Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells
Abstract
:1. Introduction
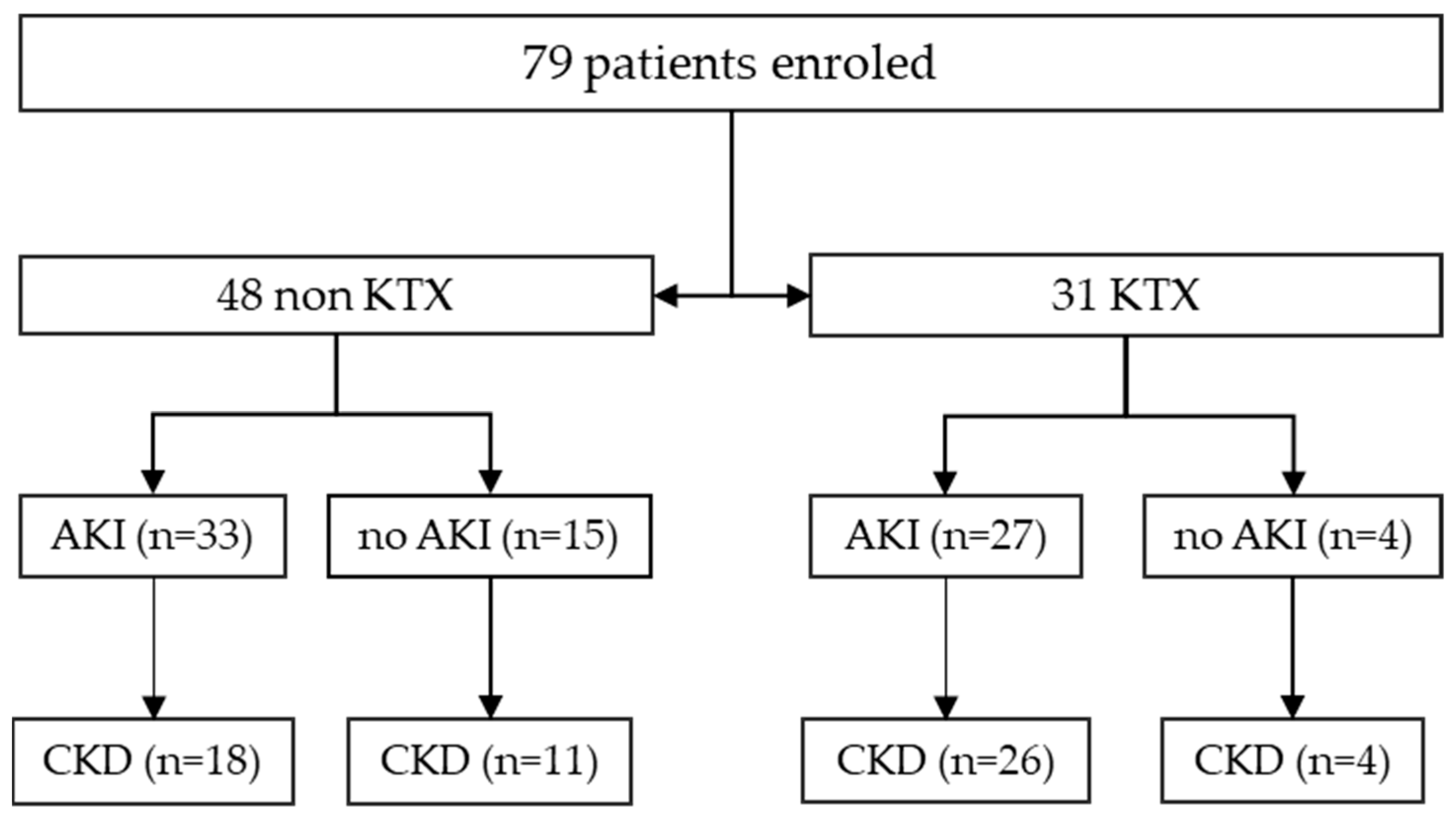
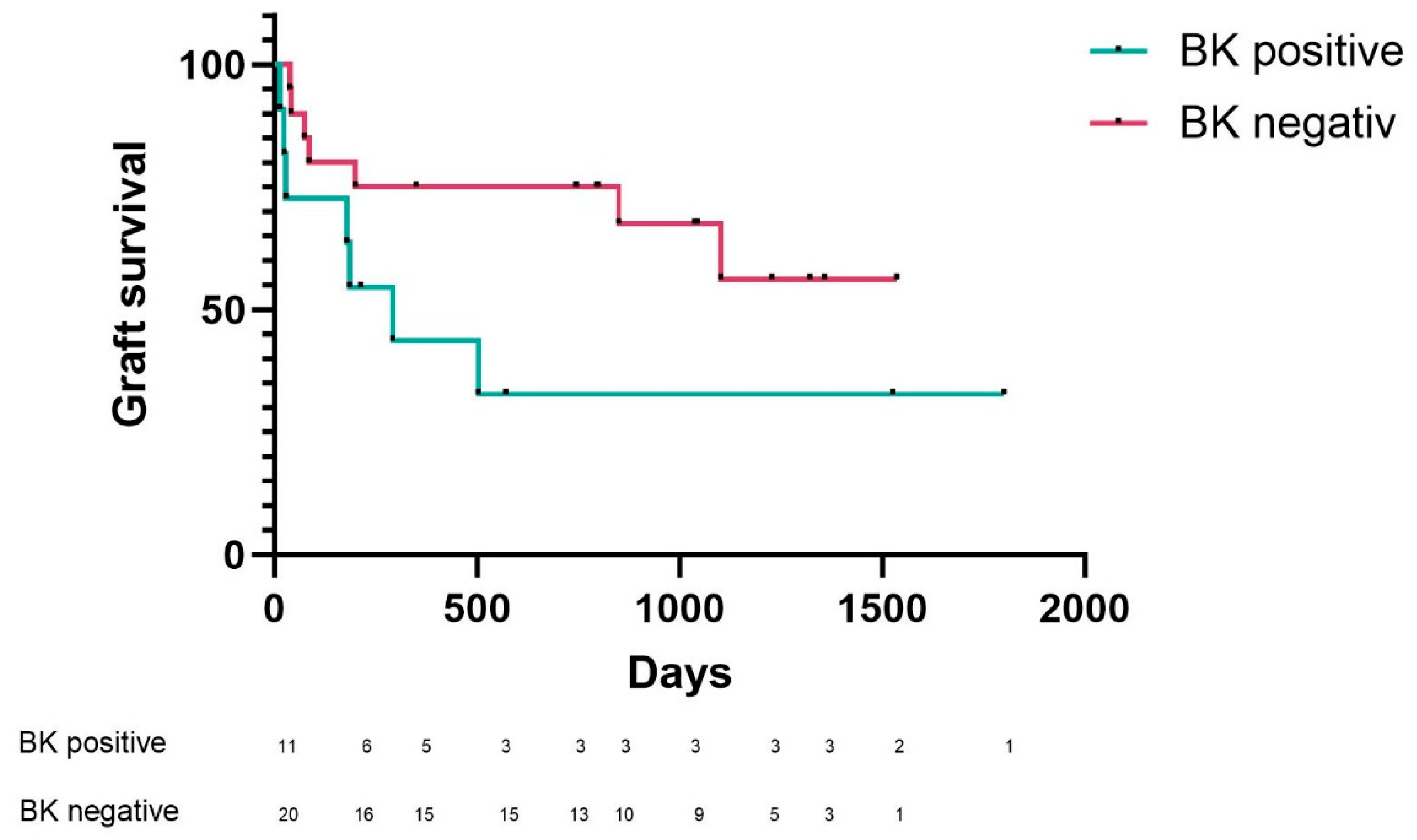
2. Results
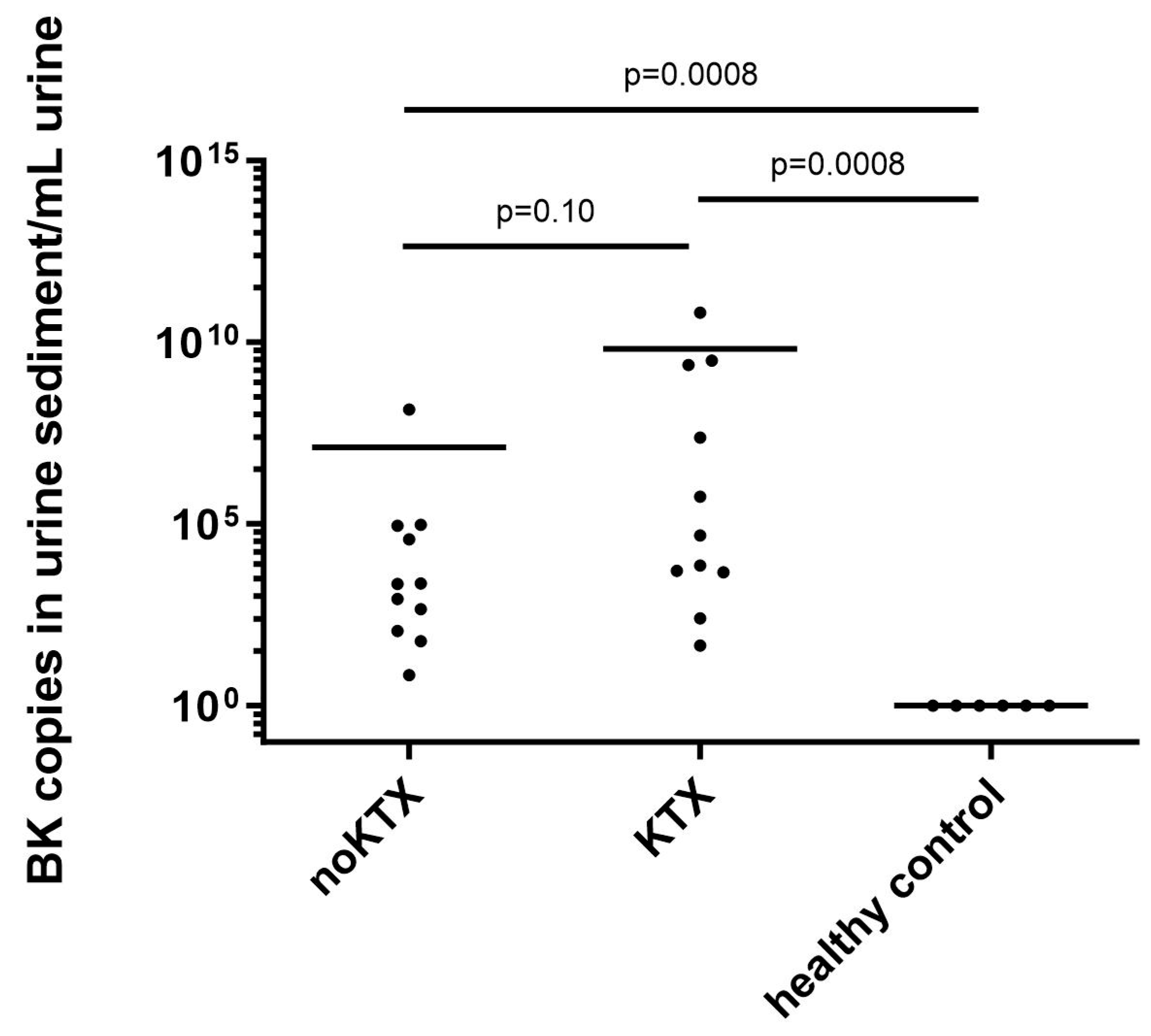
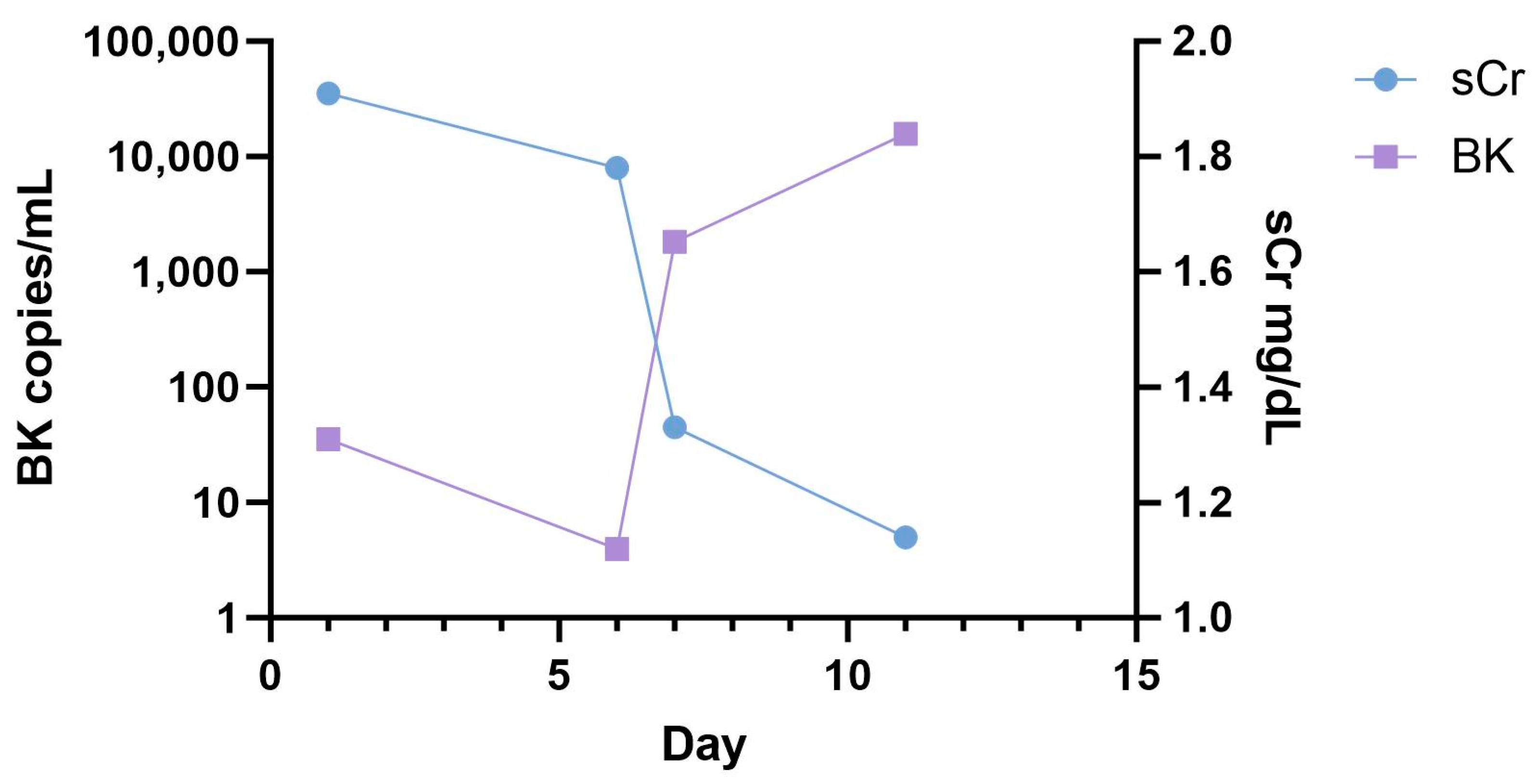
2.1. BKV Spot Measurement in Urinary Sediment Cells of Patients at Time of Admission
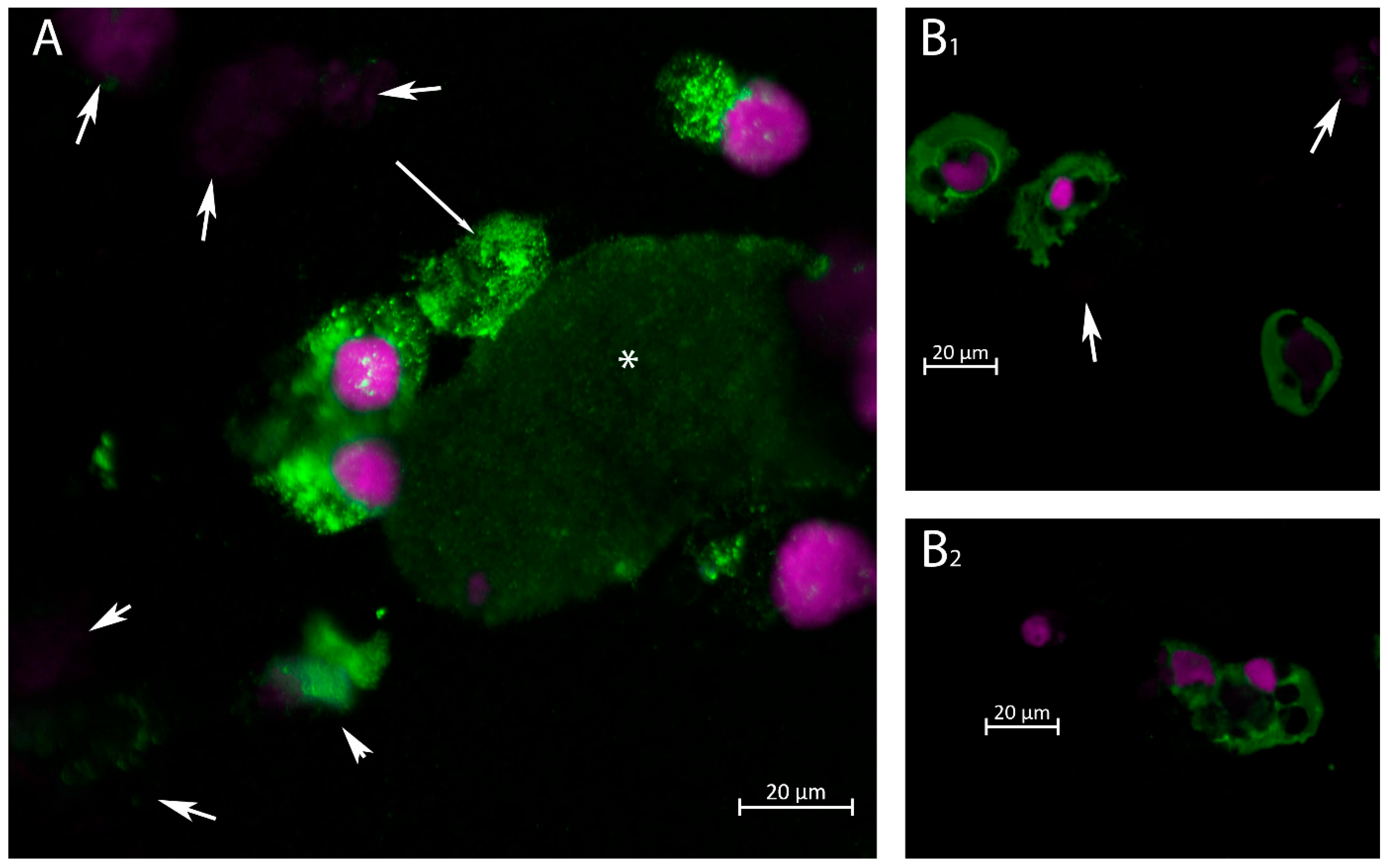
2.2. Immunofluorescence Staining for VP1 in Urinary Cells
3. Discussion
4. Materials and Methods
4.1. Reverse Transcription
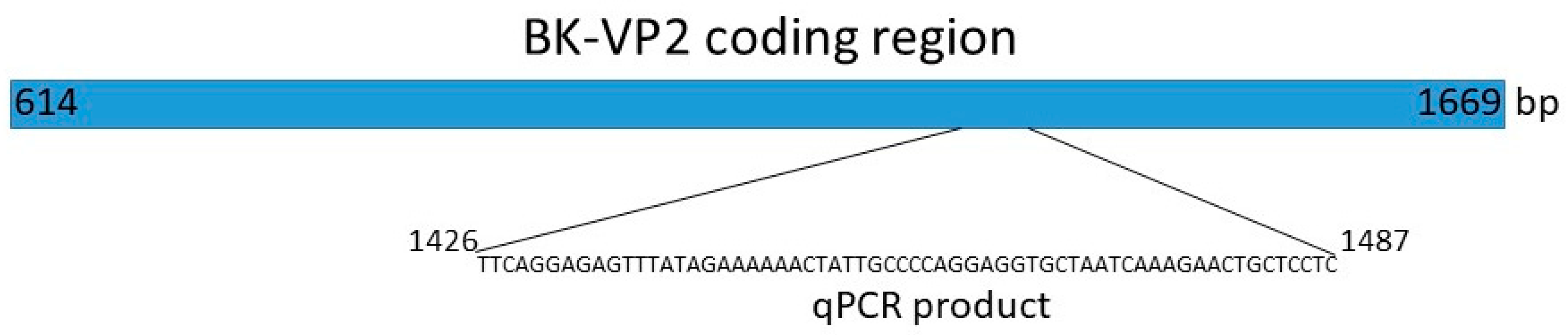
qPCR
4.2. BK Polyoma Virus Copy Number Quantification
4.3. BK—VP1 PCR
4.4. Animal Care and Immunization
4.5. Rabbit Immunization
4.6. Immunofluorescence Staining of Urinary Sediment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stolt, A.; Sasnauskas, K.; Koskela, P.; Lehtinen, M.; Dillner, J. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 2003, 84, 1499–1504. [Google Scholar] [CrossRef]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 2009, 199, 837–846. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Vincenti, F.; Friman, S.; Tuncer, M.; Citterio, F.; Wiecek, A.; Scheuermann, E.H.; Klinger, M.; Russ, G.; Pescovitz, M.D.; et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study. Am. J. Transplant. 2013, 13, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P.S.; Practice, A.I.D.C.o. BK polyomavirus in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef] [PubMed]
- Balba, G.P.; Javaid, B.; Timpone, J.G., Jr. BK polyomavirus infection in the renal transplant recipient. Infect. Dis. Clin. N. Am. 2013, 27, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Kato, I.; Kawaguchi, K.; Tasaka, K.; Kamitori, T.; Ogata, H.; Mikami, T.; Hiramatsu, H.; Saito, R.; Ogawa, O.; et al. High incidence of BK virus-associated hemorrhagic cystitis in children after second or third allogeneic hematopoietic stem cell transplantation. Pediatr. Transplant. 2018, 22, e13183. [Google Scholar] [CrossRef]
- Hirsch, H.H.; Knowles, W.; Dickenmann, M.; Passweg, J.; Klimkait, T.; Mihatsch, M.J.; Steiger, J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N. Engl. J. Med. 2002, 347, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Drachenberg, C.B.; Papadimitriou, J.C.; Hamze, O.; Fink, J.C.; Klassen, D.K.; Drachenberg, R.C.; Wiland, A.; Wali, R.; Cangro, C.B.; et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J. Am. Soc. Nephrol. 2002, 13, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, B.; Hariharan, S.; Hussain, S.A.; Zhu, Y.-R.; Bresnahan, B.A.; Cohen, E.P. BK virus nephritis: Risk factors, timing, and outcome in renal transplant recipients. Kidney Int. 2005, 68, 1834–1839. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Dadhania, D.; Cornea, V.; El-Husseini, A.; Castellanos, A.; Davis, V.G.; Waid, T.; Seshan, S.V. The 2018 Banff Working Group classification of definitive polyomavirus nephropathy: A multicenter validation study in the modern era. Am. J. Transplant. 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Nickeleit, V.; Singh, H.K.; Randhawa, P.; Drachenberg, C.B.; Bhatnagar, R.; Bracamonte, E.; Chang, A.; Chon, W.J.; Dadhania, D.; Davis, V.G.; et al. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J. Am. Soc. Nephrol. 2018, 29, 680–693. [Google Scholar] [CrossRef]
- Azar, M.M.; Assi, R.; Valika, A.K.; Banach, D.B.; Hall, I.E.; Landry, M.L.; Malinis, M.F. Graft loss among renal-transplant recipients with early reduction of immunosuppression for BK viremia. World J. Transplant. 2017, 7, 269–275. [Google Scholar] [CrossRef]
- Howell, D.N.; Smith, S.R.; Butterly, D.W.; Klassen, P.S.; Krigman, H.R.; Burchette, J.L., Jr.; Miller, S.E. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation 1999, 68, 1279–1288. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Hirsch, H.H.; Papadimitriou, J.C.; Gosert, R.; Wali, R.K.; Munivenkatappa, R.; Nogueira, J.; Cangro, C.B.; Haririan, A.; Mendley, S.; et al. Polyomavirus BK Versus JC Replication and Nephropathy in Renal Transplant Recipients: A Prospective Evaluation. Transplantation 2007, 84, 323–330. [Google Scholar] [CrossRef]
- Abuhelaiqa, E.; Snopkowski, C.; Li, C.; Salvatore, S.; Lee, J.R.; Muthukumar, T.; Lee, J.B.; Hartono, C.; Ding, R.; Seshan, S.V.; et al. Validation of a noninvasive prognostic signature for allograft failure following BK virus associated nephropathy. Clin. Transplant. 2021, 35, e14200. [Google Scholar] [CrossRef]
- Dadhania, D.; Snopkowski, C.; Ding, R.; Muthukumar, T.; Lee, J.; Bang, H.; Sharma, V.K.; Seshan, S.; August, P.; Kapur, S.; et al. Validation of Noninvasive Diagnosis of BK Virus Nephropathy and Identification of Prognostic Biomarkers. Transplantation 2010, 90, 189–197. [Google Scholar] [CrossRef]
- Ding, R.; Medeiros, M.; Dadhania, D.; Muthukumar, T.; Kracker, D.; Kong, J.M.; Epstein, S.R.; Sharma, V.K.; Seshan, S.V.; Li, B.; et al. Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine1. Transplantation 2002, 74, 987–994. [Google Scholar] [CrossRef]
- Chen, X.-T.; Chen, W.-F.; Hou, X.-T.; Yang, S.-C.; Yang, H.-F.; Li, J.; Deng, R.-H.; Huang, Y.; Nuertai, Y.; Wang, C.-X.; et al. Non-invasive urinary sediment double-immunostaining predicts BK polyomavirus associated-nephropathy in kidney transplant recipients. Ann. Transl. Med. 2020, 8, 235. [Google Scholar] [CrossRef]
- Yan, L.; Guo, H.; Han, L.; Huang, H.; Shen, Y.; He, J.; Liu, J. Sternheimer-Malbin Staining to Detect Decoy Cells in Urine of 213 Kidney Transplant Patients. Transplant. Proc. 2020, 52, 823–828. [Google Scholar] [CrossRef]
- Evans, G.L.; Caller, L.G.; Foster, V.; Crump, C.M. Anion homeostasis is important for non-lytic release of BK polyomavirus from infected cells. Open Biol. 2015, 5, 150041. [Google Scholar] [CrossRef]
- Bär, S.; Daeffler, L.; Rommelaere, J.; Nüesch, J.P.F. Vesicular Egress of Non-Enveloped Lytic Parvoviruses Depends on Gelsolin Functioning. PLoS Pathog. 2008, 4, e1000126. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.-H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Clayson, E.T.; Brando, L.V.; Compans, R.W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J. Virol. 1989, 63, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Kujat, J.; Langhans, V.; Brand, H.; Freund, P.; Görlich, N.; Wagner, L.; Metzke, D.; Timm, S.; Ochs, M.; Grützkau, A.; et al. Monitoring tubular epithelial cell damage in AKI via urine flow cytometry. medRxiv 2022. [Google Scholar] [CrossRef]
- Grellier, J.; Hirsch, H.H.; Mengelle, C.; Esposito, L.; Hebral, A.L.; Bellière, J.; Weissbach, F.; Izopet, J.; Del Bello, A.; Kamar, N. Impact of donor BK polyomavirus replication on recipient infections in living donor transplantation. Transplant. Infect. Dis. 2018, 20, e12917. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Linnenweber-Held, S.; Heim, A.; Framke, T.; Haller, H.; Schmitt, C. Viral Origin, Clinical Course, and Renal Outcomes in Patients With BK Virus Infection After Living-Donor Renal Transplantation. Transplantation 2016, 100, 844–853. [Google Scholar] [CrossRef]
- Schmitt, C.; Raggub, L.; Linnenweber-Held, S.; Adams, O.; Schwarz, A.; Heim, A. Donor origin of BKV replication after kidney transplantation. J. Clin. Virol. 2014, 59, 120–125. [Google Scholar] [CrossRef]
- Nüesch, J.P.F.; Lachmann, S.; Rommelaere, J. Selective alterations of the host cell architecture upon infection with parvovirus minute virus of mice. Virology 2005, 331, 159–174. [Google Scholar] [CrossRef]
- Radtke, K.; Döhner, K.; Sodeik, B. Viral interactions with the cytoskeleton: A hitchhiker’s guide to the cell. Cell Microbiol. 2006, 8, 387–400. [Google Scholar] [CrossRef]
- Mineeva-Sangwo, O.; Van Loon, E.; Andrei, G.; Kuypers, D.; Naesens, M.; Snoeck, R. Time-dependent variations in BK polyomavirus genome from kidney transplant recipients with persistent viremia. Sci. Rep. 2023, 13, 13534. [Google Scholar] [CrossRef]
- Caller, L.G.; Davies, C.T.R.; Antrobus, R.; Lehner, P.J.; Weekes, M.P.; Crump, C.M. Temporal Proteomic Analysis of BK Polyomavirus Infection Reveals Virus-Induced G2 Arrest and Highly Effective Evasion of Innate Immune Sensing. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Cantalupo, P.G.; Zheng, W.; Saenz-Robles, M.T.; Duray, A.M.; Weitz, D.; Pipas, J.M. Single-Cell Transcriptomics Reveals a Heterogeneous Cellular Response to BK Virus Infection. J. Virol. 2021, 95. [Google Scholar] [CrossRef] [PubMed]

| KTX | nonKTX | Controls | ||
|---|---|---|---|---|
| Characteristics | 31 | 48 | 6 | |
| Gender | ||||
| Male | 22 | 37 | 4 | |
| Female | 9 | 11 | 2 | |
| Age ± SD | 55.97 ± 12.98 | 58.35 ± 19.24 | 46.17 ± 12.00 | |
| Comorbidities | ||||
| Hypertension | 28 (93.32%) | 35 (72.91%) | ||
| Diabetes mellitus | 11 (35.48%) | 11 (22.91%) | ||
| Cardiovascular disease | 15 (48.39%) | 14 (29.16%) | ||
| Cerebrovascular disease | 7 (22.58%) | 3 (6.25%) | ||
| Malignancy | 2 (66.45%) | 13 (27.08%) | ||
| Immunosuppression | 30 (96.77%) | 8 (16.66%) | ||
| Chronic kidney disease | ||||
| stage 1 | 1 (3.23%) | 19 (39.58%) | ||
| stage 2 | 3 (9.68%) | 7 (14.58%) | ||
| stage 3 | 9 (29.03%) | 11 (22.91%) | ||
| stage 4 | 4 (12.90%) | 4 (8.33%) | ||
| stage 5 | 14 (45.16%) | 7 (14.58%) | ||
| Acute Kidney injury | ||||
| No AKI | 4 (12.90%) | 15 (31.25%) | ||
| stage 1 | 5 (16.13%) | 5 (10.41%) | ||
| stage 2 | 2 (6.45%) | 4 (8.33%) | ||
| stage 3 | 10 (32.26%) | 24 (50.00%) | ||
| post TX DGF | 10 (32.26%) | n.a. | ||
| KTX | nonKTX | |
|---|---|---|
| Number of participants with urinary | ||
| sediment positive for polyoma | ||
| n = 11 | n = 11 | |
| Chronic kidney disease | ||
| stage 1 | 1 | 5 |
| stage 2 | 2 | 3 |
| stage 3 | 4 | 3 |
| stage 4 | 1 | 0 |
| stage 5 | 3 | 0 |
| Acute kidney injury | ||
| stage 1 | 5 | 1 |
| stage 2 | 1 | 1 |
| stage 3 | 5 | 9 |
| Immunosuppression | ||
| No immunosuppression | n.a. | 8 |
| Triple IS | 11 | 0 |
| Dual IS | 0 | 2 |
| Mono IS | 0 | 1 |
| KTX induction therapy (ATG) | 1 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajenda, S.; Gerges, D.A.; Freire, R.; Wagner, L.; Hevesi, Z.; Aiad, M.; Eder, M.; Schmidt, A.; Winnicki, W.; Eskandary, F.A. Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells. Int. J. Mol. Sci. 2023, 24, 17511. https://doi.org/10.3390/ijms242417511
Pajenda S, Gerges DA, Freire R, Wagner L, Hevesi Z, Aiad M, Eder M, Schmidt A, Winnicki W, Eskandary FA. Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells. International Journal of Molecular Sciences. 2023; 24(24):17511. https://doi.org/10.3390/ijms242417511
Chicago/Turabian StylePajenda, Sahra, Daniela Anna Gerges, Raimundo Freire, Ludwig Wagner, Zsofia Hevesi, Monika Aiad, Michael Eder, Alice Schmidt, Wolfgang Winnicki, and Farsad Alexander Eskandary. 2023. "Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells" International Journal of Molecular Sciences 24, no. 24: 17511. https://doi.org/10.3390/ijms242417511
APA StylePajenda, S., Gerges, D. A., Freire, R., Wagner, L., Hevesi, Z., Aiad, M., Eder, M., Schmidt, A., Winnicki, W., & Eskandary, F. A. (2023). Acute Kidney Injury and BK Polyomavirus in Urine Sediment Cells. International Journal of Molecular Sciences, 24(24), 17511. https://doi.org/10.3390/ijms242417511






