Blinatumomab Redirects Donor Lymphocytes against CD19+ Acute Lymphoblastic Leukemia without Relevant Bystander Alloreactivity after Haploidentical Hematopoietic Stem Cell Transplantation
Abstract
:1. Introduction
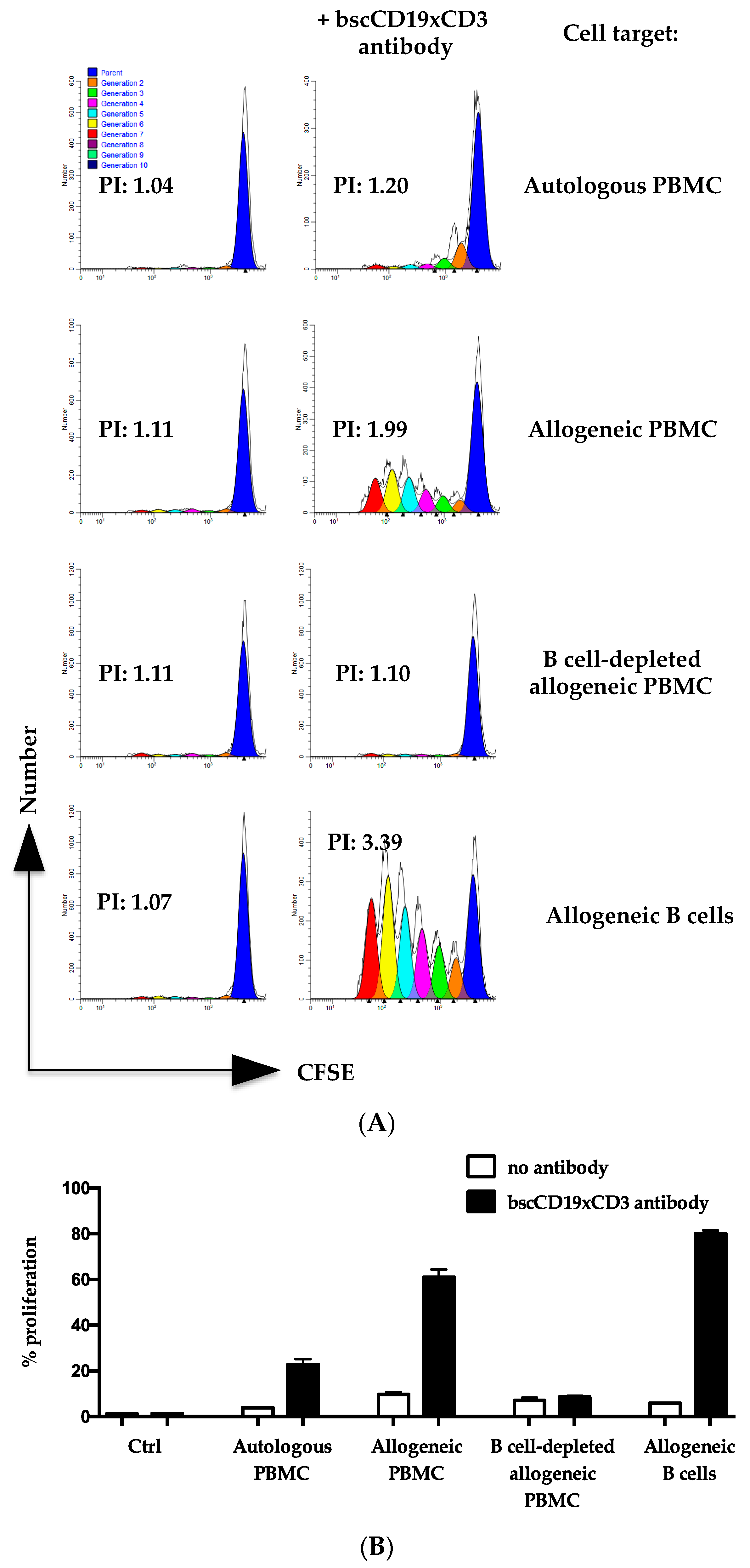
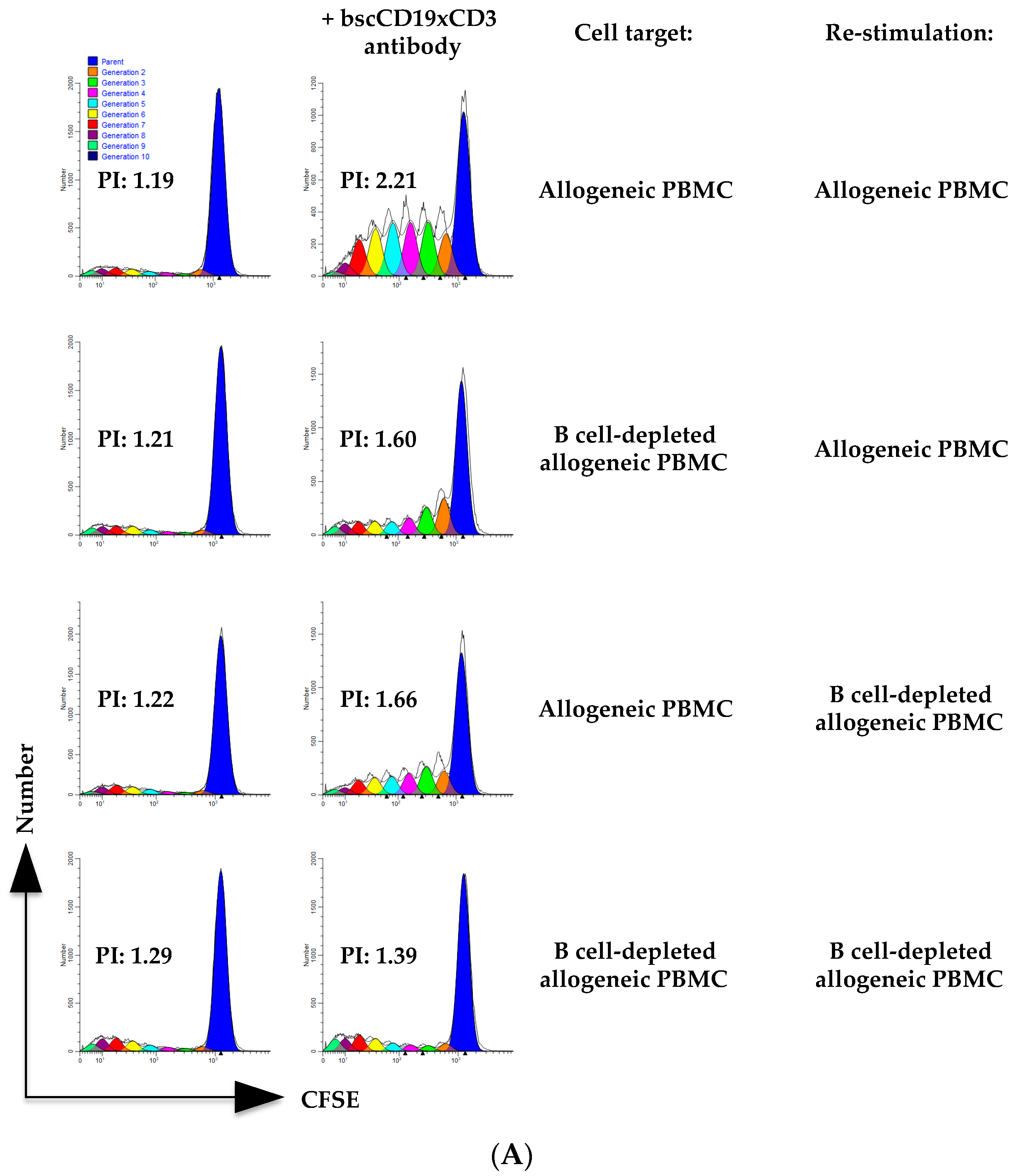
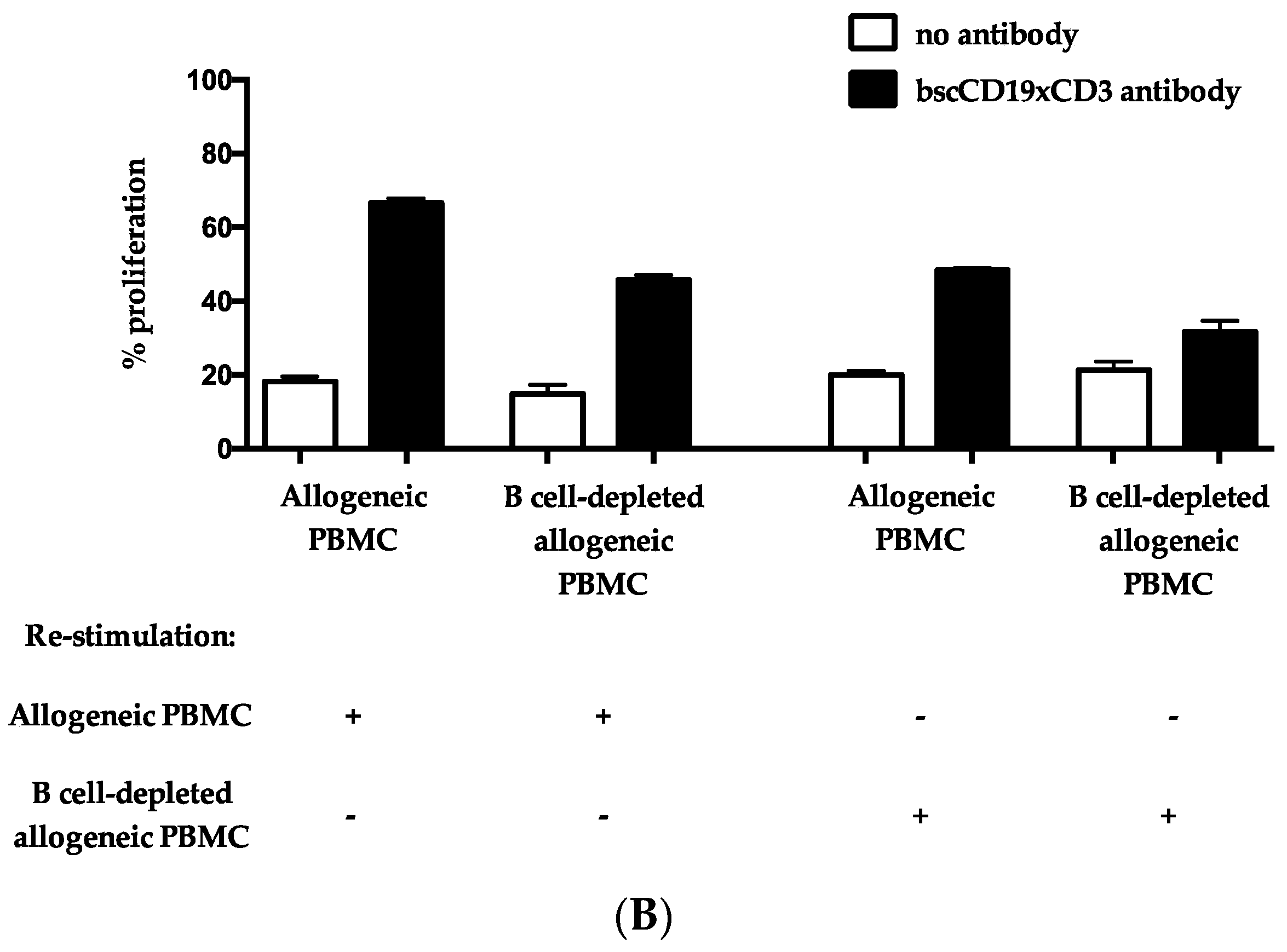
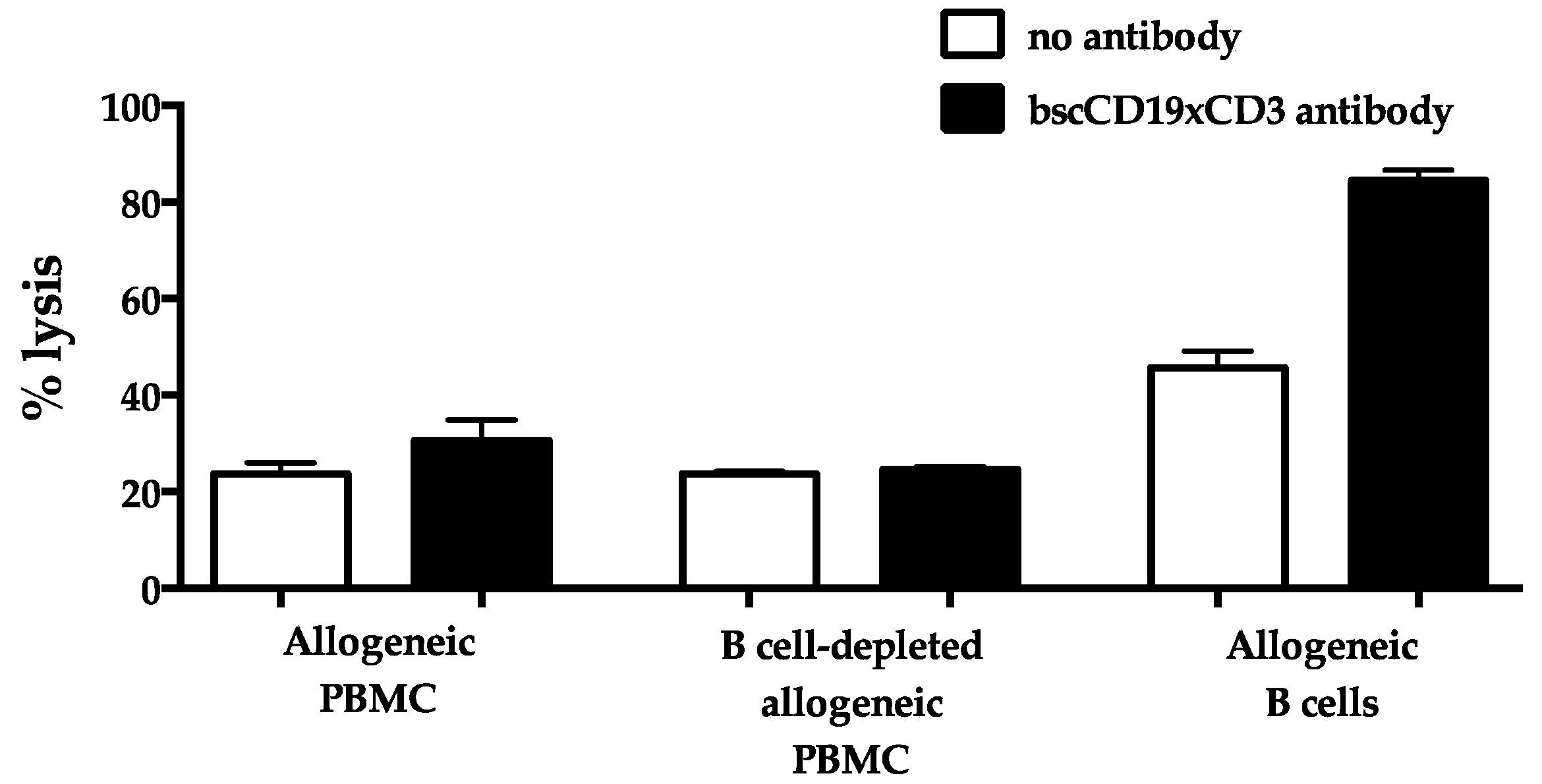
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. CFSE Dilution Assay
4.3. 51Cr Release Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appelbaum, F.R.; Forman, S.J.; Negrin, R.S.; Blume, K.G. (Eds.) Thomas’ Hematopoietic Cell Transplantation, 4th ed.; Wiley-Blackwell Publishing: Oxford, UK, 2009. [Google Scholar]
- Löffler, A.; Gruen, M.; Wuchter, C.; Schriever, F.; Kufer, P.; Dreier, T.; Hanakam, F.; Baeuerle, P.A.; Bommert, K.; Karawajew, L.; et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia 2003, 17, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; Noppeney, R.; Viardot, A.; Hess, G.; Schuler, M.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- von Stackelberg, A.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; Trippett, T.M.; Rizzari, C.; Bader, P.; O’Brien, M.M.; Brethon, B.; Bhojwani, D.; et al. Phase i/phase ii study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J. Clin. Oncol. 2016, 34, 4381–4389. [Google Scholar] [CrossRef]
- Gökbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Brüggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef]
- Jen, E.Y.; Xu, Q.; Schetter, A.; Przepiorka, D.; Shen, Y.L.; Roscoe, D.; Sridhara, R.; Deisseroth, A.; Philip, R.; Farrell, A.T.; et al. FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin. Cancer Res. 2019, 25, 473–477. [Google Scholar] [CrossRef]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of Postreinduction Therapy Consolidation with Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults with First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Stein, A.S.; Kantarjian, H.; Gökbuget, N.; Bargou, R.; Litzow, M.R.; Rambaldi, A.; Ribera, J.M.; Zhang, A.; Zimmerman, Z.; Zugmaier, G.; et al. Blinatumomab for Acute Lymphoblastic Leukemia Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1498–1504. [Google Scholar] [CrossRef]
- Schlegel, P.; Lang, P.; Zugmaier, G.; Ebinger, M.; Kreyenberg, H.; Witte, K.E.; Feucht, J.; Pfeiffer, M.; Teltschik, H.M.; Kyzirakos, C.; et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica 2014, 99, 1212–1219. [Google Scholar] [CrossRef]
- Handgretinger, R.; Zugmaier, G.; Henze, G.; Kreyenberg, H.; Lang, P.; von Stackelberg, A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia 2011, 25, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Gul, Z. Blinatumomab may induce graft versus host leukemia in patients with pre-B ALL relapsing after hematopoietic stem cell transplant. Clin. Case Rep. 2016, 4, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; de Lima, M.; Caimi, P.; Tomlinson, B.; Little, J.; Creger, R.; Lazarus, H.; Cooper, B. Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transpl. 2016, 51, 1253–1255. [Google Scholar] [CrossRef]
- Durer, S.; Durer, C.; Shafqat, M.; Comba, I.Y.; Malik, S.; Faridi, W.; Aslam, S.; Ijaz, A.; Tariq, M.J.; Fraz, M.A.; et al. Concomitant use of blinatumomab and donor lymphocyte infusion for mixed-phenotype acute leukemia: A case report with literature review. Immunotherapy 2019, 11, 373–378. [Google Scholar] [CrossRef]
- Chauvet, P.; Paviglianiti, A.; Labopin, M.; Labussière, H.; Boissel, N.; Robin, M.; Maillard, N.; Ouachée-Chardin, M.; Forcade, E.; Poiré, X.; et al. Combining blinatumomab and donor lymphocyte infusion in B-ALL patients relapsing after allogeneic hematopoietic cell transplantation: A study of the SFGM-TC. Bone Marrow Transpl. 2023, 58, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Kumar, A.; Stanton, N.A.; Katsanis, E. Concurrent application of blinatumomab and haploidentical donor leukocyte infusions for refractory primary mediastinal large B-cell lymphoma. Ther. Adv. Hematol. 2021, 12, 2040620721994348. [Google Scholar] [CrossRef] [PubMed]
- Di Ianni, M.; Falzetti, F.; Carotti, A.; Terenzi, A.; Castellino, F.; Bonifacio, E.; Del Papa, B.; Zei, T.; Ostini, R.I.; Cecchini, D.; et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011, 117, 3921–3928. [Google Scholar] [CrossRef]
- Martelli, M.F.; Di Ianni, M.; Ruggeri, L.; Falzetti, F.; Carotti, A.; Terenzi, A.; Pierini, A.; Massei, M.S.; Amico, L.; Urbani, E.; et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014, 124, 638–644. [Google Scholar] [CrossRef]
- Pierini, A.; Ruggeri, L.; Carotti, A.; Falzetti, F.; Saldi, S.; Terenzi, A.; Zucchetti, C.; Ingrosso, G.; Zei, T.; Iacucci Ostini, R.; et al. Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv. 2021, 5, 1199–1208. [Google Scholar] [CrossRef]
- Meyer, E.H.; Hsu, A.R.; Liliental, J.; Löhr, A.; Florek, M.; Zehnder, J.L.; Strober, S.; Lavori, P.; Miklos, D.B.; Johnson, D.S.; et al. A distinct evolution of the T-cell repertoire categorizes treatment refractory gastrointestinal acute graft-versus-host disease. Blood 2013, 121, 4955–4962. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Zeiser, R.; Dasilva, D.L.; Chang, D.S.; Beilhack, A.; Contag, C.H.; Negrin, R.S. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood 2007, 109, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Pierini, A.; Colonna, L.; Alvarez, M.; Schneidawind, D.; Nishikii, H.; Baker, J.; Baker, J.; Pan, Y.; Florek, M.; Kim, B.S. Donor requirements for regulatory T cell suppression of murine graft-versus-host disease. J. Immunol. 2015, 195, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Zakrzewski, J.; James, S.; Sadelain, M. Posttransplant chimeric antigen receptor therapy. Blood 2018, 131, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Del Bufalo, F.; Becilli, M.; Rosignoli, C.; De Angelis, B.; Algeri, M.; Hanssens, L.; Gunetti, M.; Iacovelli, S.; Li Pira, G.; Girolami, E.; et al. Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL. Blood 2023, 142, 146–157. [Google Scholar] [CrossRef]
- Gaballa, M.R.; Banerjee, P.; Milton, D.R.; Jiang, X.; Ganesh, C.; Khazal, S.; Nandivada, V.; Islam, S.; Kaplan, M.; Daher, M.; et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood 2022, 139, 1908–1919. [Google Scholar] [CrossRef]
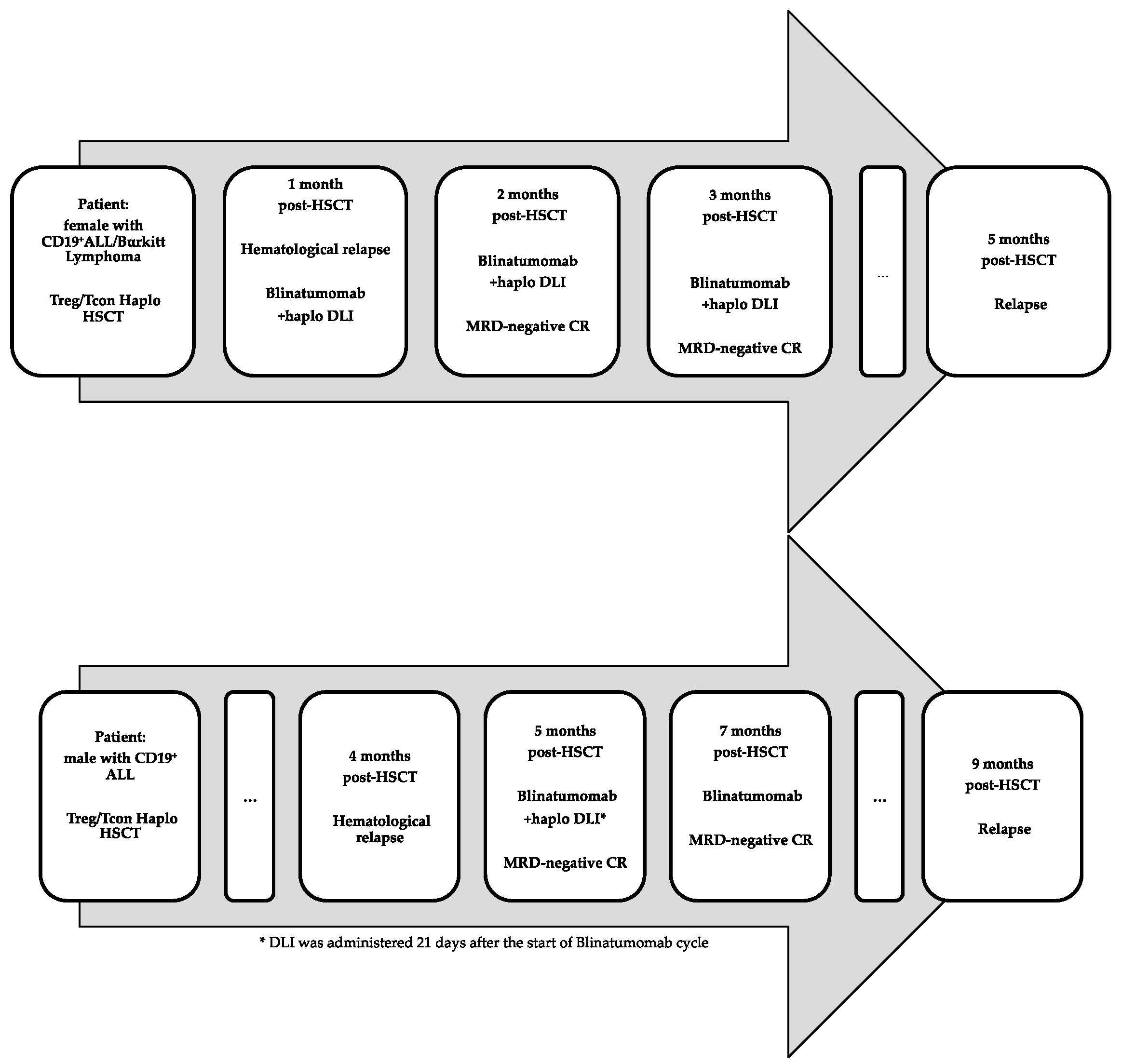
| Patient | Diagnosis | Time of Post-HSCT Relapse | Blinatumomab | DLIs (Dose) | CRS | Neurotoxicity | Acute or Chronic GvHD | Response |
|---|---|---|---|---|---|---|---|---|
| Female, 50 years | CD19+ ALL/Burkitt lymphoma | 1 month | 3 cycles | 3 (0.5–1 × 106/Kg) | No | No | No | MRD- CR |
| Male, 42 years | CD19+ ALL | 4 months | 2 cycles | 1 (0.5 × 106/Kg) | No | Yes § | No | MRD- CR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancusi, A.; Zorutti, F.; Ruggeri, L.; Bonato, S.; Tricarico, S.; Zei, T.; Iacucci Ostini, R.; Viglione, V.; Sembenico, R.; Sciabolacci, S.; et al. Blinatumomab Redirects Donor Lymphocytes against CD19+ Acute Lymphoblastic Leukemia without Relevant Bystander Alloreactivity after Haploidentical Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2023, 24, 16105. https://doi.org/10.3390/ijms242216105
Mancusi A, Zorutti F, Ruggeri L, Bonato S, Tricarico S, Zei T, Iacucci Ostini R, Viglione V, Sembenico R, Sciabolacci S, et al. Blinatumomab Redirects Donor Lymphocytes against CD19+ Acute Lymphoblastic Leukemia without Relevant Bystander Alloreactivity after Haploidentical Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences. 2023; 24(22):16105. https://doi.org/10.3390/ijms242216105
Chicago/Turabian StyleMancusi, Antonella, Francesco Zorutti, Loredana Ruggeri, Samanta Bonato, Sara Tricarico, Tiziana Zei, Roberta Iacucci Ostini, Valerio Viglione, Rebecca Sembenico, Sofia Sciabolacci, and et al. 2023. "Blinatumomab Redirects Donor Lymphocytes against CD19+ Acute Lymphoblastic Leukemia without Relevant Bystander Alloreactivity after Haploidentical Hematopoietic Stem Cell Transplantation" International Journal of Molecular Sciences 24, no. 22: 16105. https://doi.org/10.3390/ijms242216105
APA StyleMancusi, A., Zorutti, F., Ruggeri, L., Bonato, S., Tricarico, S., Zei, T., Iacucci Ostini, R., Viglione, V., Sembenico, R., Sciabolacci, S., Cardinali, V., Martelli, M. F., Mecucci, C., Carotti, A., Martelli, M. P., Velardi, A., & Pierini, A. (2023). Blinatumomab Redirects Donor Lymphocytes against CD19+ Acute Lymphoblastic Leukemia without Relevant Bystander Alloreactivity after Haploidentical Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences, 24(22), 16105. https://doi.org/10.3390/ijms242216105






