Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review
Abstract
:1. Introduction
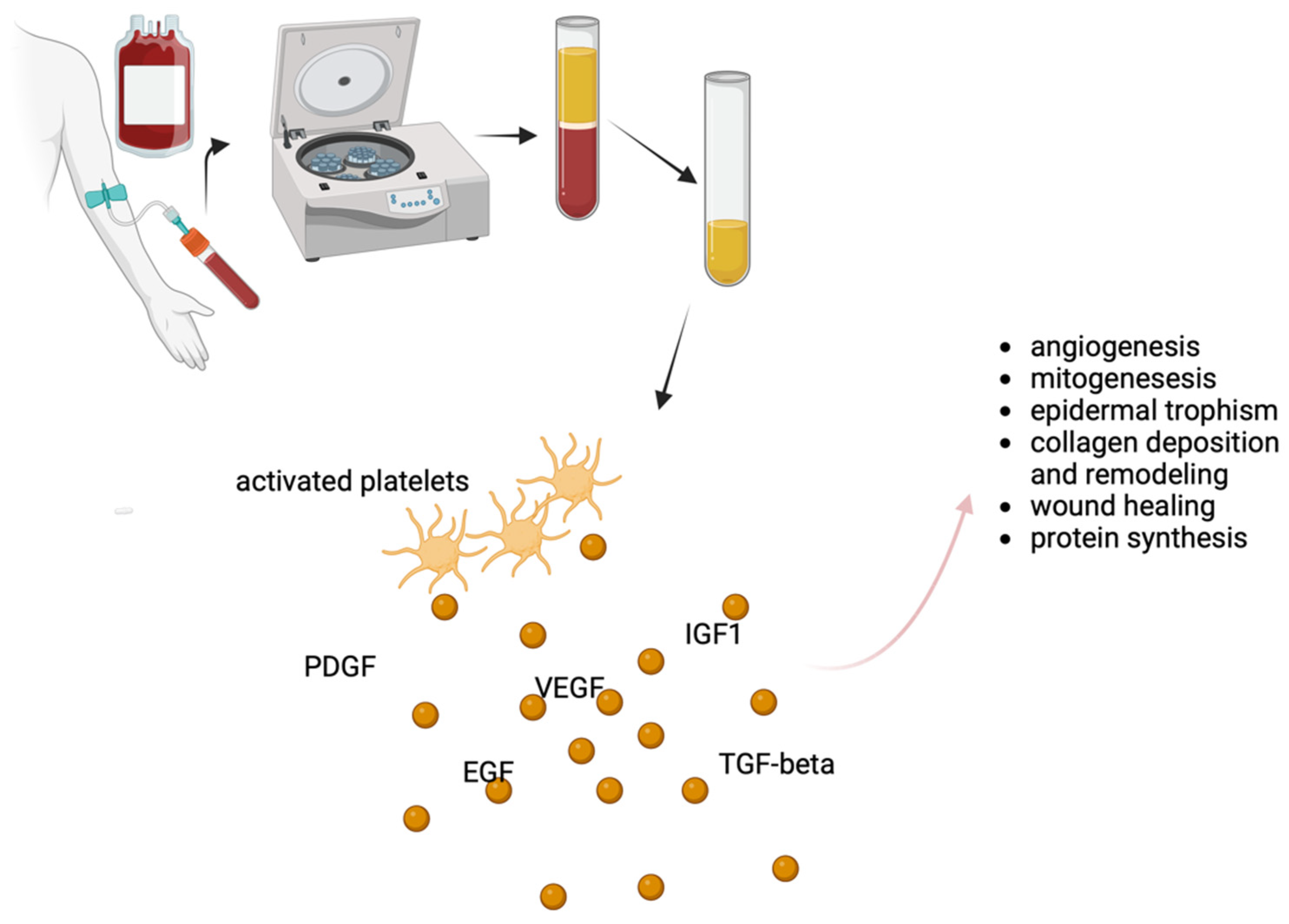
2. Platelet-Rich Plasma (PRP) Therapy
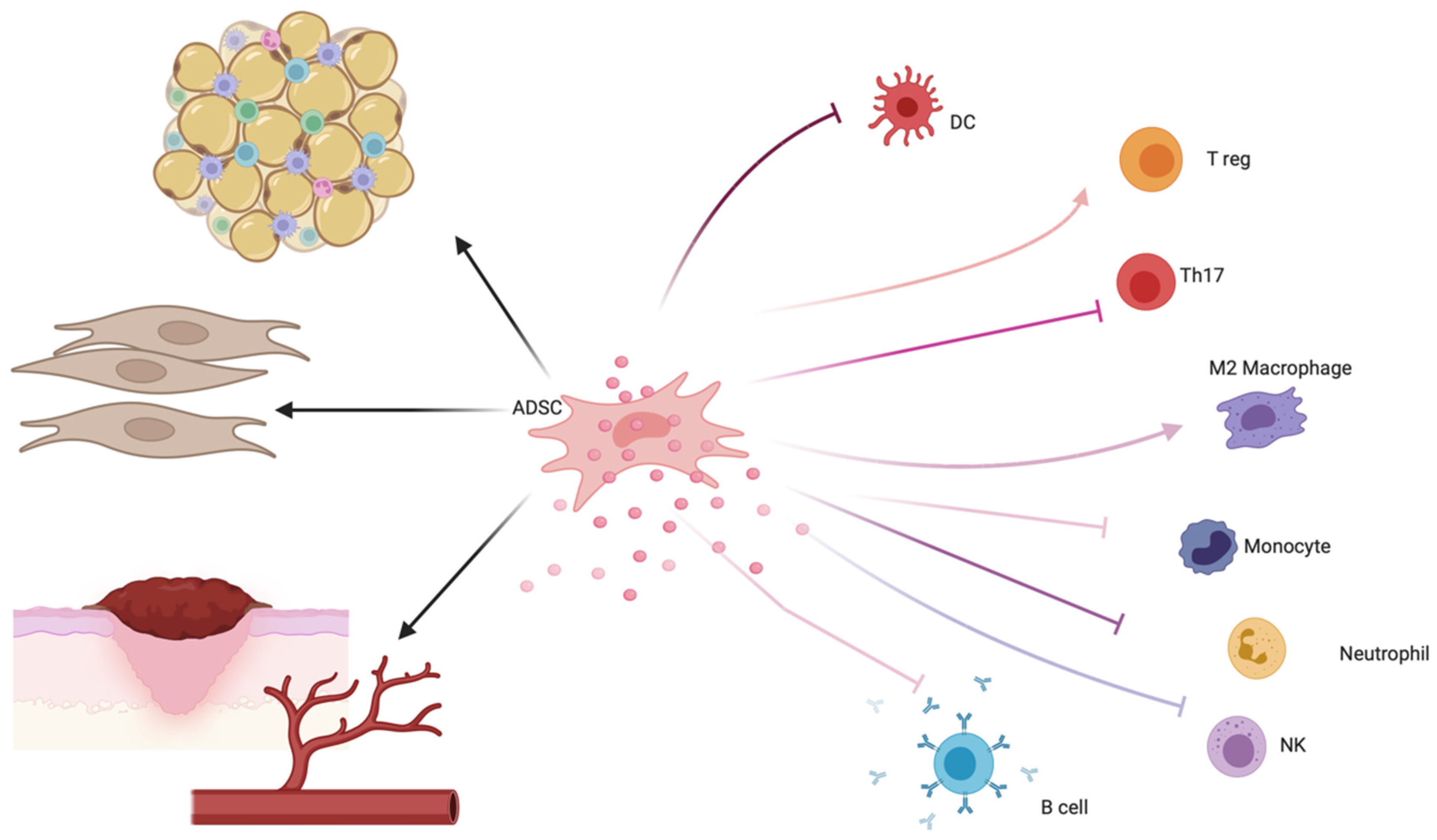
3. Adipose-Derived Stem Cell (ADSC) Therapy
4. Combined Treatment: ADSCs and PRP
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kirtschig, G. Lichen Sclerosus-Presentation, Diagnosis and Management. Dtsch. Arztebl. Int. 2016, 113, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Steben, M. Lichen Sclerosus: Why Do Most Women Struggle With Their Diagnosis? J. Obstet. Gynaecol. Can. 2022, 44, 119–120.e1. [Google Scholar] [CrossRef] [PubMed]
- Meffert, J.J.; Davis, B.M.; Grimwood, R.E. Lichen Sclerosus. J. Am. Acad. Dermatol. 1995, 32, 393–416; quiz 417–418. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Fatima, R.; Sami, M. Extragenital Lichen Sclerosus: A Comprehensive Review. Aust. J. Dermatol. 2022, 63, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Tasker, G.L.; Wojnarowska, F. Lichen Sclerosus. Clin. Exp. Dermatol. 2003, 28, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ranum, A.; Pearson, D.R. The Impact of Genital Lichen Sclerosus and Lichen Planus on Quality of Life: A Review. Int. J. Womens Dermatol. 2022, 8, e042. [Google Scholar] [CrossRef]
- Felmingham, C.; Chan, L.; Doyle, L.W.; Veysey, E. The Vulval Disease Quality of Life Index in Women with Vulval Lichen Sclerosus Correlates with Clinician and Symptom Scores. Australas. J. Dermatol. 2020, 61, 110–118. [Google Scholar] [CrossRef]
- Vittrup, G.; Mørup, L.; Heilesen, T.; Jensen, D.; Westmark, S.; Melgaard, D. Quality of Life and Sexuality in Women with Lichen Sclerosus: A Cross-Sectional Study. Clin. Exp. Dermatol. 2022, 47, 343–350. [Google Scholar] [CrossRef]
- Casabona, F.; Gasparini, G.; Cozzani, E.; Barbazza, A.; Casabona, F.; Carmisciano, L.; Parodi, A. Improvement in Quality of Life and Sexual Function in Patients Affected by Vulvar Lichen Sclerosus Treated with Combined Autologous Platelet-Rich Plasma and Fat Grafting. Eur. J. Dermatol. 2023, 33, 249–254. [Google Scholar] [CrossRef]
- Khan Mohammad Beigi, P. The Immunogenetics of Morphea and Lichen Sclerosus. Adv. Exp. Med. Biol. 2022, 1367, 155–172. [Google Scholar] [CrossRef]
- Veronesi, G.; Virdi, A.; Leuzzi, M.; Gurioli, C.; Chessa, M.A.; Guglielmo, A.; Neri, I. Vulvar Vitiligo and Lichen Sclerosus in Children: A Clinical Challenge. Pediatr. Dermatol. 2021, 38, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Vieira-Baptista, P. Lichen Sclerosus in Women: A Review. Climacteric 2017, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fistarol, S.K.; Itin, P.H. Diagnosis and Treatment of Lichen Sclerosus: An Update. Am. J. Clin. Dermatol. 2013, 14, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Kirtschig, G.; Becker, K.; Günthert, A.; Jasaitiene, D.; Cooper, S.; Chi, C.-C.; Kreuter, A.; Rall, K.K.; Aberer, W.; Riechardt, S.; et al. Evidence-Based (S3) Guideline on (Anogenital) Lichen Sclerosus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e43. [Google Scholar] [CrossRef]
- White, C.; Brahs, A.; Dorton, D.; Witfill, K. Platelet-Rich Plasma: A Comprehensive Review of Emerging Applications in Medical and Aesthetic Dermatology. J. Clin. Aesthet. Dermatol. 2021, 14, 44–57. [Google Scholar]
- Eshtiaghi, P.; Sadownik, L.A. Fact or Fiction? Adipose-Derived Stem Cells and Platelet-Rich Plasma for the Treatment of Vulvar Lichen Sclerosus. J. Low. Genit. Tract. Dis. 2019, 23, 65–70. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Ther. Lett. 2019, 24, 1–6. [Google Scholar]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic Review of the Use of Platelet-Rich Plasma in Aesthetic Dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef]
- Cook, C.S.; Smith, P.A. Clinical Update: Why PRP Should Be Your First Choice for Injection Therapy in Treating Osteoarthritis of the Knee. Curr. Rev. Musculoskelet. Med. 2018, 11, 583–592. [Google Scholar] [CrossRef]
- Ince, B.; Yildirim, M.E.C.; Dadaci, M.; Avunduk, M.C.; Savaci, N. Comparison of the Efficacy of Homologous and Autologous Platelet-Rich Plasma (PRP) for Treating Androgenic Alopecia. Aesth Plast. Surg. 2018, 42, 297–303. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Goszka, M.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Dołęgowska, B. Applications of the Regenerative Capacity of Platelets in Modern Medicine. Cytokine Growth Factor. Rev. 2022, 64, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Irmak, G.; Demirtaş, T.T.; Gümüşderelioğlu, M. Sustained Release of Growth Factors from Photoactivated Platelet Rich Plasma (PRP). Eur. J. Pharm. Biopharm. 2020, 148, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Grigoriadis, T.; Athanasiou, S. Platelet Rich Plasma for the Management of Urogynecological Disorders: The Current Evidence. Curr. Opin. Obstet. Gynecol. 2022, 34, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Casabona, F.; Gambelli, I.; Casabona, F.; Santi, P.; Santori, G.; Baldelli, I. Autologous Platelet-Rich Plasma (PRP) in Chronic Penile Lichen Sclerosus: The Impact on Tissue Repair and Patient Quality of Life. Int. Urol. Nephrol. 2017, 49, 573–580. [Google Scholar] [CrossRef]
- Behnia-Willison, F.; Pour, N.R.; Mohamadi, B.; Willison, N.; Rock, M.; Holten, I.W.; O’Shea, R.; Miller, J. Use of Platelet-Rich Plasma for Vulvovaginal Autoimmune Conditions Like Lichen Sclerosus. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e1124. [Google Scholar] [CrossRef]
- Medina Garrido, C.; Cano García, A.; De La Cruz Cea, L.; Oreja Cuesta, A.B. Mid-Term Symptomatic Relief after Platelet-Rich Plasma Infiltration in Vulvar Lichen Sclerosus. Arch. Dermatol. Res. 2023, 315, 1527–1532. [Google Scholar] [CrossRef]
- Goldstein, A.T.; Mitchell, L.; Govind, V.; Heller, D. A Randomized Double-Blind Placebo-Controlled Trial of Autologous Platelet-Rich Plasma Intradermal Injections for the Treatment of Vulvar Lichen Sclerosus. J. Am. Acad. Dermatol. 2019, 80, 1788–1789. [Google Scholar] [CrossRef]
- Goldstein, A.T.; King, M.; Runels, C.; Gloth, M.; Pfau, R. Intradermal Injection of Autologous Platelet-Rich Plasma for the Treatment of Vulvar Lichen Sclerosus. J. Am. Acad. Dermatol. 2017, 76, 158–160. [Google Scholar] [CrossRef]
- Tedesco, M.; Pranteda, G.; Chichierchia, G.; Paolino, G.; Latini, A.; Orsini, D.; Cristaudo, A.; Foddai, M.L.; Migliano, E.; Morrone, A. The Use of PRP (Platelet-rich Plasma) in Patients Affected by Genital Lichen Sclerosus: Clinical Analysis and Results. Acad. Dermatol. Venereol. 2019, 33, e58–e59. [Google Scholar] [CrossRef]
- Franic, D.; Iternička, Z.; Franić-Ivanišević, M. Platelet-Rich Plasma (PRP) for the Treatment of Vulvar Lichen Sclerosus in a Premenopausal Woman: A Case Report. Case Rep. Women’s Health 2018, 18, e00062. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.; Garelli, V.; Elia, F.; Chicherchia, G.; Foddai, M.L.; Latini, A.; Morrone, A.; Migliano, E. Usefulness of Video Thermography in the Evaluation of Platelet-Rich Plasma Effectiveness in Vulvar Lichen Sclerosus: Preliminary Study. J. Dermatol. Treat. 2021, 32, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, J.; Echarte, L.; Sujanov, A.; Guillones, A.; Vola, M.; Bunker, C.B.; Agorio, C.; Touriño, C. Platelet-rich Plasma for Male Genital Lichen Sclerosus Resistant to Conventional Therapy: First Prospective Study. Dermatol. Ther. 2020, 33, e14032. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.; Garelli, V.; Bellei, B.; Sperduti, I.; Chichierchia, G.; Latini, A.; Foddai, M.L.; Bertozzi, E.; Bonadies, A.; Pallara, T.; et al. Platelet-Rich Plasma for Genital Lichen Sclerosus: Analysis and Results of 94 Patients. Are There Gender-Related Differences in Symptoms and Therapeutic Response to PRP? J. Dermatol. Treat. 2022, 33, 1558–1562. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef]
- Gronthos, S.; Zannettino, A.C.W.; Hay, S.J.; Shi, S.; Graves, S.E.; Kortesidis, A.; Simmons, P.J. Molecular and Cellular Characterisation of Highly Purified Stromal Stem Cells Derived from Human Bone Marrow. J. Cell Sci. 2003, 116, 1827–1835. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Housman, T.S.; Lawrence, N.; Mellen, B.G.; George, M.N.; Filippo, J.S.; Cerveny, K.A.; DeMarco, M.; Feldman, S.R.; Fleischer, A.B. The Safety of Liposuction: Results of a National Survey. Dermatol. Surg. 2002, 28, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Benassi, L.; Pastar, I.; Pellegrini, M.; Azzoni, P.; Vaschieri, C.; Pisciotta, A.; Carnevale, G.; Pellacani, G.; Magnoni, C. In Vitro Engineering of a Skin Substitute Based on Adipose-Derived Stem Cells. Cells Tissues Organs 2019, 207, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.C.; Du, W.J.; Han, Z.B.; Liang, L. New Insights into the Heterogeneity and Functional Diversity of Human Mesenchymal Stem Cells. Biomed. Mater. Eng. 2017, 28, S29–S45. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Tomic, S.; Djokic, J.; Vasilijic, S.; Vucevic, D.; Todorovic, V.; Supic, G.; Colic, M. Immunomodulatory Properties of Mesenchymal Stem Cells Derived from Dental Pulp and Dental Follicle Are Susceptible to Activation by Toll-like Receptor Agonists. Stem Cells Dev. 2011, 20, 695–708. [Google Scholar] [CrossRef]
- Zhao, S.; Wehner, R.; Bornhäuser, M.; Wassmuth, R.; Bachmann, M.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells and Their Therapeutic Consequences for Immune-Mediated Disorders. Stem Cells Dev. 2010, 19, 607–614. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal Stem Cells: Immunomodulatory Capability and Clinical Potential in Immune Diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef]
- Spiekman, M.; van Dongen, J.A.; Willemsen, J.C.; Hoppe, D.L.; van der Lei, B.; Harmsen, M.C. The Power of Fat and Its Adipose-Derived Stromal Cells: Emerging Concepts for Fibrotic Scar Treatment. J. Tissue Eng. Regen. Med. 2017, 11, 3220–3235. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Migliano, E.; Picardo, M. Therapeutic Potential of Adipose Tissue-Derivatives in Modern Dermatology. Exp. Dermatol. 2022, 31, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Jiang, D.; Rinkevich, Y. Genital Wound Repair and Scarring. Med. Sci. 2022, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Bellei, B.; Migliano, E.; Picardo, M. Research Update of Adipose Tissue-Based Therapies in Regenerative Dermatology. Stem Cell Rev. Rep. 2022, 18, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Giuseppina Onesti, M.; Carella, S.; Ceccarelli, S.; Marchese, C.; Scuderi, N. The Use of Human Adipose-Derived Stem Cells in the Treatment of Physiological and Pathological Vulvar Dystrophies. Stem Cells Int. 2016, 2016, 2561461. [Google Scholar] [CrossRef]
- Almadori, A.; Hansen, E.; Boyle, D.; Zenner, N.; Swale, V.; Reid, W.; Maclane, A.; Butler, P.E.M. Fat Grafting Improves Fibrosis and Scarring in Vulvar Lichen Sclerosus: Results From a Prospective Cohort Study. J. Low. Genit. Tract. Dis. 2020, 24, 305–310. [Google Scholar] [CrossRef]
- Boero, V.; Brambilla, M.; Sipio, E.; Liverani, C.A.; Di Martino, M.; Agnoli, B.; Libutti, G.; Cribiù, F.M.; Del Gobbo, A.; Ragni, E.; et al. Vulvar Lichen Sclerosus: A New Regenerative Approach through Fat Grafting. Gynecol. Oncol. 2015, 139, 471–475. [Google Scholar] [CrossRef]
- Boero, V.; Brambilla, M.; Di Loreto, E.; Cetera, G.E.; Cipriani, S.; Boggio, F.; Monti, E.; Libutti, G.; Caia, C.; Parazzini, F. Fat Grafting in Vulvar Lichen Sclerosus: Long Term Follow-Up. J. Low. Genit. Tract. Dis. 2023, 27, 365–372. [Google Scholar] [CrossRef]
- Monreal, J. Safety and Efficacy of Stromal Vascular Fraction Enriched Fat Grafting Therapy for Vulvar Lichen Sclerosus. Cureus 2020, 12, e7096. [Google Scholar] [CrossRef]
- Lai, F.; Dai, S.; Zhao, Y.; Sun, Y. Combination of PDGF-BB and Adipose-Derived Stem Cells Accelerated Wound Healing through Modulating PTEN/AKT Pathway. Injury 2023, 54, 1451–1461. [Google Scholar] [CrossRef]
- Carvalho Schweich-Adami, L.; Silva, R.A.D.; Menezes, J.N.D.S.; Baranoski, A.; Kassuya, C.A.L.; Bernardi, L.; Juliano Oliveira, R.; Conceição Milan Brochado Antoniolli-Silva, A. The Intra-articular Injection of Adipose-derived Stem Cells Decreases Pain and Reduces Inflammation in Knee Osteoarthritis, with or without the Addition of Platelet-rich Plasma Also Improves Functionality. J. Tissue Eng. Regen. Med. 2022, 16, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Riahi, R.R. Platelet-Rich Plasma and Genital Rejuvenation. Skinmed 2019, 17, 272–274. [Google Scholar] [PubMed]
- Kim, S.H.; Park, E.S.; Kim, T.H. Rejuvenation Using Platelet-Rich Plasma and Lipofilling for Vaginal Atrophy and Lichen Sclerosus. J. Menopausal Med. 2017, 23, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Casabona, F.; Priano, V.; Vallerino, V.; Cogliandro, A.; Lavagnino, G. New Surgical Approach to Lichen Sclerosus of the Vulva: The Role of Adipose-Derived Mesenchymal Cells and Platelet-Rich Plasma in Tissue Regeneration. Plast. Reconstr. Surg. 2010, 126, 210e–211e. [Google Scholar] [CrossRef]
- Gutierrez-Ontalvilla, P.; Giner, F.; Vidal, L.; Iborra, M. The Effect of Lipofilling and Platelet-Rich Plasma on Patients with Moderate–Severe Vulvar Lichen Sclerosus Who Were Non-Responders to Topical Clobetasol Propionate: A Randomized Pilot Study. Aesth. Plast. Surg. 2022, 46, 2469–2479. [Google Scholar] [CrossRef]
- Tedesco, M.; Bellei, B.; Garelli, V.; Caputo, S.; Latini, A.; Giuliani, M.; Cota, C.; Chichierchia, G.; Romani, C.; Foddai, M.L.; et al. Adipose Tissue Stromal Vascular Fraction and Adipose Tissue Stromal Vascular Fraction plus Platelet-Rich Plasma Grafting: New Regenerative Perspectives in Genital Lichen Sclerosus. Dermatol. Ther. 2020, 33, e14277. [Google Scholar] [CrossRef]
| Author | Y | M | F | ADSCs/Fat | PRP | ADSC + PRP |
|---|---|---|---|---|---|---|
| Almadori | 2020 | 33 | 33 | |||
| Behnia-Willison | 2016 | 28 | 28 | |||
| Boero | 2015 | 36 | 36 | |||
| Casabona | 2010 | 15 | 15 | |||
| Casabona | 2017 | 45 | 45 | |||
| Casabona | 2023 | 72 | 72 | |||
| Cohen | 2019 | 1 | 1 | |||
| Franic | 2018 | 1 | 1 | |||
| Gutierrez-Ontalvilla | 2022 | 9 | 9 | |||
| Goldstein | 2016 | 15 | 15 | |||
| Goldstein | 2019 | 19 | 19 | |||
| Kim | 2017 | 1 | 1 | |||
| Medina Garrido | 2023 | 28 | 28 | |||
| Monreal | 2020 | 39 | 39 | |||
| Navarrete | 2020 | 5 | 5 | |||
| Onesti | 2016 | 8 | 8 | |||
| Tedesco | 2019 | 13 | 18 | 31 | ||
| Tedesco | 2020 | 40 | 20 | 20 | ||
| Tedesco | 2021 | 6 | 6 | |||
| Tedesco | 2022 | 43 | 51 | 94 | ||
| 106 | 420 | 136 | 272 | 118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganelli, A.; Contu, L.; Condorelli, A.; Ficarelli, E.; Motolese, A.; Paganelli, R.; Motolese, A. Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16107. https://doi.org/10.3390/ijms242216107
Paganelli A, Contu L, Condorelli A, Ficarelli E, Motolese A, Paganelli R, Motolese A. Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. International Journal of Molecular Sciences. 2023; 24(22):16107. https://doi.org/10.3390/ijms242216107
Chicago/Turabian StylePaganelli, Alessia, Luca Contu, Alessandra Condorelli, Elena Ficarelli, Alfonso Motolese, Roberto Paganelli, and Alberico Motolese. 2023. "Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review" International Journal of Molecular Sciences 24, no. 22: 16107. https://doi.org/10.3390/ijms242216107
APA StylePaganelli, A., Contu, L., Condorelli, A., Ficarelli, E., Motolese, A., Paganelli, R., & Motolese, A. (2023). Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. International Journal of Molecular Sciences, 24(22), 16107. https://doi.org/10.3390/ijms242216107








