute Kidney Injury and Chronic Kidney Disease. Nephron Clin. Pr. 2014, 127, 139–143. [Google Scholar] [CrossRef]
- Poesen, R.; Windey, K.; Neven, E.; Kuypers, D.; De Preter, V.; Augustijns, P.; D’Haese, P.; Evenepoel, P.; Verbeke, K.; Meijers, B. The Influence of CKD on Colonic Microbial Metabolism. J. Am. Soc. Nephrol. 2016, 27, 1389–1399. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska-Rosołowska, A.; Sroka, N.; Kakareko, K.; Rosołowski, M.; Zbroch, E.; Hryszko, T. The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review. Toxins 2020, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moturi, K.R.; Wang, L.; Zhang, K.; Yu, C. Gut Derived-Endotoxin Contributes to Inflammation in Severe Ischemic Acute Kidney Injury. BMC Nephrol. 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation Between Intraluminal Oxygen Gradient and Radial Partitioning of Intestinal Microbiota. Gastroenterology 2014, 147, 1055–1063.e8. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen Battle in the Gut: Hypoxia and Hypoxia-Inducible Factors in Metabolic and Inflammatory Responses in the Intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Saranya, G.; Viswanathan, P. Gut Microbiota Dysbiosis in AKI to CKD Transition. Biomed. Pharmacother. 2023, 161, 114447. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, L.; Yang, T.; Feng, Y.-L.; Vaziri, N.D.; Liu, B.-L.; Liu, Q.-Q.; Guo, Y.; Zhao, Y.-Y. Aryl Hydrocarbon Receptor Activation Mediates Kidney Disease and Renal Cell Carcinoma. J. Transl. Med. 2019, 17, 302. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and Function of Myofibroblasts in Kidney Fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Musiał, K.; Bargenda, A.; Zwolińska, D. Urine Survivin, E-Cadherin and Matrix Metalloproteinases as Novel Biomarkers in Children with Chronic Kidney Disease. Biomarkers 2015, 20, 177–182. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Ferrario, F.; Giardino, L.; Dell’Antonio, G.; Grillo, C.; Grillo, P.; Strutz, F.; Müller, G.A.; Colasanti, G.; D’Amico, G. Epithelial-Mesenchymal Transition of Tubular Epithelial Cells in Human Renal Biopsies. Kidney Int. 2002, 62, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.; Ali, S.; McDonnell, B.J.; Burt, A.D.; Kirby, J.A. Chronic Renal Allograft Dysfunction: The Role of T Cell–Mediated Tubular Epithelial to Mesenchymal Cell Transition. J. Am. Soc. Nephrol. 2004, 15, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Nakamura, S.; Miglinas, M.; Enomoto, A.; Niwa, T. Accumulation of Indoxyl Sulfate in OAT1/3-Positive Tubular Cells in Kidneys of Patients with Chronic Renal Failure. J. Ren. Nutr. 2006, 16, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yu, M.-A.; Ryu, E.S.; Jang, Y.-H.; Kang, D.-H. Indoxyl Sulfate-Induced Epithelial-to-Mesenchymal Transition and Apoptosis of Renal Tubular Cells as Novel Mechanisms of Progression of Renal Disease. Lab. Investig. 2012, 92, 488–498. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic Toxins Induce Kidney Fibrosis by Activating Intrarenal Renin–Angiotensin–Aldosterone System Associated Epithelial-to-Mesenchymal Transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef]
- Chang, J.-F.; Hsieh, C.-Y.; Lu, K.-C.; Chen, Y.-W.; Liang, S.-S.; Lin, C.-C.; Hung, C.-F.; Liou, J.-C.; Wu, M.-S. Therapeutic Targeting of Aristolochic Acid Induced Uremic Toxin Retention, SMAD 2/3 and JNK/ERK Pathways in Tubulointerstitial Fibrosis: Nephroprotective Role of Propolis in Chronic Kidney Disease. Toxins 2020, 12, 364. [Google Scholar] [CrossRef]
- Milanesi, S.; Garibaldi, S.; Saio, M.; Ghigliotti, G.; Picciotto, D.; Ameri, P.; Garibotto, G.; Barisione, C.; Verzola, D. Indoxyl Sulfate Induces Renal Fibroblast Activation through a Targetable Heat Shock Protein 90-Dependent Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 2050183. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Higashiyama, Y.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Induces Epithelial-to-Mesenchymal Transition in Rat Kidneys and Human Proximal Tubular Cells. Am. J. Nephrol. 2011, 34, 318–323. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Niwa, T. AST-120 Ameliorates Epithelial-to-Mesenchymal Transition and Interstitial Fibrosis in the Kidneys of Chronic Kidney Disease Rats. J. Ren. Nutr. 2012, 22, 176–180. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chao, C.-T.; Huang, J.-W.; Hung, K.-Y.; Liu, S.-H.; Tarng, D.-C.; Chiang, C.-K. Early Elimination of Uremic Toxin Ameliorates AKI-to-CKD Transition. Clin. Sci. 2021, 135, 2643–2658. [Google Scholar] [CrossRef]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uremic Toxins of Organic Anions Up-Regulate PAI-1 Expression by Induction of NF-κB and Free Radical in Proximal Tubular Cells. Kidney Int. 2003, 63, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Enhances P53-TGF-β1-Smad3 Pathway in Proximal Tubular Cells. Am. J. Nephrol. 2013, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Aoyama, I.; Ise, M.; Seo, H.; Niwa, T. An Oral Sorbent Reduces Overload of Indoxyl Sulphate and Gene Expression of TGF-β1 in Uraemic Rat Kidneys. Nephrol. Dial. Transplant. 2000, 15, 1773–1781. [Google Scholar] [CrossRef]
- Aoyama, I.; Shimokata, K.; Niwa, T. An Oral Adsorbent Downregulates Renal Expression of Genes That Promote Interstitial Inflammation and Fibrosis in Diabetic Rats. Nephron 2002, 92, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic Kidney Disease-Induced Cardiac Fibrosis Is Ameliorated by Reducing Circulating Levels of a Non-Dialysable Uremic Toxin, Indoxyl Sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef]
- Shimoishi, K.; Anraku, M.; Kitamura, K.; Tasaki, Y.; Taguchi, K.; Hashimoto, M.; Fukunaga, E.; Maruyama, T.; Otagiri, M. An Oral Adsorbent, AST-120 Protects Against the Progression of Oxidative Stress by Reducing the Accumulation of Indoxyl Sulfate in the Systemic Circulation in Renal Failure. Pharm. Res. 2007, 24, 1283–1289. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, S.; Higashiyama, Y.; Nishijima, F.; Niwa, T. CREB, NF-κB, and NADPH Oxidase Coordinately Upregulate Indoxyl Sulfate-Induced Angiotensinogen Expression in Proximal Tubular Cells. Am. J. Physiol. Cell Physiol. 2013, 304, C685–C692. [Google Scholar] [CrossRef]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl Sulfate, a Uremic Toxin, Downregulates Renal Expression of Nrf2 through Activation of NF-κB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef]
- Nakagawa, N.; Hasebe, N.; Sumitomo, K.; Fujino, T.; Fukuzawa, J.; Hirayama, T.; Kikuchi, K. An Oral Adsorbent, AST-120, Suppresses Oxidative Stress in Uremic Rats. Am. J. Nephrol. 2006, 26, 455–461. [Google Scholar] [CrossRef]
- Palm, F.; Nangaku, M.; Fasching, A.; Tanaka, T.; Nordquist, L.; Hansell, P.; Kawakami, T.; Nishijima, F.; Fujita, T. Uremia Induces Abnormal Oxygen Consumption in Tubules and Aggravates Chronic Hypoxia of the Kidney via Oxidative Stress. Am. J. Physiol. Ren. Physiol. 2010, 299, F380–F386. [Google Scholar] [CrossRef]
- Mutsaers, H.A.M.; Wilmer, M.J.G.; Reijnders, D.; Jansen, J.; Van Den Broek, P.H.H.; Forkink, M.; Schepers, E.; Glorieux, G.; Vanholder, R.; Van Den Heuvel, L.P.; et al. Uremic Toxins Inhibit Renal Metabolic Capacity through Interference with Glucuronidation and Mitochondrial Respiration. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Cheng, M.-L.; Pan, H.-C.; Lee, J.-H.; Lee, C.-C. Protein-Bound Uremic Toxins Impaired Mitochondrial Dynamics and Functions. Oncotarget 2017, 8, 77722–77733. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.-J.; Yang, H.-M.; Lyu, Y.-S.; Pae, H.-O.; Ju, S.-M.; Jeon, B.-H. Apigenin Inhibits Indoxyl Sulfate-Induced Endoplasmic Reticulum Stress and Anti-Proliferative Pathways, CHOP and IL-6/P21, in Human Renal Proximal Tubular Cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2303–2310. [Google Scholar] [PubMed]
- Kawakami, T.; Inagi, R.; Wada, T.; Tanaka, T.; Fujita, T.; Nangaku, M. Indoxyl Sulfate Inhibits Proliferation of Human Proximal Tubular Cells via Endoplasmic Reticulum Stress. Am. J. Physiol. Ren. Physiol. 2010, 299, F568–F576. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, D.H.; Lee, S.J.; Kang, Y.J.; Kim, G.; Koh, H.B.; Ko, Y.E.; Shin, H.M.; Lee, H.; Yoo, T.-H.; et al. Uremic Toxin Indoxyl Sulfate Induces Trained Immunity via the AhR-Dependent Arachidonic Acid Pathway in ESRD. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hopp, K.; Kleczko, E.K.; Gitomer, B.Y.; Chonchol, M.; Klawitter, J.; Christians, U.; Klawitter, J. Metabolic Reprogramming in a Slowly Developing Orthologous Model of Polycystic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2022, 322, F258–F267. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, X.; Nie, L.; Liu, Y.; He, T.; Xiong, J.; Xu, X.; Li, Y.; Yang, K.; Wang, Y.; et al. DNA Hypermethylation of sFRP5 Contributes to Indoxyl Sulfate-Induced Renal Fibrosis. J. Mol. Med. 2017, 95, 601–613. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Suppression of Klotho Expression by Protein-Bound Uremic Toxins Is Associated with Increased DNA Methyltransferase Expression and DNA Hypermethylation. Kidney Int. 2012, 81, 640–650. [Google Scholar] [CrossRef]
- Shimizu, H.; Bolati, D.; Adijiang, A.; Adelibieke, Y.; Muteliefu, G.; Enomoto, A.; Higashiyama, Y.; Higuchi, Y.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Downregulates Renal Expression of Klotho through Production of ROS and Activation of Nuclear Factor-ĸB. Am. J. Nephrol. 2011, 33, 319–324. [Google Scholar] [CrossRef]
- Shimizu, H.; Bolati, D.; Adijiang, A.; Muteliefu, G.; Enomoto, A.; Nishijima, F.; Dateki, M.; Niwa, T. NF-κB Plays an Important Role in Indoxyl Sulfate-Induced Cellular Senescence, Fibrotic Gene Expression, and Inhibition of Proliferation in Proximal Tubular Cells. Am. J. Physiol. Cell Physiol. 2011, 301, C1201–C1212. [Google Scholar] [CrossRef]
- Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Stat3 Contributes to Indoxyl Sulfate-Induced Inflammatory and Fibrotic Gene Expression and Cellular Senescence. Am. J. Nephrol. 2012, 36, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Hsu, H.-H.; Wu, M.-S. P-Cresol Sulfate and Indoxyl Sulfate Induce Similar Cellular Inflammatory Gene Expressions in Cultured Proximal Renal Tubular Cells. Nephrol. Dial. Transplant. 2013, 28, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Takeshita, K.; Saito, S.; Murohara, T.; Niwa, T. Indole-3-Propionic Acid Suppresses Indoxyl Sulfate-Induced Expression of Fibrotic and Inflammatory Genes in Proximal Tubular Cells. Nagoya J. Med. Sci. 2017, 79, 477. [Google Scholar] [CrossRef]
- Ito, S.; Higuchi, Y.; Yagi, Y.; Nishijima, F.; Yamato, H.; Ishii, H.; Osaka, M.; Yoshida, M. Reduction of Indoxyl Sulfate by AST-120 Attenuates Monocyte Inflammation Related to Chronic Kidney Disease. J. Leukoc. Biol. 2013, 93, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Barisione, C.; Garibaldi, S.; Furfaro, A.L.; Nitti, M.; Palmieri, D.; Passalacqua, M.; Garuti, A.; Verzola, D.; Parodi, A.; Ameri, P.; et al. Moderate Increase of Indoxyl Sulfate Promotes Monocyte Transition into Profibrotic Macrophages. PLoS ONE 2016, 11, e0149276. [Google Scholar] [CrossRef]
- Shimizu, H.; Bolati, D.; Higashiyama, Y.; Nishijima, F.; Shimizu, K.; Niwa, T. Indoxyl Sulfate Upregulates Renal Expression of MCP-1 via Production of ROS and Activation of NF-κB, P53, ERK, and JNK in Proximal Tubular Cells. Life Sci. 2012, 90, 525–530. [Google Scholar] [CrossRef]
- Hamano, H.; Ikeda, Y.; Watanabe, H.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Imanishi, M.; Zamami, Y.; Takechi, K.; Miyamoto, L.; Ishizawa, K.; et al. The Uremic Toxin Indoxyl Sulfate Interferes with Iron Metabolism by Regulating Hepcidin in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2018, 33, 586–597. [Google Scholar] [CrossRef]
- Ahmed, M.S.E.; Abed, M.; Voelkl, J.; Lang, F. Triggering of Suicidal Erythrocyte Death by Uremic Toxin Indoxyl Sulfate. BMC Nephrol. 2013, 14, 244. [Google Scholar] [CrossRef]
- Tozoni, S.S.; Dias, G.F.; Bohnen, G.; Grobe, N.; Pecoits-Filho, R.; Kotanko, P.; Moreno-Amaral, A.N. Uremia and Hypoxia Independently Induce Eryptosis and Erythrocyte Redox Imbalance. Cell Physiol. Biochem. 2019, 53, 794–804. [Google Scholar] [CrossRef]
- Gao, C.; Ji, S.; Dong, W.; Qi, Y.; Song, W.; Cui, D.; Shi, J. Indolic Uremic Solutes Enhance Procoagulant Activity of Red Blood Cells through Phosphatidylserine Exposure and Microparticle Release. Toxins 2015, 7, 4390–4403. [Google Scholar] [CrossRef]
- Rutkowski, P.; Maria Słomińska, E.; Szołkiewicz, M.; Aleksandrowicz, E.; Tomasz Smoleński, R.; Wołyniec, W.; Renke, M.; Wisterowicz, K.; Świerczyński, J.; Rutkowski, B. Relationship between Uremic Toxins and Oxidative Stress in Patients with Chronic Renal Failure. Scand. J. Urol. Nephrol. 2007, 41, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Aiuchi, T.; Nakaya, K.; Emoto, Y.; Miyazaki, T.; Maeda, K. Inhibition of Mitochondrial Respiration by Furancarboxylic Acid Accumulated in Uremic Serum in Its Albumin-Bound and Non-Dialyzable Form. Clin. Nephrol. 1993, 39, 92–96. [Google Scholar] [PubMed]
- Park, J.S.; Kim, D.-H.; Choi, H.-I.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. 3-Carboxy-4-Methyl-5-Propyl-2-Furanpropanoic Acid (CMPF) Induces Cell Death through Ferroptosis and Acts as a Trigger of Apoptosis in Kidney Cells. Cell Death Dis. 2023, 14, 78. [Google Scholar] [CrossRef]
- Okada, A.; Nangaku, M.; Jao, T.-M.; Maekawa, H.; Ishimono, Y.; Kawakami, T.; Inagi, R. D-Serine, a Novel Uremic Toxin, Induces Senescence in Human Renal Tubular Cells via GCN2 Activation. Sci. Rep. 2017, 7, 11168. [Google Scholar] [CrossRef] [PubMed]
- Mihout, F.; Shweke, N.; Bigé, N.; Jouanneau, C.; Dussaule, J.-C.; Ronco, P.; Chatziantoniou, C.; Boffa, J.-J. Asymmetric Dimethylarginine (ADMA) Induces Chronic Kidney Disease through a Mechanism Involving Collagen and TGF-Β1 Synthesis. J. Pathol. 2011, 223, 37–45. [Google Scholar] [CrossRef]
- Schurek, H.J.; Jost, U.; Baumgartl, H.; Bertram, H.; Heckmann, U. Evidence for a Preglomerular Oxygen Diffusion Shunt in Rat Renal Cortex. Am. J. Physiol. Ren. Physiol. 1990, 259, F910–F915. [Google Scholar] [CrossRef]
- Welch, W.J.; Baumgärtl, H.; Lübbers, D.; Wilcox, C.S. Nephron pO2 and Renal Oxygen Usage in the Hypertensive Rat Kidney. Kidney Int. 2001, 59, 230–237. [Google Scholar] [CrossRef]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal Oxygenation: Unique Challenges and the Biophysical Basis of Homeostasis. Am. J. Physiol. Ren. Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef]
- Mimura, I.; Nangaku, M. The Suffocating Kidney: Tubulointerstitial Hypoxia in End-Stage Renal Disease. Nat. Rev. Nephrol. 2010, 6, 667–678. [Google Scholar] [CrossRef]
- Shoji, K.; Tanaka, T.; Nangaku, M. Role of Hypoxia in Progressive Chronic Kidney Disease and Implications for Therapy. Curr. Opin. Nephrol. Hypertens. 2014, 23, 161–168. [Google Scholar] [CrossRef]
- Orphanides, C.; Fine, L.G.; Norman, J.T. Hypoxia Stimulates Proximal Tubular Cell Matrix Production via a TGF-Β1-Independent Mechanism. Kidney Int. 1997, 52, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Manotham, K.; Tanaka, T.; Matsumoto, M.; Ohse, T.; Inagi, R.; Miyata, T.; Kurokawa, K.; Fujita, T.; Ingelfinger, J.R.; Nangaku, M. Transdifferentiation of Cultured Tubular Cells Induced by Hypoxia. Kidney Int. 2004, 65, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, R.; Dobberfuhl, A.D.; Cooley, C.; Overstreet, J.M.; Patel, S.; Goldschmeding, R.; Meldrum, K.K.; Higgins, P.J. Induction of Renal Fibrotic Genes by TGF-Β1 Requires EGFR Activation, P53 and Reactive Oxygen Species. Cell. Signal. 2013, 25, 2198–2209. [Google Scholar] [CrossRef]
- Han, W.-Q.; Zhu, Q.; Hu, J.; Li, P.-L.; Zhang, F.; Li, N. Hypoxia-Inducible Factor Prolyl-Hydroxylase-2 Mediates Transforming Growth Factor Beta 1-Induced Epithelial–Mesenchymal Transition in Renal Tubular Cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seok, Y.M.; Jung, K.-J.; Park, K.M. Reactive Oxygen Species/Oxidative Stress Contributes to Progression of Kidney Fibrosis Following Transient Ischemic Injury in Mice. Am. J. Physiol. Ren. Physiol. 2009, 297, F461–F470. [Google Scholar] [CrossRef] [PubMed]
- Nezu, M.; Souma, T.; Yu, L.; Suzuki, T.; Saigusa, D.; Ito, S.; Suzuki, N.; Yamamoto, M. Transcription Factor Nrf2 Hyperactivation in Early-Phase Renal Ischemia-Reperfusion Injury Prevents Tubular Damage Progression. Kidney Int. 2017, 91, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Small, D.M.; Vesey, D.A.; Johnson, D.W.; Francis, R.; Vitetta, L.; Gobe, G.C.; Morais, C. Indoxyl Sulphate and Kidney Disease: Causes, Consequences and Interventions: Indoxyl Sulphate and the Kidneys. Nephrology 2016, 21, 170–177. [Google Scholar] [CrossRef]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef]
- Vida, C.; Oliva, C.; Yuste, C.; Ceprián, N.; Caro, P.J.; Valera, G.; González De Pablos, I.; Morales, E.; Carracedo, J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants 2021, 10, 1155. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial Diseases: The Contribution of Organelle Stress Responses to Pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial Dysfunction in the Pathophysiology of Renal Diseases. Am. J. Physiol. Ren. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Tapia, E.; Rojas-Morales, P.; León-Contreras, J.C.; Martínez-Klimova, E.; Hernández-Pando, R.; Sánchez- Lozada, L.G.; Pedraza-Chaverri, J. Chronic Impairment of Mitochondrial Bioenergetics and β-Oxidation Promotes Experimental AKI-to-CKD Transition Induced by Folic Acid. Free Radic. Biol. Med. 2020, 154, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.M.; Huang, L.; Wilson, R.J.; Bajwa, A.; Sesaki, H.; Yan, Z.; Rosin, D.L.; Kashatus, D.F.; Okusa, M.D. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Ren, H.; Wu, J.; Wu, X.; Liu, S.; Wang, G.; Gu, G.; Guo, K.; Li, J. Urinary Mitochondrial DNA Identifies Renal Dysfunction and Mitochondrial Damage in Sepsis-Induced Acute Kidney Injury. Oxidative Med. Cell. Longev. 2018, 2018, 8074936. [Google Scholar] [CrossRef]
- Gallazzini, M.; Pallet, N. Endoplasmic Reticulum Stress and Kidney Dysfunction: Endoplasmic Reticulum Stress. Biol. Cell 2018, 110, 205–216. [Google Scholar] [CrossRef]
- Mo, J.-S.; Choi, D.; Han, Y.-R.; Kim, N.; Jeong, H.-S. Morin Has Protective Potential against ER Stress Induced Apoptosis in Renal Proximal Tubular HK-2 Cells. Biomed. Pharmacother. 2019, 112, 108659. [Google Scholar] [CrossRef]
- Porter, A.W.; Nguyen, D.N.; Clayton, D.R.; Ruiz, W.G.; Mutchler, S.M.; Ray, E.C.; Marciszyn, A.L.; Nkashama, L.J.; Subramanya, A.R.; Gingras, S.; et al. The Molecular Chaperone GRP170 Protects against ER Stress and Acute Kidney Injury in Mice. JCI Insight 2022, 7, e151869. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, W.; Lee, K.; Salem, F.; Wen, J.; He, L.; Zhang, J.; Fei, Y.; Cheng, D.; Bao, H.; et al. Inhibition of Reticulon-1A–Mediated Endoplasmic Reticulum Stress in Early AKI Attenuates Renal Fibrosis Development. J. Am. Soc. Nephrolog. 2017, 28, 2007–2021. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.-J.; Manson, S.R.; Molina, C.A.F.; Kidd, K.; Thiessen-Philbrook, H.; Perry, R.J.; Liapis, H.; Kmoch, S.; Parikh, C.R.; et al. Elevated Urinary CRELD2 Is Associated with Endoplasmic Reticulum Stress–Mediated Kidney Disease. JCI Insight 2017, 2, e92896. [Google Scholar] [CrossRef]
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094. [Google Scholar] [CrossRef] [PubMed]
- Thome, T.; Kumar, R.A.; Burke, S.K.; Khattri, R.B.; Salyers, Z.R.; Kelley, R.C.; Coleman, M.D.; Christou, D.D.; Hepple, R.T.; Scali, S.T.; et al. Impaired Muscle Mitochondrial Energetics Is Associated with Uremic Metabolite Accumulation in Chronic Kidney Disease. JCI Insight 2021, 6, e139826. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ishimori, N.; Takada, S.; Saito, A.; Kadoguchi, T.; Furihata, T.; Fukushima, A.; Matsushima, S.; Yokota, T.; Kinugawa, S.; et al. AST-120 Ameliorates Lowered Exercise Capacity and Mitochondrial Biogenesis in the Skeletal Muscle from Mice with Chronic Kidney Disease via Reducing Oxidative Stress. Nephrol. Dial. Transplant. 2015, 30, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Kopp, J.B. The Complexity of Nicotinamide Adenine Dinucleotide (NAD), Hypoxic, and Aryl Hydrocarbon Receptor Cell Signaling in Chronic Kidney Disease. J. Transl. Med. 2023, 21, 706. [Google Scholar] [CrossRef]
- Hasegawa, S.; Inagi, R. Harnessing Metabolomics to Describe the Pathophysiology Underlying Progression in Diabetic Kidney Disease. Curr. Diab. Rep. 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Dagar, N.; Shelke, V.; Lech, M.; Khare, P.; Gaikwad, A.B. Metabolic Reprogramming: Unveiling the Therapeutic Potential of Targeted Therapies against Kidney Disease. Drug Discov. Today 2023, 28, 103765. [Google Scholar] [CrossRef]
- Schaub, J.A.; Venkatachalam, M.A.; Weinberg, J.M. Proximal Tubular Oxidative Metabolism in Acute Kidney Injury and the Transition to CKD. Kidney360 2021, 2, 355–364. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Li, X. The Role of Metabolic Reprogramming in Tubular Epithelial Cells during the Progression of Acute Kidney Injury. Cell. Mol. Life Sci. 2021, 78, 5731–5741. [Google Scholar] [CrossRef]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins 2016, 8, 376. [Google Scholar] [CrossRef]
- Tanaka, T. Epigenetic Changes Mediating Transition to Chronic Kidney Disease: Hypoxic Memory. Acta Physiol. 2018, 222, e13023. [Google Scholar] [CrossRef]
- Rodríguez-Romo, R.; Berman, N.; Gómez, A.; Bobadilla, N.A. Epigenetic Regulation in the Acute Kidney Injury to Chronic Kidney Disease Transition. Nephrology 2015, 20, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Mar, D.; Gharib, S.A.; Zager, R.A.; Johnson, A.; Denisenko, O.; Bomsztyk, K. Heterogeneity of Epigenetic Changes at Ischemia/Reperfusion- and Endotoxin-Induced Acute Kidney Injury Genes. Kidney Int. 2015, 88, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Tampe, B.; Steinle, U.; Tampe, D.; Carstens, J.L.; Korsten, P.; Zeisberg, E.M.; Müller, G.A.; Kalluri, R.; Zeisberg, M. Low-Dose Hydralazine Prevents Fibrosis in a Murine Model of Acute Kidney Injury–to–Chronic Kidney Disease Progression. Kidney Int. 2017, 91, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation Determines Fibroblast Activation and Fibrogenesis in the Kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Soofi, A.; Kutschat, A.P.; Azam, M.; Laszczyk, A.M.; Dressler, G.R. Regeneration after Acute Kidney Injury Requires PTIP-Mediated Epigenetic Modifications. JCI Insight 2020, 5, e130204. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-A.; Mohtat, D.; Suzuki, M.; Park, A.; Izquierdo, M.; Han, S.; Kang, H.; Si, H.; Hostetter, T.; Pullman, J.M.; et al. Cytosine Methylation Changes in Enhancer Regions of Core Pro-Fibrotic Genes Characterize Kidney Fibrosis Development. Genome Biol. 2013, 14, R108. [Google Scholar] [CrossRef]
- Kang, S.W.; Shih, P.B.; Mathew, R.O.; Mahata, M.; Biswas, N.; Rao, F.; Yan, L.; Bouchard, J.; Malhotra, R.; Tolwani, A.; et al. Renal Kallikrein Excretion and Epigenetics in Human Acute Kidney Injury: Expression, Mechanisms and Consequences. BMC Nephrol. 2011, 12, 27. [Google Scholar] [CrossRef]
- Kölling, M.; Seeger, H.; Haddad, G.; Kistler, A.; Nowak, A.; Faulhaber-Walter, R.; Kielstein, J.; Haller, H.; Fliser, D.; Mueller, T.; et al. The Circular RNA ciRs-126 Predicts Survival in Critically Ill Patients with Acute Kidney Injury. Kidney Int. Rep. 2018, 3, 1144–1152. [Google Scholar] [CrossRef]
- Wing, M.R.; Devaney, J.M.; Joffe, M.M.; Xie, D.; Feldman, H.I.; Dominic, E.A.; Guzman, N.J.; Ramezani, A.; Susztak, K.; Herman, J.G.; et al. DNA Methylation Profile Associated with Rapid Decline in Kidney Function: Findings from the CRIC Study. Nephrol. Dial. Transplant. 2014, 29, 864–872. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell Cycle Arrest and the Evolution of Chronic Kidney Disease from Acute Kidney Injury. Nephrol. Dial. Transplant. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- Crépin, T.; Legendre, M.; Carron, C.; Vachey, C.; Courivaud, C.; Rebibou, J.-M.; Ferrand, C.; Laheurte, C.; Vauchy, C.; Gaiffe, E.; et al. Uraemia-Induced Immune Senescence and Clinical Outcomes in Chronic Kidney Disease Patients. Nephrol. Dial. Transplant. 2020, 35, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-B.; Lee, D.-S.; Padanilam, B.J.; Kim, J. Repeated Administration of Cisplatin Transforms Kidney Fibroblasts through G2/M Arrest and Cellular Senescence. Cells 2022, 11, 3472. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement Component C5a Induces Aberrant Epigenetic Modifications in Renal Tubular Epithelial Cells Accelerating Senescence by Wnt4/Βcatenin Signaling after Ischemia/Reperfusion Injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial Cell Cycle Arrest in G2/M Mediates Kidney Fibrosis after Injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Canaud, G.; Brooks, C.R.; Kishi, S.; Taguchi, K.; Nishimura, K.; Magassa, S.; Scott, A.; Hsiao, L.-L.; Ichimura, T.; Terzi, F.; et al. Cyclin G1 and TASCC Regulate Kidney Epithelial Cell G 2 -M Arrest and Fibrotic Maladaptive Repair. Sci. Transl. Med. 2019, 11, eaav4754. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, N.; Li, Y.; Liu, C.; Hou, F.F.; Wang, J. N-Acetylcysteine Ameliorates Cisplatin-Induced Renal Senescence and Renal Interstitial Fibrosis through Sirtuin1 Activation and P53 Deacetylation. Free Radic. Biol. Med. 2019, 130, 512–527. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and Validation of Cell Cycle Arrest Biomarkers in Human Acute Kidney Injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Lacroix, J.S.; Urena-Torres, P. Potentielle application de l’axe fibroblast growth factor 23-Klotho dans la maladie rénale chronique. Néphrologie Thérapeutique 2020, 16, 83–92. [Google Scholar] [CrossRef]
- Gewin, L.; Vadivelu, S.; Neelisetty, S.; Srichai, M.B.; Paueksakon, P.; Pozzi, A.; Harris, R.C.; Zent, R. Deleting the TGF-β Receptor Attenuates Acute Proximal Tubule Injury. J. Am. Soc. Nephrol. 2012, 23, 2001–2011. [Google Scholar] [CrossRef]
- Chung, S.; Overstreet, J.M.; Li, Y.; Wang, Y.; Niu, A.; Wang, S.; Fan, X.; Sasaki, K.; Jin, G.-N.; Khodo, S.N.; et al. TGF-β Promotes Fibrosis after Severe Acute Kidney Injury by Enhancing Renal Macrophage Infiltration. JCI Insight 2018, 3, e123563. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, G.; Wei, B.; Jin, J.; Huang, X.R.; Shao, W.; Li, J.; Meng, X.; Lan, H.Y. Conditional Knockout of TGF-βRII /Smad2 Signals Protects against Acute Renal Injury by Alleviating Cell Necroptosis, Apoptosis and Inflammation. Theranostics 2019, 9, 8277–8293. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L.S. Transforming Growth Factor-β in the Acute Kidney Injury to Chronic Kidney Disease Transition. Nephron 2019, 143, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Squires, P.E. The Role of TGF-β and Epithelial-to Mesenchymal Transition in Diabetic Nephropathy. Cytokine Growth Factor. Rev. 2011, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Boo, C.-S.; Jo, S.-K.; Cho, W.Y.; Kim, H.K. Macrophages Contribute to the Development of Renal Fibrosis Following Ischaemia/Reperfusion-Induced Acute Kidney Injury. Nephrol. Dial. Transplant. 2007, 23, 842–852. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martín-Sánchez, D.; Martinez-Moreno, J.M.; Carrasco, S.; Ruiz-Andrés, O.; Monsalve, M.; Sanchez-Ramos, C.; Gómez, M.J.; Ruiz-Ortega, M.; Sánchez-Niño, M.D.; et al. PGC-1α Deficiency Causes Spontaneous Kidney Inflammation and Increases the Severity of Nephrotoxic AKI. J. Pathol. 2019, 249, 65–78. [Google Scholar] [CrossRef]
- Maekawa, H.; Inoue, T.; Ouchi, H.; Jao, T.-M.; Inoue, R.; Nishi, H.; Fujii, R.; Ishidate, F.; Tanaka, T.; Tanaka, Y.; et al. Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep. 2019, 29, 1261–1273.e6. [Google Scholar] [CrossRef]
- Lerolle, N.; Nochy, D.; Guérot, E.; Bruneval, P.; Fagon, J.-Y.; Diehl, J.-L.; Hill, G. Histopathology of Septic Shock Induced Acute Kidney Injury: Apoptosis and Leukocytic Infiltration. Intensive Care Med. 2010, 36, 471–478. [Google Scholar] [CrossRef]
- Murashima, M.; Nishimoto, M.; Kokubu, M.; Hamano, T.; Matsui, M.; Eriguchi, M.; Samejima, K.; Akai, Y.; Tsuruya, K. Inflammation as a Predictor of Acute Kidney Injury and Mediator of Higher Mortality after Acute Kidney Injury in Non-Cardiac Surgery. Sci. Rep. 2019, 9, 20260. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, Y.; Sun, L. The Cross-Link between Ferroptosis and Kidney Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 6654887. [Google Scholar] [CrossRef]
- Guo, R.; Duan, J.; Pan, S.; Cheng, F.; Qiao, Y.; Feng, Q.; Liu, D.; Liu, Z. The Road from AKI to CKD: Molecular Mechanisms and Therapeutic Targets of Ferroptosis. Cell Death Dis. 2023, 14, 426. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria Regulation in Ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J. Ferroptosis, a Rising Force against Renal Fibrosis. Oxidative Med. Cell. Longev. 2022, 2022, 7686956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xue, X.; Hou, Q.; Dai, C. Targeting Ferroptosis Attenuates Interstitial Inflammation and Kidney Fibrosis. Kidney Dis. 2022, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, B.; Di Costanzo, D.; Dwyer, D.F. Macrophage-Mediated Immune Responses: From Fatty Acids to Oxylipins. Molecules 2021, 27, 152. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Kobayashi, Y.; Ide, K.; Strausser, S.A.; Abe, K.; Herbek, S.; O’Brien, L.L.; Crowley, S.D.; Barisoni, L.; Tata, A.; et al. Ferroptotic Stress Promotes the Accumulation of Pro-Inflammatory Proximal Tubular Cells in Maladaptive Renal Repair. eLife 2021, 10, e68603. [Google Scholar] [CrossRef]
- Proneth, B.; Conrad, M. Ferroptosis and Necroinflammation, a yet Poorly Explored Link. Cell Death Differ. 2019, 26, 14–24. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The Release and Activity of HMGB1 in Ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Li, X.; Zou, Y.; Xing, J.; Fu, Y.-Y.; Wang, K.-Y.; Wan, P.-Z.; Zhai, X.-Y. Pretreatment with Roxadustat (FG-4592) Attenuates Folic Acid-Induced Kidney Injury through Antiferroptosis via Akt/GSK-3 β/Nrf2 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 6286984. [Google Scholar] [CrossRef]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef]
- Tonnus, W.; Meyer, C.; Steinebach, C.; Belavgeni, A.; Von Mässenhausen, A.; Gonzalez, N.Z.; Maremonti, F.; Gembardt, F.; Himmerkus, N.; Latk, M.; et al. Dysfunction of the Key Ferroptosis-Surveilling Systems Hypersensitizes Mice to Tubular Necrosis during Acute Kidney Injury. Nat. Commun. 2021, 12, 4402. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- Dreischer, P.; Duszenko, M.; Stein, J.; Wieder, T. Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells. Cells 2022, 11, 503. [Google Scholar] [CrossRef]
- Föller, M.; Lang, F. Ion Transport in Eryptosis, the Suicidal Death of Erythrocytes. Front. Cell Dev. Biol. 2020, 8, 597. [Google Scholar] [CrossRef]
- Li, D.; Zheng, X.; Zhang, Y.; Li, X.; Chen, X.; Yin, Y.; Hu, J.; Li, J.; Guo, M.; Wang, X. What Should Be Responsible for Eryptosis in Chronic Kidney Disease? Kidney Blood Press. Res. 2022, 47, 375–390. [Google Scholar] [CrossRef]
- Basile, D.P. The Endothelial Cell in Ischemic Acute Kidney Injury: Implications for Acute and Chronic Function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal Ischemic Injury Results in Permanent Damage to Peritubular Capillaries and Influences Long-Term Function. Am. J. Physiol. Ren. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Sutton, T.A.; Fisher, C.J.; Molitoris, B.A. Microvascular Endothelial Injury and Dysfunction during Ischemic Acute Renal Failure. Kidney Int. 2002, 62, 1539–1549. [Google Scholar] [CrossRef]
- Sutton, T.A.; Mang, H.E.; Campos, S.B.; Sandoval, R.M.; Yoder, M.C.; Molitoris, B.A. Injury of the Renal Microvascular Endothelium Alters Barrier Function after Ischemia. Am. J. Physiol. Ren. Physiol. 2003, 285, F191–F198. [Google Scholar] [CrossRef]
- Yang, B.; Lan, S.; Dieudé, M.; Sabo-Vatasescu, J.-P.; Karakeussian-Rimbaud, A.; Turgeon, J.; Qi, S.; Gunaratnam, L.; Patey, N.; Hébert, M.-J. Caspase-3 Is a Pivotal Regulator of Microvascular Rarefaction and Renal Fibrosis after Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2018, 29, 1900–1916. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-Pericyte Interactions in Angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, C.; Xin, C.; Campanholle, G.; Gill, S.E.; Stallcup, W.; Lin, S.-L.; Davis, G.E.; Gharib, S.A.; Humphreys, B.D.; Duffield, J.S. Pericyte TIMP3 and ADAMTS1 Modulate Vascular Stability after Kidney Injury. J. Am. Soc. Nephrol. 2012, 23, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Wongboonsin, J.; Chang-Panesso, M.; Machado, F.G.; Humphreys, B.D. Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. J. Am. Soc. Nephrol. 2017, 28, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Koller, G.M.; Schafer, C.; Kemp, S.S.; Aguera, K.N.; Lin, P.K.; Forgy, J.C.; Griffin, C.T.; Davis, G.E. Proinflammatory Mediators, IL (Interleukin)-1β, TNF (Tumor Necrosis Factor) α, and Thrombin Directly Induce Capillary Tube Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 365–377. [Google Scholar] [CrossRef]
- Sato, Y.; Yanagita, M. Immune Cells and Inflammation in AKI to CKD Progression. Am. J. Physiol. Ren. Physiol. 2018, 315, F1501–F1512. [Google Scholar] [CrossRef]
- Ohashi, R.; Shimizu, A.; Masuda, Y.; Kitamura, H.; Ishizaki, M.; Sugisaki, Y.; Yamanaka, N. Peritubular Capillary Regression during the Progression of Experimental Obstructive Nephropathy. J. Am. Soc. Nephrol. 2002, 13, 1795–1805. [Google Scholar] [CrossRef]
- Loganathan, K.; Salem Said, E.; Winterrowd, E.; Orebrand, M.; He, L.; Vanlandewijck, M.; Betsholtz, C.; Quaggin, S.E.; Jeansson, M. Angiopoietin-1 Deficiency Increases Renal Capillary Rarefaction and Tubulointerstitial Fibrosis in Mice. PLoS ONE 2018, 13, e0189433. [Google Scholar] [CrossRef]
- Kang, D.-H.; Joly, A.H.; Oh, S.-W.; Hugo, C.; Kerjaschki, D.; Gordon, K.L.; Mazzali, M.; Jefferson, J.A.; Hughes, J.; Madsen, K.M.; et al. Impaired Angiogenesis in the Remnant Kidney Model: I. Potential Role of Vascular Endothelial Growth Factor and Thrombospondin-1. J. Am. Soc. Nephrol. 2001, 12, 1434–1447. [Google Scholar] [CrossRef]
- Chen, J.; Hamm, L.L.; Kleinpeter, M.A.; Husserl, F.; Khan, I.E.; Chen, C.-S.; Liu, Y.; Mills, K.T.; He, C.; Rifai, N.; et al. Elevated Plasma Levels of Endostatin Are Associated with Chronic Kidney Disease. Am. J. Nephrol. 2012, 35, 335–340. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 Controls Endothelial Angiogenic Functions during Vascular Growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 Modulates Premature Senescence-like Phenotype in Human Endothelial Cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Zullo, J.A.; Goligorsky, M.S. Endothelial Sirtuin 1 Inactivation Enhances Capillary Rarefaction and Fibrosis Following Kidney Injury through Notch Activation. Biochem. Biophys. Res. Commun. 2016, 478, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ibrahim, M.M.; Kranz, J.; Zhang, X.; Ziegler, S.; Perales-Patón, J.; Jansen, J.; Reimer, K.C.; Smith, J.R.; Dobie, R.; et al. Decoding Myofibroblast Origins in Human Kidney Fibrosis. Nature 2021, 589, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-W.; Hsu, K.-H.; Hsu, H.-J.; Lee, C.-C.; Sun, C.-Y.; Tsai, C.-J.; Wu, M.-S. Serum Free P-Cresyl Sulfate Levels Predict Cardiovascular and All-Cause Mortality in Elderly Hemodialysis Patients--a Prospective Cohort Study. Nephrology Dialysis Transplantation 2012, 27, 1169–1175. [Google Scholar] [CrossRef]
- Fan, P.-C.; Chang, J.C.-H.; Lin, C.-N.; Lee, C.-C.; Chen, Y.-T.; Chu, P.-H.; Kou, G.; Lu, Y.-A.; Yang, C.-W.; Chen, Y.-C. Serum Indoxyl Sulfate Predicts Adverse Cardiovascular Events in Patients with Chronic Kidney Disease. J. Formos. Med. Assoc. 2019, 118, 1099–1106. [Google Scholar] [CrossRef]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Tumur, Z.; Niwa, T. Indoxyl Sulfate Inhibits Nitric Oxide Production and Cell Viability by Inducing Oxidative Stress in Vascular Endothelial Cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl Sulfate Upregulates Expression of ICAM-1 and MCP-1 by Oxidative Stress-Induced NF-ĸB Activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef]
- Six, I.; Gross, P.; Rémond, M.C.; Chillon, J.M.; Poirot, S.; Drueke, T.B.; Massy, Z.A. Deleterious Vascular Effects of Indoxyl Sulfate and Reversal by Oral Adsorbent AST-120. Atherosclerosis 2015, 243, 248–256. [Google Scholar] [CrossRef]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van De Voorde, J.; De Vriese, A.; et al. Protein-Bound Uremic Toxins Stimulate Crosstalk between Leukocytes and Vessel Wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef]
- Adijiang, A.; Goto, S.; Uramoto, S.; Nishijima, F.; Niwa, T. Indoxyl Sulphate Promotes Aortic Calcification with Expression of Osteoblast-Specific Proteins in Hypertensive Rats. Nephrol. Dial. Transplant. 2008, 23, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallah, J.; Zibara, K.; Issa, H.; Lenglet, G.; Kchour, G.; Caus, T.; Six, I.; Choukroun, G.; Kamel, S.; Bennis, Y. Endothelial Cells Exposed to Phosphate and Indoxyl Sulphate Promote Vascular Calcification through Interleukin-8 Secretion. Nephrol. Dial. Transplant. 2019, 34, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic Uremic Solutes Increase Tissue Factor Production in Endothelial Cells by the Aryl Hydrocarbon Receptor Pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Salyers, Z.R.; Coleman, M.; Balestrieri, N.P.; Ryan, T.E. Indoxyl Sulfate Impairs Angiogenesis via Chronic Aryl Hydrocarbon Receptor Activation. Am. J. Physiol. Cell Physiol. 2021, 320, C240–C249. [Google Scholar] [CrossRef]
- Esquivias-Motta, E.; Martín-Malo, A.; Buendia, P.; Álvarez-Lara, M.A.; Soriano, S.; Crespo, R.; Carracedo, J.; Ramírez, R.; Aljama, P. Hemodiafiltration with Endogenous Reinfusion Improved Microinflammation and Endothelial Damage Compared with Online-Hemodiafiltration: A Hypothesis Generating Study: Thoughts and Progress. Artif. Organs 2017, 41, 88–98. [Google Scholar] [CrossRef]
- Glorieux, G.; Vanholder, R.; Van Biesen, W.; Pletinck, A.; Schepers, E.; Neirynck, N.; Speeckaert, M.; De Bacquer, D.; Verbeke, F. Free p-Cresyl Sulfate Shows the Highest Association with Cardiovascular Outcome in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, 998–1005. [Google Scholar] [CrossRef]
- Jing, Y.J.; Ni, J.W.; Ding, F.H.; Fang, Y.H.; Wang, X.Q.; Wang, H.B.; Chen, X.N.; Chen, N.; Zhan, W.W.; Lu, L.; et al. P-Cresyl Sulfate Is Associated with Carotid Arteriosclerosis in Hemodialysis Patients and Promotes Atherogenesis in apoE−/− Mice. Kidney Int. 2016, 89, 439–449. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wang, C.-H.; Kuo, C.-H.; Lin, Y.-L.; Tsai, J.-P.; Hsu, B.-G. Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis. Toxins 2019, 12, 10. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The Uremic Retention Solute P-Cresyl Sulfate and Markers of Endothelial Damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Zhu, Z.; Su, X.; Ni, J.; Du, R.; Zhang, R.; Jin, W. P-Cresyl Sulfate Promotes the Formation of Atherosclerotic Lesions and Induces Plaque Instability by Targeting Vascular Smooth Muscle Cells. Front. Med. 2016, 10, 320–329. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Zhu, J.; Ni, J.; Sun, J.; Zhang, R. Atorvastatin Attenuates P-Cresyl Sulfate-Induced Atherogenesis and Plaque Instability in ApoE Knockout Mice. Mol. Med. Rep. 2016, 14, 3122–3128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hung, S.-C.; Kuo, K.-L.; Huang, H.-L.; Lin, C.-C.; Tsai, T.-H.; Wang, C.-H.; Chen, J.-W.; Lin, S.-J.; Huang, P.-H.; Tarng, D.-C. Indoxyl Sulfate Suppresses Endothelial Progenitor Cell–Mediated Neovascularization. Kidney Int. 2016, 89, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Young, G.-H.; Huang, P.-H.; Lo, S.-C.; Wang, K.-C.; Sun, C.-Y.; Liang, C.-J.; Huang, T.-M.; Chen, J.-H.; Chang, F.-C.; et al. In Acute Kidney Injury, Indoxyl Sulfate Impairs Human Endothelial Progenitor Cells: Modulation by Statin. Angiogenesis 2013, 16, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Liang, C.-J.; Huang, T.-M.; Liu, C.-W.; Wang, S.-H.; Young, G.-H.; Tsai, J.-S.; Tseng, Y.-C.; Peng, Y.-S.; Wu, V.-C.; et al. Indoxyl Sulfate Enhances IL-1β-Induced E-Selectin Expression in Endothelial Cells in Acute Kidney Injury by the ROS/MAPKs/NFκB/AP-1 Pathway. Arch. Toxicol. 2016, 90, 2779–2792. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takayanagi, K.; Kojima, M.; Taguchi, K.; Kobayashi, T. Acute Exposure to Indoxyl Sulfate Impairs Endothelium-Dependent Vasorelaxation in Rat Aorta. Int. J. Mol. Sci. 2019, 20, 338. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takayanagi, K.; Kojima, M.; Katome, T.; Taguchi, K.; Kobayashi, T. Direct Impairment of the Endothelial Function by Acute Indoxyl Sulfate through Declined Nitric Oxide and Not Endothelium-Derived Hyperpolarizing Factor or Vasodilator Prostaglandins in the Rat Superior Mesenteric Artery. Biol. Pharm. Bull. 2019, 42, 1236–1242. [Google Scholar] [CrossRef]
- Tang, W.-H.; Wang, C.-P.; Yu, T.-H.; Tai, P.-Y.; Liang, S.-S.; Hung, W.-C.; Wu, C.-C.; Huang, S.-H.; Lee, Y.-J.; Chen, S.-C. Protein-Bounded Uremic Toxin p-Cresylsulfate Induces Vascular Permeability Alternations. Histochem. Cell Biol. 2018, 149, 607–617. [Google Scholar] [CrossRef]
- Zhang, M.; Malik, A.B.; Rehman, J. Endothelial Progenitor Cells and Vascular Repair. Curr. Opin. Hematol. 2014, 21, 224–228. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, L.; Tao, X.; Song, Y.; Cui, J.; Wan, J. The Role of Podocyte Damage in the Etiology of Ischemia-Reperfusion Acute Kidney Injury and Post-Injury Fibrosis. BMC Nephrol. 2019, 20, 106. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Petrosyan, A.; Andrighetto, S.; Budge, K.; D’Agati, V.D.; Hartzell, S.; Malvi, D.; Donadei, C.; Thurman, J.M.; et al. Loss of Decay-Accelerating Factor Triggers Podocyte Injury and Glomerulosclerosis. J. Exp. Med. 2020, 217, e20191699. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, T.; Murea, M.; Susztak, K. The Pathogenic Role of Notch Activation in Podocytes. Nephron Exp. Nephrol. 2009, 111, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hitomi, H.; Diah, S.; Deguchi, K.; Mori, H.; Masaki, T.; Nakano, D.; Kobori, H.; Nishiyama, A. Roles of Na+/H+ Exchanger Type 1 and Intracellular pH in Angiotensin II-Induced Reactive Oxygen Species Generation and Podocyte Apoptosis. J. Pharmacol. Sci. 2013, 122, 176–183. [Google Scholar] [CrossRef]
- Nishad, R.; Meshram, P.; Singh, A.K.; Reddy, G.B.; Pasupulati, A.K. Activation of Notch1 Signaling in Podocytes by Glucose-Derived AGEs Contributes to Proteinuria. BMJ Open Diab Res. Care 2020, 8, e001203. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jin, H.; Chai, Y.; Shou, S. Cellular Senescence and Acute Kidney Injury. Pediatr. Nephrol. 2022, 37, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Eymael, J.; Sharma, S.; Loeven, M.A.; Wetzels, J.F.; Mooren, F.; Florquin, S.; Deegens, J.K.; Willemsen, B.K.; Sharma, V.; Van Kuppevelt, T.H.; et al. CD44 Is Required for the Pathogenesis of Experimental Crescentic Glomerulonephritis and Collapsing Focal Segmental Glomerulosclerosis. Kidney Int. 2018, 93, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Roeder, S.S.; Stefanska, A.; Eng, D.G.; Kaverina, N.; Sunseri, M.W.; McNicholas, B.A.; Rabinovitch, P.; Engel, F.B.; Daniel, C.; Amann, K.; et al. Changes in Glomerular Parietal Epithelial Cells in Mouse Kidneys with Advanced Age. Am. J. Physiol. Ren. Physiol. 2015, 309, F164–F178. [Google Scholar] [CrossRef]
- Appel, D.; Kershaw, D.B.; Smeets, B.; Yuan, G.; Fuss, A.; Frye, B.D.; Elger, M.; Kriz, W.; Floege, J.D.; Moeller, M.J. Recruitment of Podocytes from Glomerular Parietal Epithelial Cells. J. Am. Soc. Nephrol. 2009, 20, 333–343. [Google Scholar] [CrossRef]
- Miesen, L.; Steenbergen, E.; Smeets, B. Parietal Cells—New Perspectives in Glomerular Disease. Cell Tissue Res. 2017, 369, 237–244. [Google Scholar] [CrossRef]
- Gelasco, A.K.; Raymond, J.R. Indoxyl Sulfate Induces Complex Redox Alterations in Mesangial Cells. Am. J. Physiol. Ren. Physiol. 2006, 290, F1551–F1558. [Google Scholar] [CrossRef]
- Owada, S.; Goto, S.; Bannai, K.; Hayashi, H.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Reduces Superoxide Scavenging Activity in the Kidneys of Normal and Uremic Rats. Am. J. Nephrol. 2008, 28, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M.; Miyazaki, T. Progression of Glomerular Sclerosis in Experimental Uremic Rats by Administration of Indole, a Precursor of Indoxyl Sulfate. Am. J. Nephrol. 1994, 14, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M. Indoxyl Sulfate, a Circulating Uremic Toxin, Stimulates the Progression of Glomerular Sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. P-Cresyl Sulfate Causes Renal Tubular Cell Damage by Inducing Oxidative Stress by Activation of NADPH Oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Arita, K.; Kato, A.; Shimizu, M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J. Am. Soc. Nephrol. 2015, 26, 1732–1746. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Tanaka, T.; Inagi, R. Effect of AST-120 in Chronic Kidney Disease Treatment: Still a Controversy? Nephron 2017, 135, 201–206. [Google Scholar] [CrossRef]
- Bennis, Y.; Cluet, Y.; Titeca-Beauport, D.; El Esper, N.; Ureña, P.; Bodeau, S.; Combe, C.; Dussol, B.; Fouque, D.; Choukroun, G.; et al. The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Toxins 2019, 11, 279. [Google Scholar] [CrossRef]
- Cha, R.; Kang, S.W.; Park, C.W.; Cha, D.R.; Na, K.Y.; Kim, S.G.; Yoon, S.A.; Han, S.Y.; Chang, J.H.; Park, S.K.; et al. A Randomized, Controlled Trial of Oral Intestinal Sorbent AST-120 on Renal Function Deterioration in Patients with Advanced Renal Dysfunction. Clin. J. Am. Soc. Nephrol. 2016, 11, 559–567. [Google Scholar] [CrossRef]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What Would You Like to Eat, Mr CKD Microbiota? A Mediterranean Diet, Please! Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Xu, H.; Carrero, J.J.; Pascoe, E.; French, C.; Campbell, K.L. Dietary Protein-Fiber Ratio Associates with Circulating Levels of Indoxyl Sulfate and p-Cresyl Sulfate in Chronic Kidney Disease Patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; Di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; De Carvalho Ferreira, D.; Rosado, A.; Carraro Eduardo, J.C.; Mafra, D. Does Low-Protein Diet Influence the Uremic Toxin Serum Levels from the Gut Microbiota in Nondialysis Chronic Kidney Disease Patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very Low Protein Diet Reduces Indoxyl Sulfate Levels in Chronic Kidney Disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.; Masereeuw, R.; Lowenstein, J. Effects of an SGLT Inhibitor on the Production, Toxicity, and Elimination of Gut-Derived Uremic Toxins: A Call for Additional Evidence. Toxins 2022, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Fukuda, S.; Kanemitsu, Y.; Saigusa, D.; Mukawa, C.; Asaji, K.; Matsumoto, Y.; Tsukamoto, H.; Tachikawa, T.; Tsukimi, T.; et al. Canagliflozin Reduces Plasma Uremic Toxins and Alters the Intestinal Microbiota Composition in a Chronic Kidney Disease Mouse Model. Am. J. Physiol. Ren. Physiol. 2018, 315, F824–F833. [Google Scholar] [CrossRef]
- Ho, H.; Kikuchi, K.; Oikawa, D.; Watanabe, S.; Kanemitsu, Y.; Saigusa, D.; Kujirai, R.; Ikeda-Ohtsubo, W.; Ichijo, M.; Akiyama, Y.; et al. SGLT-1-specific Inhibition Ameliorates Renal Failure and Alters the Gut Microbial Community in Mice with Adenine-induced Renal Failure. Physiol. Rep. 2021, 9, e15092. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Lu, J.-R.; Chen, B.-J.; Wu, C.; Chen, Y.-P.; Chen, M.-J. Selection of Uremic Toxin-Reducing Probiotics in Vitro and in Vivo. J. Funct. Foods 2014, 7, 407–415. [Google Scholar] [CrossRef]
- De Faria Barros, A.; Borges, N.A.; Nakao, L.S.; Dolenga, C.J.; Do Carmo, F.L.; De Carvalho Ferreira, D.; Stenvinkel, P.; Bergman, P.; Lindholm, B.; Mafra, D. Effects of Probiotic Supplementation on Inflammatory Biomarkers and Uremic Toxins in Non-Dialysis Chronic Kidney Patients: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Funct. Foods 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Hatch, S.T.; Bathini, T.; Sharma, K.; Wijarnpreecha, K.; Ungprasert, P.; D’Costa, M.; Mao, M.A.; Cheungpasitporn, W. Effects of Probiotics on Inflammation and Uremic Toxins Among Patients on Dialysis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2019, 64, 469–479. [Google Scholar] [CrossRef]
- Saxena, A.; Srinivasa, S.; Veerappan, I.; Jacob, C.; Mahaldar, A.; Gupta, A.; Rajagopal, A. Enzobiotics—A Novel Therapy for the Elimination of Uremic Toxins in Patients with CKD (EETOX Study): A Multicenter Double-Blind Randomized Controlled Trial. Nutrients 2022, 14, 3804. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Stasi, A.; Franzin, R.; Fiorentino, M.; Cimmarusti, M.T.; Deleonardis, A.; Palieri, R.; Pontrelli, P.; Gesualdo, L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins 2023, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Soulage, C.O.; Caggiano, G.; Glorieux, G.; Fouque, D.; Koppe, L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant Gut Microbiota Alters Host Metabolome and Impacts Renal Failure in Humans and Rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrolog. 2017, 28, 1450–1461. [Google Scholar] [CrossRef]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut Microbiota–Derived D-Serine Protects against Acute Kidney Injury. JCI Insight 2018, 3, e97957. [Google Scholar] [CrossRef]
- Nazzal, L.; Soiefer, L.; Chang, M.; Tamizuddin, F.; Schatoff, D.; Cofer, L.; Aguero-Rosenfeld, M.E.; Matalon, A.; Meijers, B.; Holzman, R.; et al. Effect of Vancomycin on the Gut Microbiome and Plasma Concentrations of Gut-Derived Uremic Solutes. Kidney Int. Rep. 2021, 6, 2122–2133. [Google Scholar] [CrossRef]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef]
- Saito, H.; Saigo, C.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Matsunaga, R.; Jono, H.; Nishi, K. Meclofenamate Elicits a Nephropreventing Effect in a Rat Model of Ischemic Acute Kidney Injury by Suppressing Indoxyl Sulfate Production and Restoring Renal Organic Anion Transporters. Drug Des. Dev. Ther. 2014, 8, 1073–1082. [Google Scholar] [CrossRef]
- Saito, H.; Yoshimura, M.; Saigo, C.; Komori, M.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Wakida, A.; Chuman, E.; Nishi, K.; et al. Hepatic Sulfotransferase as a Nephropreventing Target by Suppression of the Uremic Toxin Indoxyl Sulfate Accumulation in Ischemic Acute Kidney Injury. Toxicol. Sci. 2014, 141, 206–217. [Google Scholar] [CrossRef]
- Steinke, I.; Ghanei, N.; Govindarajulu, M.; Yoo, S.; Zhong, J.; Amin, R.H. Drug Discovery and Development of Novel Therapeutics for Inhibiting TMAO in Models of Atherosclerosis and Diabetes. Front. Physiol. 2020, 11, 567899. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.C.; Eloot, S.; Glorieux, G.L.R.L. Future Avenues to Decrease Uremic Toxin Concentration. Am. J. Kidney Dis. 2016, 67, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Paats, J.; Adoberg, A.; Arund, J.; Dhondt, A.; Fernström, A.; Fridolin, I.; Glorieux, G.; Leis, L.; Luman, M.; Gonzalez-Parra, E.; et al. Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients. Int. J. Mol. Sci. 2020, 21, 1522. [Google Scholar] [CrossRef] [PubMed]
- Meert, N.; Eloot, S.; Schepers, E.; Lemke, H.-D.; Dhondt, A.; Glorieux, G.; Van Landschoot, M.; Waterloos, M.-A.; Vanholder, R. Comparison of Removal Capacity of Two Consecutive Generations of High-Flux Dialysers during Different Treatment Modalities. Nephrol. Dial. Transplant. 2011, 26, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, S.; Tonelli, M.; Unsworth, L.D. Adsorption-Based Strategies for Removing Uremic Toxins from Blood. Microporous Mesoporous Mater. 2021, 319, 111035. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cortés-Hernández, D.A.; Bartolo-Pérez, P.; Ávila-Orta, C.A.; Cruz-Delgado, V.J.; Zepeda-Pedreguera, A. Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins. Nanomaterials 2019, 9, 1261. [Google Scholar] [CrossRef]
- De Loor, H.; Meijers, B.K.I.; Meyer, T.W.; Bammens, B.; Verbeke, K.; Dehaen, W.; Evenepoel, P. Sodium Octanoate to Reverse Indoxyl Sulfate and P-Cresyl Sulfate Albumin Binding in Uremic and Normal Serum during Sample Preparation Followed by Fluorescence Liquid Chromatography. J. Chromatogr. A 2009, 1216, 4684–4688. [Google Scholar] [CrossRef]
- Tao, X.; Thijssen, S.; Kotanko, P.; Ho, C.-H.; Henrie, M.; Stroup, E.; Handelman, G. Improved Dialytic Removal of Protein-Bound Uraemic Toxins with Use of Albumin Binding Competitors: An in Vitro Human Whole Blood Study. Sci. Rep. 2016, 6, 23389. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Wakamatsu, T.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Narita, I. Removal of Uremic Toxins by Renal Replacement Therapies: A Review of Current Progress and Future Perspectives. Ren. Replace. Ther. 2016, 2, 43. [Google Scholar] [CrossRef]
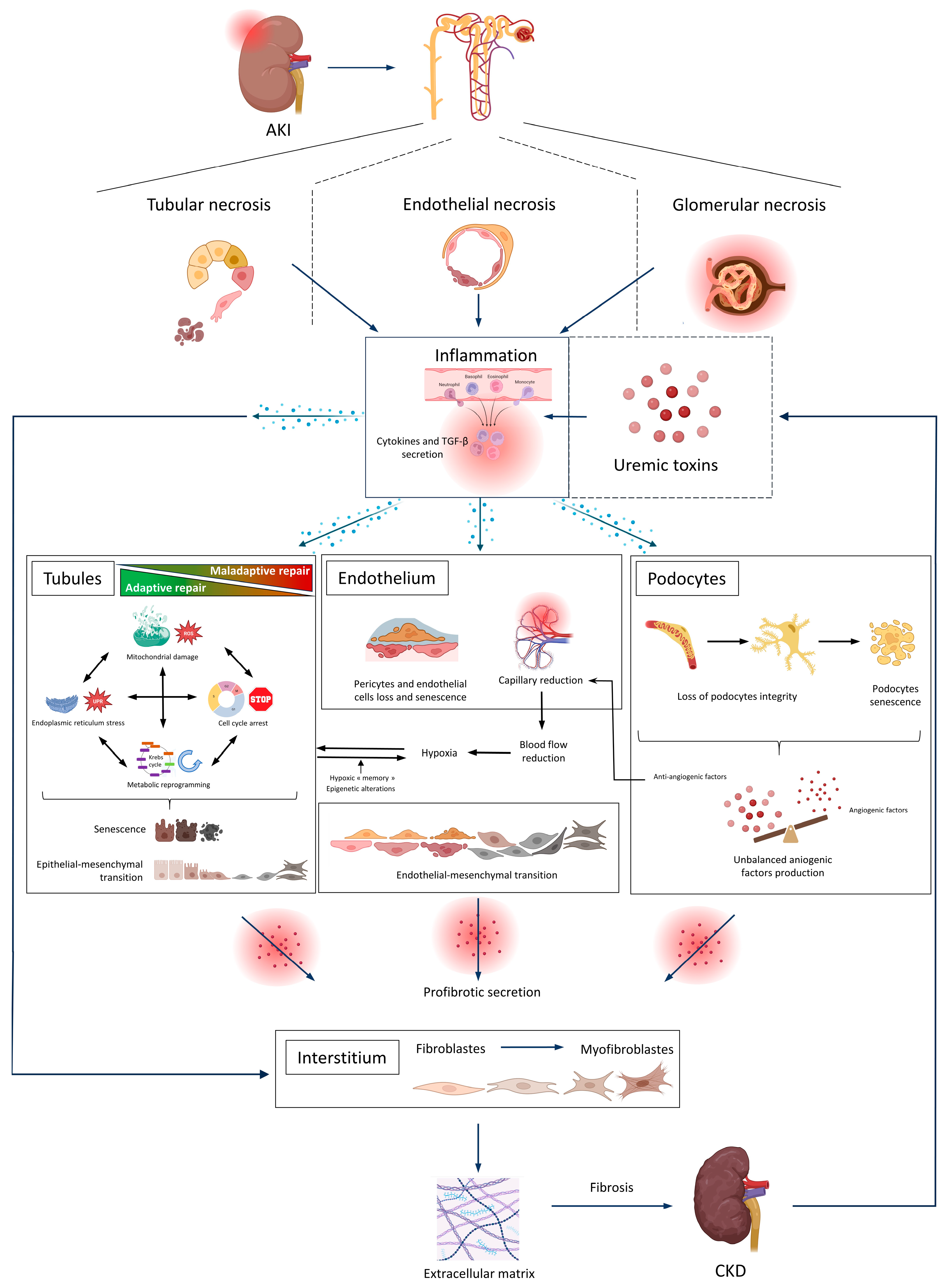
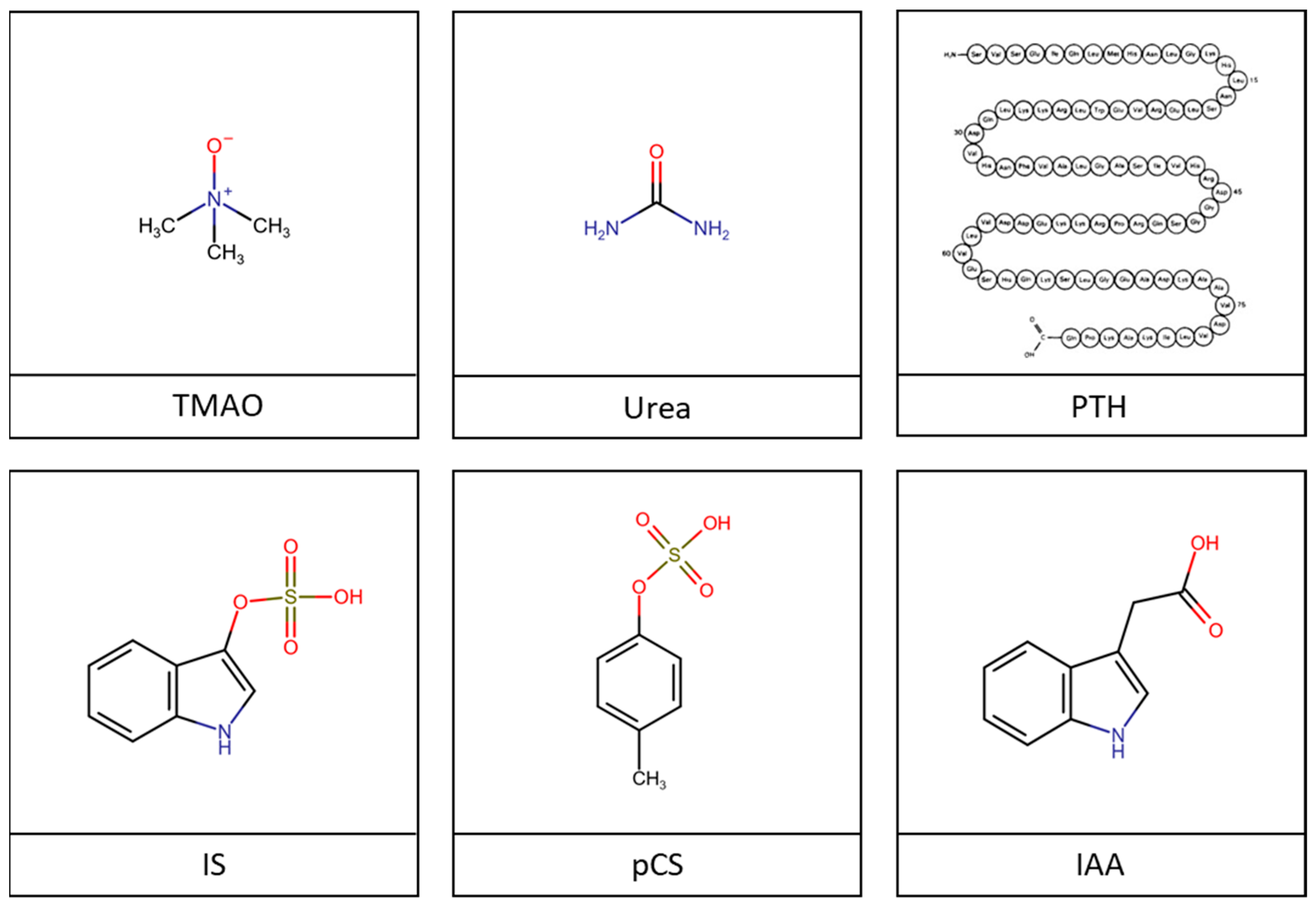
| UTs | Mechanisms Underlying the AKI-to-CKD Transition | The Tubulo-Interstitial Compartment | ||
|---|---|---|---|---|
| Models | Main Results | References | ||
| IS | EMT, TGF-β | In vitro | Extinction of the epithelial phenotype: alteration of the cubic epithelial aspect, ↓ ZO-1, ↓ E-cadherin Mesenchymal expression: ↑ α-SMA, ↑ TGF-β, ↑ Smad2/3/4 pathway, ↑ Snail, ↑ fibronectin, Inhibition of IS-induced EMT: probenecid ↓ EMT-induced MAPK/ERK pathway | [105,106,109,112,113] |
| In vivo | Extinction of the epithelial phenotype: ↓ ZO-1, ↓ E-cadherin, Mesenchymal expression: ↑ TGF-β, ↑ α-SMA, ↑ collagen, ↑ vimentin ↑ Fibrosis Inhibition of UT-induced EMT: - Losartan ↓ UTs, TGF-β and Snail and ↓ fibrosis - AST-120 ↓ IS concentration, ↑ ZO-1, ↓ α-SMA, ↓ fibrosis | [106,107,109,110,111,114,115,116,117] | ||
| Oxidative stress | In vitro | - ↑ ROS, ↑ NF-κB, ↓ Nrf2 - N-acetyl cysteine or NF-κB inhibitors or NADPH oxidase inhibitor → ↓ NF-κB, ↓ NOX4 | [112,118,119] | |
| In vivo | - AST-120 ↓ urine and serum IS concentrations, ↑ kidney oxygenation, ↓ 8-OHdG, ↓ interstitial fibrosis, ↑ Nrf2, ↑ HO-1 and ↑ NQO1 | [119,120,121] | ||
| Mitochondria dysfunction | In vitro | - ↓ Glucuronidation, ↓ complex II activity, ↓ electron transport capacity - ↓ Fusion, ↑ fission, ↑ autophagy | [122,123] | |
| In vivo | - ↓ Nitrogen metabolism, alteration of the inner membrane, ↑ fission, ↑ autophagy | [123] | ||
| ER stress | In vitro | - ↓ GRP78, ↑ CHOP | [124,125] | |
| In vivo | - AST-120 ↓ CHOP | [125] | ||
| Metabolic reprogramming | In vitro | - ↑ AhR-dependent arachidonic acid pathway-reprogramming monocytes | [126] | |
| In vivo | - Alteration of metabolic pathways, including tryptophan metabolism | [127] | ||
| Epigenetic alteration Cycle cell arrest Senescence | In vitro | - Methylation of CpG islands of sFRP => Wnt/β-catenin pathway activation - Activation of p53 and p21 - NF-κB inhibitors suppressed IS-induced p53 and p21 | [128,129,130] | |
| In vivo | - Administration of recombinant sFRP5 alleviated IS-induced fibrosis - Hypermethylation of Klotho - AST-120 decreased p65, p53, p21, β-galactosidase activation, TGF-β, and α-SMA - Stat3 siRNA suppressed IS-induced β-galactosidase activation and fibrosis | [128,129,130,131,132] | ||
| Inflammation | In vitro | - ↑ Expression of IL-1β, IL-6, IL-15, IL-6 and IL-15, TGF-β, NF-κB, Smad, Stat, B2m, Bax, and Bcl2 - Indole-3-propionic acid suppressed IS-induced MCP-1 - Correlation between CD14 + CD16+ monocytes and plasma IS concentration in AAA patients - Plasma IS from AAA patients promotes IL-10, PPARγ, TGF-β, TIMP-1, IL-6, CCL2, and COX2 | [133,134,135,136] | |
| In vivo | - ↑ MCP-1 - Indole-3-propionic acid ↓ IS-induced MCP-1 - AST-120 ↓ IS-induced Mac-1 | [134,135,137] | ||
| Iron death pathways | In vitro | - ↑ Hepcidin expression through AhR - ↑ Intracellular Ca2+ concentration and ceramide concentration in erythrocytes - ↑ Eryptosis and thrombosis | [138,139,140,141] | |
| In vivo | - AST-120 ↓ IS-induced hepcidin expression | [138] | ||
| IAA | Iron death pathways | In vitro | - ↑ Eryptosis and thrombosis | [141] |
| pCS | Inflammation | In vitro | - ↑ Expression of IL-1β, IL-6, IL-15, IL-6 and IL-15, TGF-β, NF-κB, Smad, Stat, B2m, Bax, and Bcl2 | [133,134] |
| Me2PY Me4PY | Oxidative stress | Clinical study | - Correlation between Me2PY or Me4PY and the oxidative stress marker GSH | [142] |
| CMPF | Mitochondrial dysfunction | In vitro | - Hemodialyzed patient serum with CMPF inhibited ADP-stimulated oxidation | [143] |
| Iron death pathways | In vitro | - ↓ GSH levels and GPX4, FHC, FLC, and ↑ intracellular iron concentration | [144] | |
| D-serine | Cycle cell arrest | In vitro | - Activation of GCN2 → senescence | [145] |
| ADMA | TGF-β | In vivo | - ↑ TGF-β, ↑ α-SMA, ↑ collagen, ↑ fibronectin, ↑ fibrosis | [146] |
| UTs | Mechanism Underlying the AKI-to-CKD Transition | Endothelium | ||
|---|---|---|---|---|
| Models | Main Results | References | ||
| IS | Podocyte loss and senescence | In vitro | - ↑ AhR, ↑ vimentin - Podocyte effacement, with ↓ in Actn4, Cd2ap, Myh9, Nphs1, Nphs2, Podxl, Synpo, and Wt1 mRNA - ↑ reduction rate, ↑ ROS, ↑ SOD sensitivity in mesangial cells | [69,281] |
| In vivo | - ↑ AhR, ↑ vimentin - Podocyte effacement, with ↓ in Actn4, Cd2ap, Myh9, Nphs1, Nphs2, Podxl, Synpo, and Wt1 mRNA - AST-120 ↓ glomerular sclerosis | [69,283,284] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, C.; Bodeau, S.; Kamel, S.; Bennis, Y.; Caillard, P. The AKI-to-CKD Transition: The Role of Uremic Toxins. Int. J. Mol. Sci. 2023, 24, 16152. https://doi.org/10.3390/ijms242216152
André C, Bodeau S, Kamel S, Bennis Y, Caillard P. The AKI-to-CKD Transition: The Role of Uremic Toxins. International Journal of Molecular Sciences. 2023; 24(22):16152. https://doi.org/10.3390/ijms242216152
Chicago/Turabian StyleAndré, Camille, Sandra Bodeau, Saïd Kamel, Youssef Bennis, and Pauline Caillard. 2023. "The AKI-to-CKD Transition: The Role of Uremic Toxins" International Journal of Molecular Sciences 24, no. 22: 16152. https://doi.org/10.3390/ijms242216152
APA StyleAndré, C., Bodeau, S., Kamel, S., Bennis, Y., & Caillard, P. (2023). The AKI-to-CKD Transition: The Role of Uremic Toxins. International Journal of Molecular Sciences, 24(22), 16152. https://doi.org/10.3390/ijms242216152







