B Cells Induce Early-Onset Maternal Inflammation to Protect against LPS-Induced Fetal Rejection
Abstract
:1. Introduction
2. Results
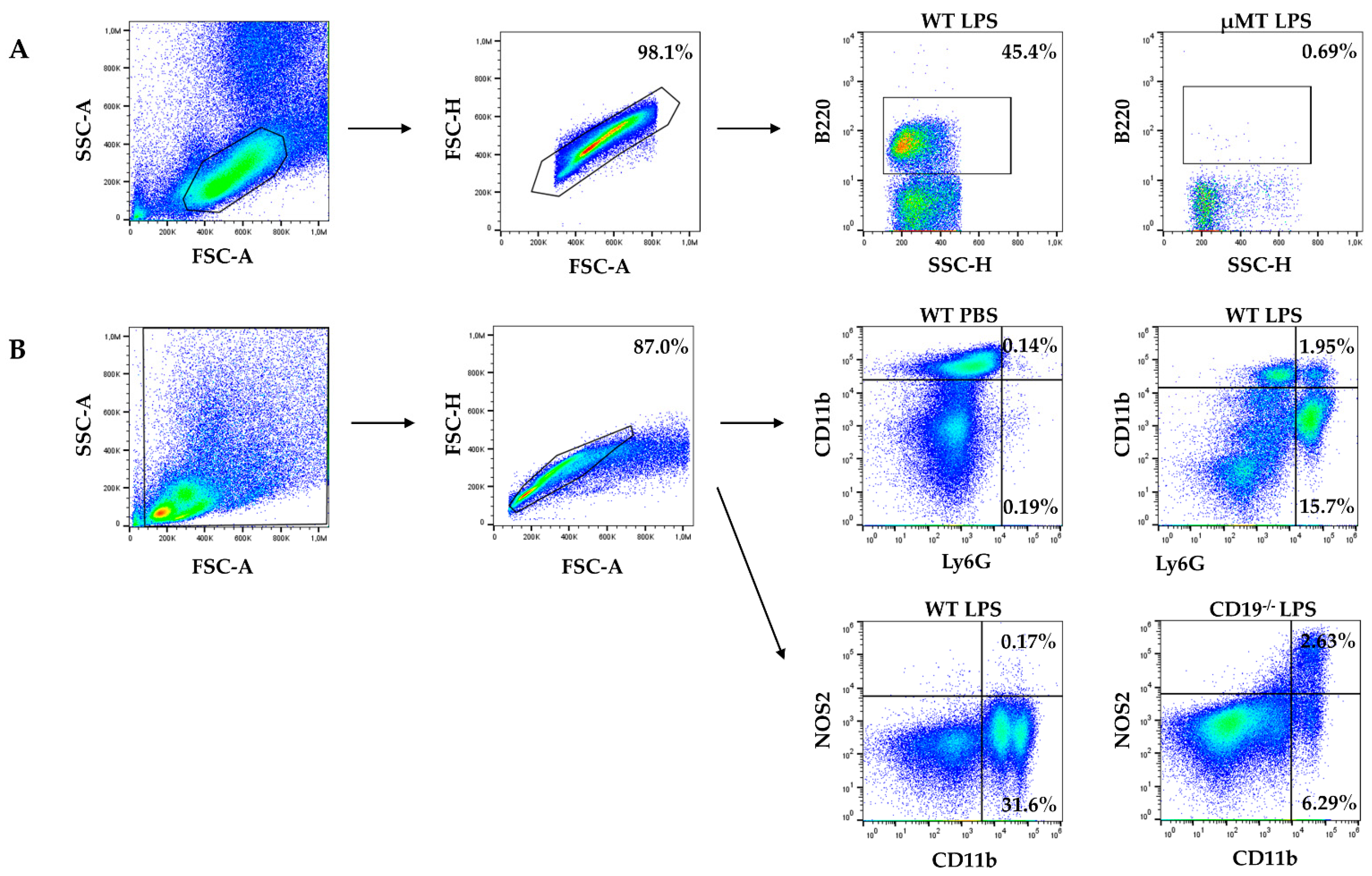
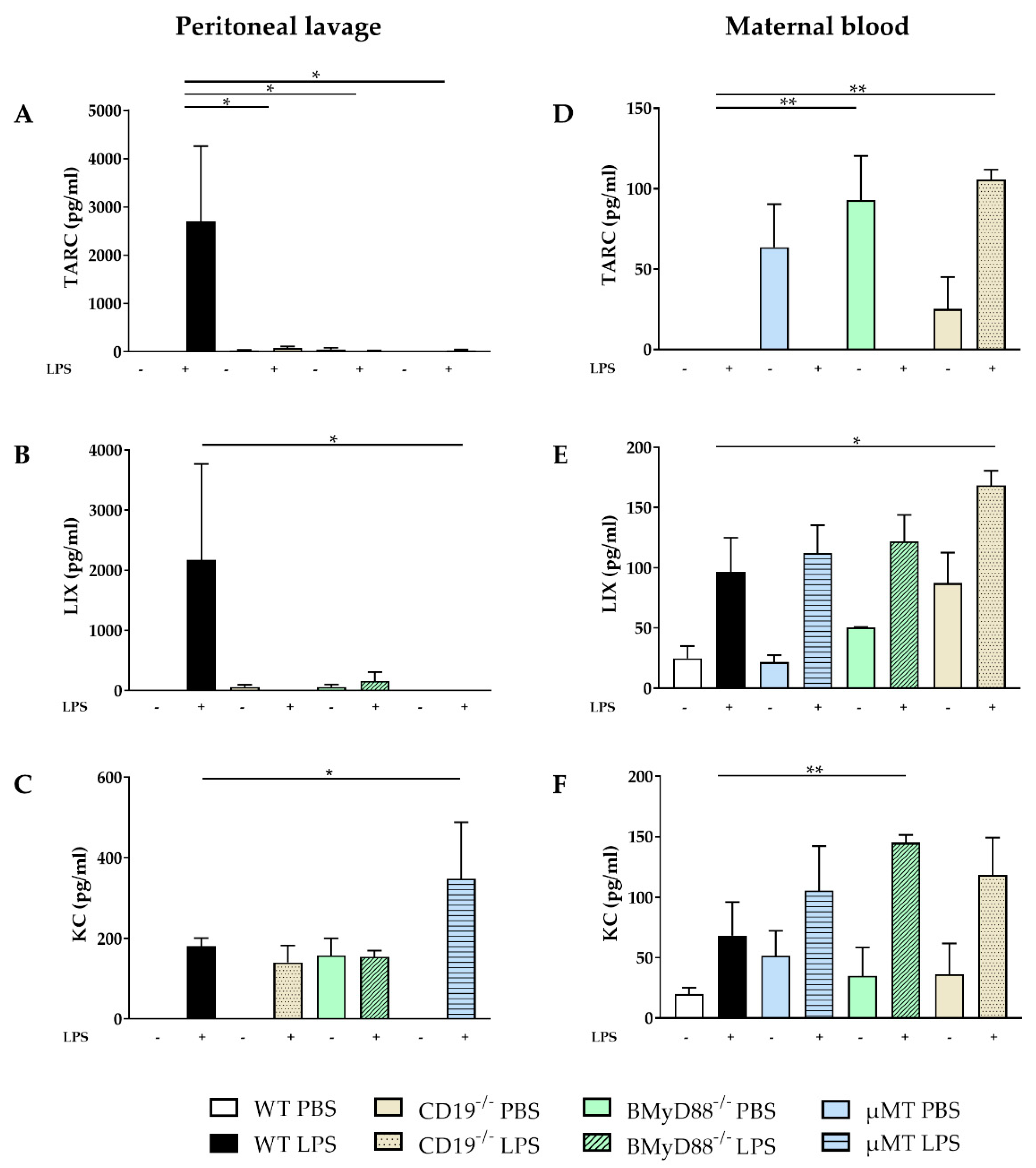
2.1. WT Dams Induce a Robust Immune Response Detectable in Peritoneal Lavage (PL) and Maternal Serum 4 h after LPS Challenge
2.2. The LPS-Induced Inflammatory Immune Response Is Attenuated in WT Dams in Placenta, Amniotic Fluid and Fetal Serum Compared to Dams Lacking B Cells or B-Cell-Specific CD19 or MyD88 Expression
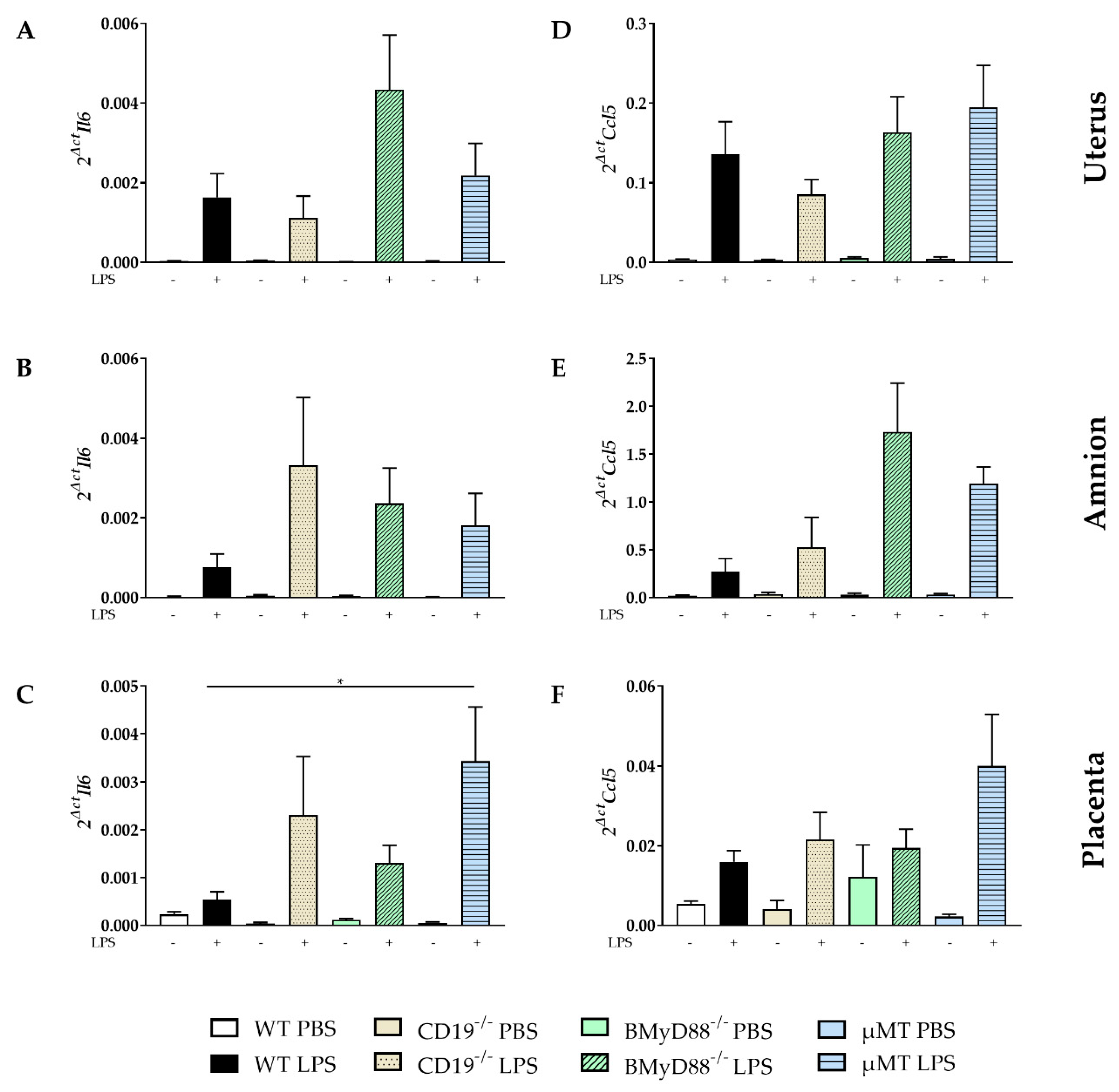
2.3. LPS Administration Altered the Expression of Inflammatory Mediators in Gestational and Fetal Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Mouse Model
4.2. The Application of LPSs and Sample Collection
4.3. Cell Staining and Flow Cytometry
4.4. Cytokine Detection in Sera and Supernatants
4.5. Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.6. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015, 20, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Su, I.; Miyake, K.; Nagai, Y.; Akashi, S.; Mecklenbrauker, I.; Rajewsky, K.; Kimoto, M.; Tarakhovsky, A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 2000, 192, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, N.; Fujimoto, M.; Sato, S.; Miyake, K.; Asano, N.; Nagai, Y.; Takeuchi, O.; Takeda, K.; Okochi, H.; Akira, S.; et al. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood 2003, 102, 1374–1380. [Google Scholar] [CrossRef]
- Lu, M.; Munford, R. LPS stimulates IgM production in vivo without help from non-B cells. Innate Immun. 2016, 22, 307–315. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef]
- Ozcan, E.; Garibyan, L.; Lee, J.J.; Bram, R.J.; Lam, K.P.; Geha, R.S. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J. Allergy Clin. Immunol. 2009, 123, 1277–1286.e5. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.; Campe, K.J.; Redlich, A.; Oettel, A.; Hartig, R.; Costa, S.D.; Zenclussen, A.C. Regulatory B Cells Are Decreased and Impaired in Their Function in Peripheral Maternal Blood in Pre-term Birth. Front. Immunol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.; Redlich, A.; Hartig, R.; Costa, S.D.; Rathert, H.; Fest, S.; Zenclussen, A.C. Imbalance between inflammatory and regulatory cord blood B cells following pre-term birth. J. Reprod. Immunol. 2021, 145, 103319. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.; Scharm, M.; Oettel, A.; Redlich, A.; Costa, S.D.; Zenclussen, A.C. Enhanced S100B expression in T and B lymphocytes in spontaneous preterm birth and preeclampsia. J. Perinat. Med. 2022, 50, 157–166. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.J.; Nowak, D.; Schumacher, A.; Plenagl, S.; Langwisch, S.; Tiegs, G.; Reinhold, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef]
- Busse, M.; Plenagl, S.; Campe, N.K.J.; Muller, A.J.; Tedford, K.; Schumacher, A.; Zenclussen, A.C. Maternal B Cell-Intrinsic MyD88 Signaling Mediates LPS-Driven Intrauterine Fetal Death. Cells 2021, 10, 2693. [Google Scholar] [CrossRef]
- Busse, M.; Zenclussen, A.C. IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells. Cells 2022, 11, 2690. [Google Scholar] [CrossRef]
- Busse, M.; Langwisch, S.; Tedford, K.; Fischer, K.D.; Zenclussen, A.C. Maternal B cell signaling orchestrates fetal development in mice. Development 2021, 149, dev199783. [Google Scholar] [CrossRef]
- Huang, B.; Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Goyert, J.W.; Zhou, J.Z.; El-Hage, N.G.; Deng, J.; Lin, J.; Yao, F.; et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017, 23, 128–135. [Google Scholar] [CrossRef]
- Valeff, N.; Juriol, L.; Quadrana, F.; Muzzio, D.O.; Zygmunt, M.; Quiroga, M.F.; Ventimiglia, M.S.; Jensen, F. Expression of IL-33 Receptor Is Significantly Up-Regulated in B Cells During Pregnancy and in the Acute Phase of Preterm Birth in Mice. Front. Immunol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Campe, K.J.; Redlich, A.; Zenclussen, A.C.; Busse, M. An increased proportion of progesterone receptor A in peripheral B cells from women who ultimately underwent spontaneous preterm birth. J. Reprod. Immunol. 2022, 154, 103756. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Romero, R.; Xu, Y.; Galaz, J.; Slutsky, R.; Arenas-Hernandez, M.; Garcia-Flores, V.; Motomura, K.; Hassan, S.S.; Reboldi, A.; et al. Are B cells altered in the decidua of women with preterm or term labor? Am. J. Reprod. Immunol. 2019, 81, e13102. [Google Scholar] [CrossRef] [PubMed]
- Galaz, J.; Romero, R.; Slutsky, R.; Xu, Y.; Motomura, K.; Para, R.; Pacora, P.; Panaitescu, B.; Hsu, C.D.; Kacerovsky, M.; et al. Cellular immune responses in amniotic fluid of women with preterm prelabor rupture of membranes. J. Perinat. Med. 2020, 48, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Joiner, D.N.; Gentile, L.F.; Cuenca, A.L.; Wynn, J.L.; Kelly-Scumpia, K.M.; Scumpia, P.O.; Behrns, K.E.; Efron, P.A.; Nacionales, D.; et al. TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J. Immunol. 2015, 194, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ono, N.; Steeber, D.A.; Pisetsky, D.S.; Tedder, T.F. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol. 1996, 157, 4371–4378. [Google Scholar] [CrossRef]
- Karmakar, U.; Vermeren, S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology 2021, 164, 689–700. [Google Scholar] [CrossRef]
- del Barrio, L.; Sahoo, M.; Lantier, L.; Reynolds, J.M.; Ceballos-Olvera, I.; Re, F. Production of anti-LPS IgM by B1a B cells depends on IL-1beta and is protective against lung infection with Francisella tularensis LVS. PLoS Pathog. 2015, 11, e1004706. [Google Scholar] [CrossRef]
- Huard, B.; McKee, T.; Bosshard, C.; Durual, S.; Matthes, T.; Myit, S.; Donze, O.; Frossard, C.; Chizzolini, C.; Favre, C.; et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Investig. 2008, 118, 2887–2895. [Google Scholar] [CrossRef]
- Scapini, P.; Bazzoni, F.; Cassatella, M.A. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol. Lett. 2008, 116, 1–6. [Google Scholar] [CrossRef]
- Scapini, P.; Nardelli, B.; Nadali, G.; Calzetti, F.; Pizzolo, G.; Montecucco, C.; Cassatella, M.A. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 2003, 197, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Motomura, K.; Romero, R.; Galaz, J.; Tao, L.; Garcia-Flores, V.; Xu, Y.; Done, B.; Arenas-Hernandez, M.; Miller, D.; Gutierrez-Contreras, P.; et al. Fetal and maternal NLRP3 signaling is required for preterm labor and birth. JCI Insight 2022, 7, 3805. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.; Taggart, M.J.; Zielnik, B.; Wong, S.; Mitchell, B.F. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J. Physiol. 2008, 586, 6063–6076. [Google Scholar] [CrossRef] [PubMed]
- Duckitt, K.; Thornton, S.; O'Donovan, O.P.; Dowswell, T. Nitric oxide donors for treating preterm labour. Cochrane Database Syst. Rev. 2014, 2014, CD002860. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhang, R.; Geng, S.; Peng, L.; Jayaraman, P.; Chen, C.; Xu, F.; Yang, J.; Li, Q.; Zheng, H.; et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015, 6, 6676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Q.; Chuang, P.Y.; Lu, G.; Liu, R.; Yang, J.; Peng, L.; Dai, Y.; Zheng, Z.; Qi, C.F.; et al. Regulation of pathogenic Th17 cell differentiation by IL-10 in the development of glomerulonephritis. Am. J. Pathol. 2013, 183, 402–412. [Google Scholar] [CrossRef]
- Tsuda, H.; Michimata, T.; Hayakawa, S.; Tanebe, K.; Sakai, M.; Fujimura, M.; Matsushima, K.; Saito, S. A Th2 chemokine, TARC, produced by trophoblasts and endometrial gland cells, regulates the infiltration of CCR4+ T lymphocytes into human decidua at early pregnancy. Am. J. Reprod. Immunol. 2002, 48, 1–8. [Google Scholar] [CrossRef]
- Laudanski, P.; Lemancewicz, A.; Kuc, P.; Charkiewicz, K.; Ramotowska, B.; Kretowska, M.; Jasinska, E.; Raba, G.; Karwasik-Kajszczarek, K.; Kraczkowski, J.; et al. Chemokines profiling of patients with preterm birth. Mediat. Inflamm. 2014, 2014, 185758. [Google Scholar] [CrossRef]
- Yu, N.; Weng, Y.; Liu, W.; Chen, L.; Iqbal, F.; Yin, Z.; He, Y.; Wang, Y. TLRs induce Th1/Th2 responses by affecting the secretion of CCL2 at the maternal-foetal interface. Int. Immunopharmacol. 2021, 100, 108070. [Google Scholar] [CrossRef]
- Margry, B.; Kersemakers, S.C.; Hoek, A.; Arkesteijn, G.J.; Wieland, W.H.; van Eden, W.; Broere, F. Activated peritoneal cavity B-1a cells possess regulatory B cell properties. PLoS ONE 2014, 9, e88869. [Google Scholar] [CrossRef] [PubMed]
- Barbeiro, D.F.; Barbeiro, H.V.; Faintuch, J.; Ariga, S.K.; Mariano, M.; Popi, A.F.; de Souza, H.P.; Velasco, I.T.; Soriano, F.G. B-1 cells temper endotoxemic inflammatory responses. Immunobiology 2011, 216, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Paananen, R.; Vuolteenaho, R.; Metsola, J.; Ojaniemi, M.; Autio-Harmainen, H.; Hallman, M. Maternal endotoxin-induced preterm birth in mice: Fetal responses in toll-like receptors, collectins, and cytokines. Pediatr. Res. 2008, 63, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hudalla, H.; Karenberg, K.; Kuon, R.J.; Poschl, J.; Tschada, R.; Frommhold, D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr. Res. 2018, 84, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Bommer, I.; Juriol, L.; Muzzio, D.; Valeff, N.; Ehrhardt, J.; Matzner, F.; Ziegler, K.; Malinowsky, K.; Ventimiglia, M.S.; Zygmunt, M.; et al. Characterization of murine amniotic fluid B cells in normal pregnancy and in preterm birth. Reproduction 2019, 158, 369–376. [Google Scholar] [CrossRef]
- Harju, K.; Ojaniemi, M.; Rounioja, S.; Glumoff, V.; Paananen, R.; Vuolteenaho, R.; Hallman, M. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr. Res. 2005, 57, 644–648. [Google Scholar] [CrossRef]
- Tulina, N.M.; Brown, A.G.; Barila, G.O.; Elovitz, M.A. The Absence of TLR4 Prevents Fetal Brain Injury in the Setting of Intrauterine Inflammation. Reprod. Sci. 2019, 26, 1082–1093. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehre, G.M.; Tchaikovski, S.; Ignatov, A.; Zenclussen, A.C.; Busse, M. B Cells Induce Early-Onset Maternal Inflammation to Protect against LPS-Induced Fetal Rejection. Int. J. Mol. Sci. 2023, 24, 16091. https://doi.org/10.3390/ijms242216091
Uehre GM, Tchaikovski S, Ignatov A, Zenclussen AC, Busse M. B Cells Induce Early-Onset Maternal Inflammation to Protect against LPS-Induced Fetal Rejection. International Journal of Molecular Sciences. 2023; 24(22):16091. https://doi.org/10.3390/ijms242216091
Chicago/Turabian StyleUehre, Gina Marie, Svetlana Tchaikovski, Atanas Ignatov, Ana Claudia Zenclussen, and Mandy Busse. 2023. "B Cells Induce Early-Onset Maternal Inflammation to Protect against LPS-Induced Fetal Rejection" International Journal of Molecular Sciences 24, no. 22: 16091. https://doi.org/10.3390/ijms242216091
APA StyleUehre, G. M., Tchaikovski, S., Ignatov, A., Zenclussen, A. C., & Busse, M. (2023). B Cells Induce Early-Onset Maternal Inflammation to Protect against LPS-Induced Fetal Rejection. International Journal of Molecular Sciences, 24(22), 16091. https://doi.org/10.3390/ijms242216091





