Abstract
Natural killer (NK) cell immunotherapy has emerged as a novel treatment modality for various cancer types, including leukemia. The modulation of inhibitory signaling pathways in T cells and NK cells has been the subject of extensive investigation in both preclinical and clinical settings in recent years. Nonetheless, further research is imperative to optimize antileukemic activities, especially regarding NK-cell-based immunotherapies. The central scientific question of this study pertains to the potential for boosting cytotoxicity in expanded and activated NK cells through the inhibition of inhibitory receptors. To address this question, we employed the CRISPR-Cas9 system to target three distinct inhibitory signaling pathways in NK cells. Specifically, we examined the roles of A2AR within the metabolic purinergic signaling pathway, CBLB as an intracellular regulator in NK cells, and the surface receptors NKG2A and CD96 in enhancing the antileukemic efficacy of NK cells. Following the successful expansion of NK cells, they were transfected with Cas9+sgRNA RNP to knockout A2AR, CBLB, NKG2A, and CD96. The analysis of indel frequencies for all four targets revealed good knockout efficiencies in expanded NK cells, resulting in diminished protein expression as confirmed by flow cytometry and Western blot analysis. Our in vitro killing assays demonstrated that NKG2A and CBLB knockout led to only a marginal improvement in the cytotoxicity of NK cells against AML and B-ALL cells. Furthermore, the antileukemic activity of CD96 knockout NK cells did not yield significant enhancements, and the blockade of A2AR did not result in significant improvement in killing efficiency. In conclusion, our findings suggest that CRISPR-Cas9-based knockout strategies for immune checkpoints might not be sufficient to efficiently boost the antileukemic functions of expanded (and activated) NK cells and, at the same time, point to the need for strong cellular activating signals, as this can be achieved, for example, via transgenic chimeric antigen receptor expression.
1. Introduction
NK cell therapies are considered an outstanding strategy in cancer immunotherapy, providing promising options to overcome the limitations of T-cell-based immunotherapy [1]. As innate immune system mediators, NK cells offer an “off-the-shelf” strategy for cancer treatment with high safety and no risk of graft-versus-host disease (GvHD) [2]. This is attributed to the unique ability of NK cells to target and eliminate cancer cells irrespective of MHC molecule presentation or the need for prior sensitization due to the presence of germline-encoded receptors [2]. NK cells mediate the killing process either through the direct release of lytic granules such as perforin and granzymes or by inducing apoptosis [3]. Moreover, NK cells secrete chemokines and cytokines such as interferon-gamma (IFN-γ), IL-10, CCL3, CCL4, and CCL5 to activate other immune cells [1,4]. A variety of inhibitory and activating surface receptors are present in NK cells. Following their binding to respective ligands, these receptors facilitate the transduction of activating or inhibitory signals, governing the regulatory mechanisms of NK cell functionality. The level of NK cell activity is determined by the balance or predomination of transduced activating or inhibitory signals [1,5]. In the clinic, using primary or expanded NK cells has advantages over NK cell lines. For instance, the poor expansion of NK-92 has been shown in vivo, which leads to transient effects and less efficiency in NK cell therapy [6].
Despite the numerous advantages of NK cells in cancer immunotherapy, there are certain limitations in their clinical application, including harvesting an adequate cell number, strategies employed by tumors to evade NK cell recognition, and difficulties associated with their infiltration into solid tumors [7,8]. It has been shown that the number of NK cells in cancer tissues is lower than in normal tissue, and they can be functionally suppressed in the tumor microenvironment [9]. Additionally, cancer cells can escape from NK cells by expressing inhibitory molecules and the immunosuppressive signals of NK cells [10]. An effective strategy to mitigate immunosuppression in cancer is the inhibition of pathways that negatively impact NK cell function. This approach has been extensively tested in preclinical and clinical trials in recent years, demonstrating encouraging results [11].
A2A adenosine receptor (A2AR), also known as ADORA2A, is a G-protein-coupled receptor (GPCR) with a high affinity for adenosine, expressed in several immune cells, including macrophages, NK cells, and T cells, and mediates the generation of adenosine from ATP. It has been shown that A2AR-mediated adenosine signaling blocks the antitumor activity of T and NK cells [12]. E3 ubiquitin ligase Cbl-b (Casitas B cell lymphoma-b) is known to be a negative regulator of immune activation. This protein plays a critical role in inhibitory signaling for the regulation of protein tyrosine kinases (PTKs), T and B-cell immune tolerance, and T-cell activation. Nonetheless, little is known about the exact role of CBLB in NK cell function [13,14,15,16]. Its regulatory function seems to be associated with the TAM receptor family, blocking NK cell activation via the ubiquitylation of LAT1 [17,18].
CD96 (TACTILE) is a member of the immunoglobulin superfamily and interacts with nectin and nectin-like proteins [19]. A high level of CD96 has been reported in myelodysplastic syndrome, acute myeloid leukemia (AML), and T-cell acute lymphoblastic leukemia (T-ALL) [20,21]. It is also defined as a cell surface marker for AML stem cells [19]. CD94/NK group 2 member A (NKG2A) is a tyrosine-based inhibitory motif (ITIM) and is expressed at the cell surface of both T and NK cells as a heterodimer with CD94 in humans [22]. NKG2A is the most prominent NK inhibitory receptor, bindinghuman leukocyte antigen (HLA)-E and blocks T and NK cell cytotoxicity [22,23]. In a previously published study, we showed that HLA-E is highly expressed in acute myeloid leukemia (AML) and B-cell acute lymphocytic leukemia (B-ALL) cell lines [24]. AML and ALL are two subsets of acute leukemia with high frequency, subsequently, in adults and children [25,26].
Taking these factors into account, we developed a CRISPR-based gene knockout method to target A2AR, CBLB, CD96, and KLRC1 (NKG2A) genes to evaluate their antitherapeutic role in AML and B-ALL cancer.
2. Results
2.1. Primary NK Cell Isolation, Expansion, and Receptor Profile Characterization
NK cells constitute 5–20% of all circulating lymphocytes in humans and can be identified by the presence of CD56 and the absence of CD3 on their surfaces [27]. Despite this small fraction, NK cells need to be infused in large numbers in patients. To acquire this huge number, an optimal protocol has to be applied for the expansion of NK cells ex vivo [28]. In this study, immunomagnetic negative selection was used to target non-NK cells and isolate NK cells. Using this method, more than 95% NK cell purity was achieved after isolation (Figure 1A). For the expansion of NK cells, isolated cells were incubated with an irradiated K562mb15-41BBL cell line as feeding cells. K562mb15-41BBL is a myeloid leukemia cell line that is genetically modified to express membrane-bound interleukin-15 and 41BB ligands in order to specifically activate NK cells but not T lymphocytes. Flow cytometry analysis showed more than 90% purity for NK cells 14 days after incubation. The proliferating rate of NK cells was monitored within 14 days via counting, and the expansion rate was calculated over 100 times on day 14 (Figure 1B).
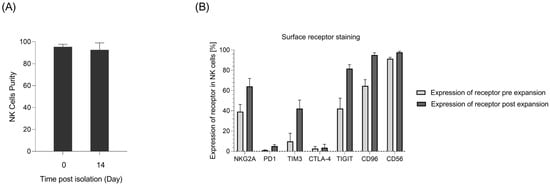
Figure 1.
Primary NK cell isolation, expansion, and receptor profile characterization. (A) NK cell purity on the day of isolation and 14 days post-expansion from merged donors measured as CD56+ cells in flow cytometry analyses (n = 6). (B) Flow cytometry analysis of receptor expression in NK cells pre- and post-in vitro expansion (n = 8).
To assess the expression level of different receptors in NK cells, receptor profile characterization was performed using flow cytometry. The data showed an increase in the expression of the investigated inhibitory receptors, namely, NKG2A, TIM3, TIGIT, and CD96, following the expansion of NK cells. In the meantime, PD-1 expression was maintained by NK cells at a low level (Figure 1).
2.2. Targeting Inhibitory Signals Involved in NK Cell Function Using CRISPR-Cas9 System
The MaxCyte GTx® instrument (MaxCyte Inc, Rockville, MD, USA) is GMP-compliant and clinically validated, enabling effortless translation into the clinic. Here, we used the MaxCyte GTx® to deliver CRISPR-Cas to difficult-to-transfect expanded NK cells. Initially, to optimize the electroporation, DsRed mRNA was transfected into expanded NK cells and the NK-92 cell line (control) using different programs. The data showed the efficient expression of DsRed using programs 4 and 5 in both expanded NK cells (up to 96%) and NK-92 cells (up to 99%) (Supplementary Figure S1).
To check the effect of CRISPR-Cas9-based gene knockout on the inhibitory signaling pathways of expanded NK cells, different sgRNAs were applied to target A2AR, CBLB, NKG2A, and CD96 genes in expanded NK cells. The selected sgRNAs were either previously published [14,29,30] or newly designed by our group using CHOPCHOP, considering high knockout efficiency and low off-target ranking. Figure 2A represents the target region of each sgRNA in the related gene sequence. Then, 14–15 days after expansion, cells were electroporated with Cas9-sgRNA RNP, and 3 days later, Sanger sequencing was performed. The results of Sanger sequencing showed up to 61% indel frequency for NKG2A, 83% for CD96, 69% for CBLB, and for 79% A2AR. The mean value of the indels for merged donors is shown in Figure 2B. Five days after electroporation, cells were harvested to check for knockout efficiency at the protein level using flow cytometry or Western blot (Figure 2C–E). Given the nonspecific binding of the antibody to Fc receptors, staining for A2AR in NK cells was not successful, although using the same sgRNA and antibody in NK-92 cells showed a reduction in the A2AR protein after knockout with 80% indel frequency (Supplementary Figure S2).
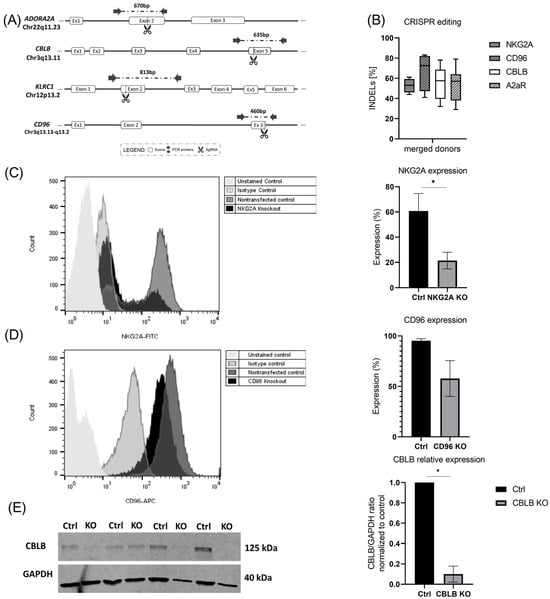
Figure 2.
CRISPR-Cas9-based knockout in expanded NK. (A) Schematic illustration showing the cutting sites of Cas9 in A2AR and CD96 genes, as well as NKG2A and CBLB (adapted from previous publication) [24]. (B) Expanded NK cells were transfected with RNP complexes containing sgRNA targeting NKG2A, CD96, CBLB, and A2AR. After Sanger sequencing, indel frequencies were analyzed using the ICE v3 software (www.ice.synthego.com, (accessed on July 2022 to July 2023)). (C,D) NKG2A and CD96 receptor expression in NK cells after knockout was measured using flow cytometry. (E) Immunoblot of CBLB and GADPH in non-transfected NK cells and transfected cells. (E) Normalized CBLB/GADPH ratio of band densitometry in non-transfected and transfected NK cells. * p < 0.05.
2.3. CRISPR-Mediated Blocking of CD96 and NKG2A, Two Inhibitory Receptors in NK Cells
In our previous publication, we showed that NKG2A knockout did not increase the cytotoxicity of the NK-92 cell line either in parental cells or in CD19-CAR- and CD276-CAR-induced NK-92 cells [24]. Here, we evaluated the effect of the CRISPR knockout of NKG2A on expanded NK cells by performing a cytotoxicity luciferase assay using U937 CD19 tag/Luc and Nalm-6 GFP/Luc cells. U937 is a pro-monocytic human myeloid leukemia cell line [31], transduced to express luciferase and Nalm-6Luc/GFP cells generated from the human precursor B-cell lymphoblast cell with the stable introduction of a GFP-firefly luciferase expression construct.
The results of the killing assay showed that a modest and statistically insignificant improvement was observed in NKG2A knockout NK cells compared with wild-type cells against the B-ALL and AML cells (Figure 3A,B). Next, the generated CD96 knockout NK cells were incubated with AML and B-ALL cells. Again, CD96 knockout did not improve the killing function of the NK cells. Yet, in some donors, even a reduction in killing was observed against both U937 CD19tag/Luc and Nalm-6 GFP/Luc cells (Figure 3C,D).
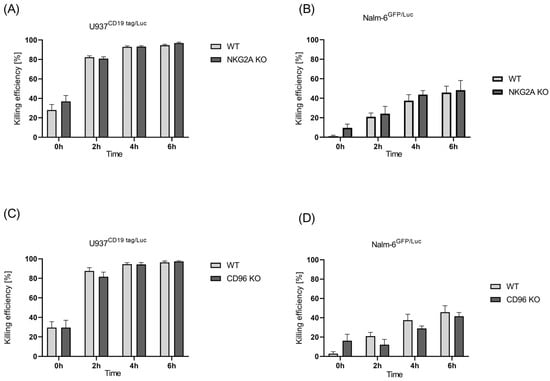
Figure 3.
Killing efficiency of unmodified primary NK cells vs. edited NKG2A and CD96 cells. Luciferase assay was performed to check for cytotoxicity of NKG2A KO NK cells against (A) U937 CD19 tag/Luc and (B) Nalm-6 GFP/Luc and CD96 KO cells against (C) U937 CD19 tag/Luc and (D) Nalm-6 GFP/Luc for a time span of 6 h at a 5:1 E:T ratio. Specific lysis is shown as mean ± SE (n = 3).
2.4. CRISPR-Mediated Blocking of CBLB as Intracellular Regulator of NK Cells
After the validation of CBLB knockout at the genetic and protein levels in NK cells, we aimed to check the cytotoxicity of edited NK cells against AML cells (U937 CD19tag/Luc) in vitro and compared this with wild-type cells. The cytotoxic performance of CBLB-KO cells against the AML cell line showed a slight but insignificant increase at 2 h, 4 h, and 6 h post-incubation compared with wild-type NK cells (Figure 4A). In contrast, CBLB knockout could not boost the antileukemic activity of NK cells against B-ALL cells (Figure 4B). These data demonstrate that although the CRISPR-based blocking of CBLB could improve the killing performance of primary T cells against Nalm-6 GFP/Luc, and especially U937 CD19tag/Luc cells (Supplementary Figure S3), it showed only a slight improvement in the killing efficacy of NK cells against these cell lines in vitro.
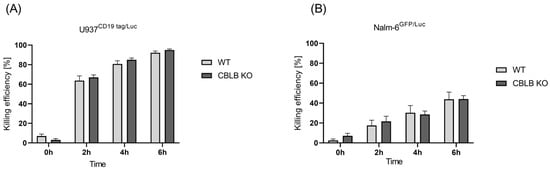
Figure 4.
Killing efficiency of unmodified primary NK cells vs. edited CBLB cells. Luciferase assay was performed to check for cytotoxicity of CBLB KO NK cells against (A) U937 CD19 tag/Luc (n = 4) and (B) Nalm-6 GFP/Luc (n = 3) at 0, 2, 4, and 6 h after coculture at a 5:1 E:T ratio. Specific lysis is shown as mean ± SE.
2.5. The Effect of Blocking Metabolic Purinergic Signaling on Antileukemic Function of NK Cells
To evaluate the cytolytic capabilities of expanded NK cells in the presence of adenosine, a cytotoxicity luciferase assay was performed. To this end, NK cells were treated with different concentrations of adenosine in the assay medium and subsequently incubated with U937 CD19tag/Luc cells. As shown in Figure 5A, a significant adenosine con-centration-dependent reduction was observed for the killing performance of NK cells against U937 CD19tag/Luc. This effect was mostly discernible at 2 and 4 h post-coculture of the effector and target cells. In contrast, the cytotoxicity of NK cells against the B-ALL cell line (Nalm-6GFP/Luc) was not affected by extracellular adenosine amount, and no improvement was observed in A2AR-depleted NK cells (Figure 5B,C).
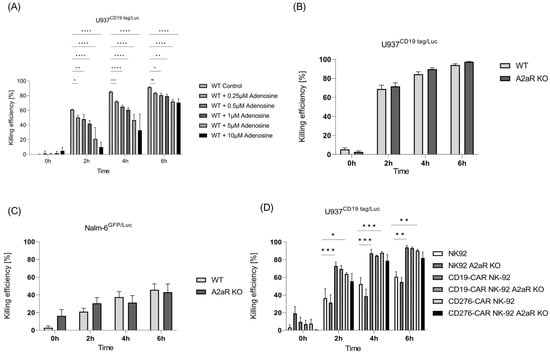
Figure 5.
Role of adenosine and its receptor A2AR in killing performance of NK cells. (A) Killing performance of NK cells in the presence of extracellular adenosine was evaluated in vitro via luciferase assay at different time points (n = 3). Killing efficiency of unmodified primary NK cells vs. edited A2AR cells against (B) U937 CD19 tag/Luc (n = 6) and (C) Nalm-6 GFP/Luc (n = 3) at 0, 2, 4, and 6 h after coculture at a 5:1 E:T ratio. (D) Luciferase killing assay for parental and A2AR KO NK-92, CD19-CAR-NK-92, and CD276-CAR-NK-92 cell lines against U937 CD19 tag/Luc cells (n = 3). Specific lysis is shown as mean ± SE. * p < 0.05; ** p < 0.01; *** p > 0.001; **** p < 0.0001, ns, non-significant (p > 0.05).
We earlier showed that the presence of chimeric antigen receptors such as CD19-CAR and CD276-CAR can strongly improve the cytotoxic effect of NK-92 cells [24]. To check if A2AR knockout can induce further killing improvement in CAR-induced NK-92 cells, a killing assay was performed. As expected, CD19- and CD276-CAR-induced NK-92 cells exhibited better killing performance than parental cells. However, A2AR knockout could not further increase their killing capacity (Figure 5D).
3. Discussion
Immunotherapy has recently emerged as the preferred strategy for cancer treatment owing to its potential to elicit long-lasting responses, mild levels of side effects, and the promising prospect of enhancing patient survival and overall quality of life [11,32,33]. NK-cell-based immunotherapy is widely considered a safe and effective therapy for solid tumors and hematologic malignancies [34]. In cancer clinical trials, a massive number of NK cells are required (ranging from 5 × 106 to 5 × 107 CD3−CD56+ NK cells per kilogram) [28]. Given the low number of NK cells in peripheral blood, an optimal ex vivo expansion of NK cells is required. It has been shown that ex vivo-expanded NK cells exhibit higher cytokine secretion profiles and have stronger cytotoxicity against tumor cells, and the maintenance of this activation depends on the presence of IL-15 or IL-2 [35]. Moreover, Mao et al. demonstrated a critical role for IL-15 in the antileukemic function of NK cells, supporting the incorporation of IL-15 in the expansion phase or through low-dose infusions following NK cell transfer in clinical settings [36]. In our study, utilizing K-562mb-IL-15 cells as feeder cells, we achieved a high expansion ratio for NK cells (up to 173.6×). Additionally, through the activation of these cells using IL-15 and IL-2, we promoted their effector function. These dual advantages overcome the limitation of using primary NK cells in terms of insufficient quantity and poor post-infusion persistence in the clinic.
Leukemia is defined as a collection of hematologic malignancies marked by the sustained proliferation of leukemic cells originating from hematopoietic stem cells affecting blood and bone marrow [11]. Enhancing the expression of inhibitory ligands and receptors is one of the mechanisms by which leukemic cells can escape from NK cell response [37,38]. Therefore, one approach to reverse NK cell dysfunction in tumors is blocking inhibitory pathways to increase the antitumor immunity of NK cells. Several monoclonal antibodies are currently employed to block NK cell checkpoint inhibitors in preclinical studies and clinical trials for leukemia [11].
CD96 and NKG2A are known as inhibitory receptors of NK and T cells. In this study, the killing capacity of expanded NK cells after the CRISPR-mediated knockout of these two receptors was addressed. We previously showed that NKG2A knockout failed to improve the killing performance of the NK-92 cell line against melanoma, AML, and B-ALL cells [24,29]. Bexte et al. performed CRISPR-based knockout in primary NK cells and reported the enhancement of tumor cell lyses in NKG2A-KO cells in comparison with WT NK cells against multiple myeloma cells after 24 h of coculturing in the FACS-based cytotoxic assay [39]. In contrast to Bexte et al. but similar to our previous publications [24,29], in this study, no significant improvement was observed in the killing of AML and B-ALL cells by NKG2A-KO-expanded NK cells, showing that NK cells might have different levels of cytotoxicity against different cancer cells.
Furthermore, CD96 knockout not only showed no increase in the cytotoxicity of NK cells against AML cells but also resulted in an insignificant reduction in killing against B-ALL cells. This observation is inconsistent with Stanietsky et al., where anti-CD96 mAb-treated NK cells could not redirect NK cytotoxicity independently and showed a slight reduction in specific lysis [40]. Other studies have reported the negative role of CD96 in the antitumor function of NK cells in mouse models [41,42], although it has been revealed that this effect is due to increased IFNγ production in CD96 knockout mice but not because of the enhancement of NK-cell-mediated cytotoxicity. Chan et al. showed the enhancement of cytokine production using NK cells in Cd96−/− mice in vivo; however, they did not observe any difference between mice with Cd96−/− and wild-type NK cells in terms of cytotoxicity in vitro, suggesting that CD96 signaling might not be strong enough to block cytotoxicity [41]. Moreover, it has been shown that cytotoxic lymphocytes have different activation thresholds for cytotoxicity and IFN-γ production [43]. This can explain the conflicting in vitro data from our group and others.
CBLB is an E3 ubiquitin ligase, and its upregulation and negative role in activated human NK cells have been reported [16]. Lu et al. showed that the siRNA-mediated downregulation of CBLB of primary NK cells resulted in an increase in cytotoxic activity against leukemia cell lines, including MV4-11 and Molm-13, in a 51Cr cytotoxicity assay [16]. However, in their study, one of the AML cell lines did not show this effect in the absence of IL-15, emphasizing the relevance of culture conditions and the type of target cells for the level of cytotoxicity evinced by NK cells. Guo et al. used the CRISPR-Cas9 system to deplete CBLB in placenta-derived CD34+ hematopoietic stem cells and differentiated them into PNK cells. They showed that CBLB knockout PNK cells have higher cytotoxicity against the myeloma cell lines RPMI8226 and U266 and the plasma cell leukemia cell line ARH77 [30]. In our study, CRISPR-Cas9-based CBLB knockout was applied to expanded NK cells from peripheral blood and showed only a slight improvement in cytotoxicity against U937 cells. Likewise, a previous study by our group showed that CBLB knockout alone is not enough to boost antileukemic activity in NK-92 cells against the B-ALL cell line [24]. However, CBLB knockout in T cells showed a significant improvement in killing performance against the U937 and Nalm-6 cell lines (Supplementary Figure S3), proving the efficiency of the applied CRISPR-Cas9 system and killing assay in our study.
The purine nucleoside adenosine is an immunosuppressive metabolite, physiologically present at very low concentrations in body fluids, and is crucial for regulating excessive immune responses during inflammation and tissue damage [44]. A2AR, expressed in leukemic and immune cells, is the main receptor that transfers signals from extracellular adenosine to apply autocrine and paracrine effects [45]. We demonstrated the significant effect of extracellular adenosine on the killing performance of expanded NK cells against AML cells (Figure 5A), indicating the role of adenosine in suppressing immune function against AML cells. It has been shown that targeting the A2AR signaling pathway by blocking A2AR alone or in combination with other molecules can restore immune competence in vivo in leukemia mouse models [45,46]. Brauneck et al. previously reported an increase in the NK-cell-mediated lysis of AML cells (MV-4-11, TF-1, and OCI-AML3) by blocking A2AR in the NK-92 cell line in vitro, which was more notable after the combinational blockage of A2AR and TIGIT [47]. These data contrast with our results, as no improvement was observed in killing efficiency after the knockout of A2AR in NK-92 cells. However, Brauneck et al. used a different method (A2AR antagonism) for blocking A2AR and different AML cell lines as target cells. Moreover, our results showed that the CRISPR-based blocking of A2AR has a minor and insignificant effect on the NK-cell-mediated killing of AML cells.
In our experiment, Fc receptors, staining for A2AR in NK cells, were not successful, although a reduction in A2AR expression in edited NK-92 cells after CRISPR-based knockout proves that the applied sgRNA can successfully target the A2AR gene. It is worth noting that difficulties in staining A2AR in primary cells were reported by another study authored by Giuffrida et al., who stated in their publication that they could not show the A2AR knockout efficiency of T cells because of a lack of A2AR antibodies suitable for flow cytometry [48]. This discrepancy could be attributed to the antibody’s specificity to particular subpopulations of NK cells, which might be predominant in NK-92 cells but less in primary NK cells. Moreover, differences in post-translational modifications of the target protein in the two cell types can affect antibody binding. Finally, NK-92 cells may express the target receptor at higher levels or in a different conformation compared with primary NK cells, making it more accessible to the antibody [49,50].
Taken together, previous data show that mixed results have been observed for the mentioned targets in improving NK cell tumor-killing efficacy. In the current study, there are several key differences compared with the published data in terms of diverse effector cells; various target cells; different cytotoxicity assays; differences in media, cytokines, and culture conditions; and cell manipulation. The central scientific question of this study is whether blocking inhibitory receptors/pathways can further increase and preserve the cytotoxicity of expanded (and activated) NK cells. To the best of our knowledge, this is the first work that has analyzed the effect of the CRISPR-mediated knockout of NKG2A, CD96, CBLB, and A2AR in expanded NK cells against the U937 CD19/Luc (AML) and Nalm-6 GFP/Luc (B-ALL) cell lines. One of the central factors might be the expansion of NK cells using K562-mbIL15 cells as feeder cells for 14 days, through which they become strongly activated in advance of editing. We speculate that this activation cannot be further boosted by CRISPR-mediated knockouts of NKG2A, CD96, CBLB, and A2AR. A similar observation was reported for another target (Tim3) by Audenaerde et al., where the authors stimulated NK cells with IL-15 and showed elevated NK-cell-mediated killing against pancreatic stellate cells (PSCs), although blocking Tim3 in the activated cells could not change the killing capacity [51]. This idea is supported by the observation that, in primary T cells, we could confirm previous publication data [52] with enhanced killing activity after CBLB knockout (Supplementary Figure S3). The data show that our methods and model work in assessing the enhancement of the tumor-killing capability of primary cells.
Overall, in the present study, we could successfully expand primary NK cells with high purity and efficiency. This study underscores the potential of the CRISPR-Cas9 system to edit expanded NK cells. However, further investigation is needed to address the exact therapeutic effects of targeting A2AR, CBLB, NKG2A, and CD96 in NK cells. Each of these proteins is part of a complex network that warrants comprehensive exploration in studies involving diverse cancer cell types. Moreover, sorting out the low-expression cells after CRISPR knockout and performing cytotoxicity tests using 100% knockout cells may lead to more convincing conclusions. Consequently, to attain a better understanding of the therapeutic potential of each target, it is advisable to conduct analyses using NK cells derived from patients with the specific cancer type.
4. Materials and Methods
4.1. Cell Line Cultures
K-562mb-IL-15, U937CD19/Luc, and Nalm-6 GFP/Luc tag were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C in a humidified 5% CO2 incubator.
4.2. Primary Human NK Cell Isolation and Culture
Heparinized peripheral blood samples were obtained from healthy donors after informed consent. Peripheral blood mononuclear cells (PBMCs) were subsequently isolated using Ficoll-density gradient centrifugation (BioColl® Separation Solution, Bio&SELL GmbH, Nuremberg, Germany) according to the manufacturer’s instructions. Isolated PBMCs were washed twice with PBS via centrifugation before evaluating the NK cell frequency with flow cytometry. CD56+ NK cells were isolated via negative selection using the EasySep™ Human NK Cell Isolation Kit (StemCell Technologies, Saint Égrève, France) according to the manufacturer’s instructions.
Isolated NK cells were cultured with irradiated K562-mb15 cells as feeder cells at a 0.66:1 ratio and maintained in NK cell medium (RPMI-1640 medium supplemented with 1% penicillin/streptomycin, 1% L-glutamine, 10% human serum, and 100 IU/mL of human recombinant IL-2 (Miltenyi Biotec, Bergisch Glattbach, Germany)) for 14 days at 37 °C and 5% CO2. In total, 5 ng/mL of soluble IL-15 was added to the medium after day 14.
4.3. Flow Cytometry
Percentages of the whole blood (WB) cell compartment containing NK cells were determined after PBMC isolation and isolation of NK cells on day 0. For the receptor analysis, harvested cells were washed and resuspended in serum-free and azide-free PBS and were characterized with specific A2AR-PE (Bio-Techne GmbH, Wiesbaden-Nordenstadt, Germany), CD3-PE, CD56-APC, CD96-APC, CTLA-4-PE, NKG2A-FITC, PD1-PE, TIGIT-PE, or TIM3-APC (Miltenyi Biotec, Bergisch Gladbach, Germany) fluorophore-labeled antibodies for 30 min at room temperature in the dark. An isotype control was used for each fluorophore used. Samples were run on a BD FACSCalibur™ flow cytometer (BD Biosciences, San Diego, CA, USA) and analyzed with the FlowJo-10 software (FlowJo LLC., BD Biosciences, Franklin Lakes, NJ, USA).
4.4. CRISPR-Cas9 Transfection
The sgRNAs were selected either from previous publications or were newly designed using the CHOPCHOP software, version 3 (www.chopchop.cbu.uib.no (accessed on June 2022); see Table 1. Before CRISPR/Cas9 electroporation, the expanded NK cells were harvested, centrifuged at 400 G for 5 min, and washed with PBS. To create the RNP complex, 1.5 µL of sgRNA and 1.23 µL of Cas9 were mixed and incubated for 15 min at room temperature. In total, 2.5 × 106 NK cells were resuspended in 50 µL of MaxCyte electroporation buffer and then mixed with RNP solution. The NK cells were electroporated with the MaxCyte GTx® instrument using the NK-4 and -5 protocols. Following electroporation, the cells were transferred to a pre-warmed 6-well plate. After 30 min of recovery time, 1.25 mL of warmed RPMI 1640 medium with 10% human serum, 1 mM of glutamine without antibiotics, and interleukins were added to the cells. Following a recovery period of 4 h, each well received 1.25 ml of RPMI medium with 200 U/mL of penicillin, 200 mg/L of streptomycin, IL-2 (200 U/mL), and IL-15 (100 U/mL). As a transfection control, DsRed mRNA was electroporated to the cells in all experiments, as described before [53].

Table 1.
List of used sgRNA sequences for target disruption.
4.5. Evaluation of CRISPR-Cas9-Induced Knockout
Three days after transfection, 5 × 105 transfected cells and WT NK cells were harvested to isolate DNA using the NucleoSpin® DNA purification Kit (Macherey Nagel, Dueren, Germany). The target sequence containing CBLB, CD96, NKG2A, or A2aR was amplified through PCR, with their respective primer sequences shown in Supplementary Table S1. Then, all the samples were submitted for Sanger sequencing, and the resulting data were analyzed using Synthego’s ICE v3 software to confirm the gene editing (https://ice.synthego.com (accessed on July 2022 to July 2023)).
4.6. Western Blot
Approximately 3 × 106 cells were suspended in RIPA Lysis (with 1× Protease and Phosphatase Inhibitor Cocktail (ThermoFisher, Waltham, MA, USA)). All samples were incubated on ice for 20 min. The soluble fraction was then obtained via centrifugation at 10,000× g for 10 min at 4 °C. The standard Bradford assay was used to measure protein concentration. In total, 20 µg of protein was loaded onto a Mini-PROTEAN TGX gel (Bio-Rad, Fort Worth, TX, USA), and the gel was blotted onto a Midi format 0.2 µm PVDF membrane utilizing the Trans-Blot Turbo Transfer System (Bio-Rad). The membrane was blocked with EveryBlot Blocking Buffer (Bio-Rad) and incubated for 1 h at room temperature (RT) or overnight at 4 °C with primary antibodies (rabbit anti-CBLB diluted at 1:250 and rat anti-GAPDH diluted at 1:1000 in blocking buffer). Following washing with PBS-T, the membrane was incubated for 1 h at room temperature with the secondary antibodies (IRDye 800CW goat anti-rat and IRDye 680RD goat anti-rabbit at 1:15,000 in a blocking buffer). The membrane was developed utilizing LI-COR.
4.7. Luciferase-Based Cytotoxicity Assay
The cytotoxicity of effector cells (WT and KO NK cells) against target cells (U937 CD19/Luc or Nalm-6Luc/GFP) was evaluated using a luciferase-based cytotoxicity assay. U937 CD19/Luc and Nalm-6Luc/GFP are capable of metabolizing luciferin and expressing luciferase, which was added at specific time intervals during the assay. In total, 1 × 104 target cells (U937 CD19/Luc or Nalm-6Luc/GFP) were resuspended in 100 µL of assay medium (RPMI 10% FBS10% FBS, 1% L-glutamine, and 1% penicillin/streptomycin), and 5 × 104 effector cells (WT or KO NK cells) were resuspended in 50 µL of assay medium and cocultured on a black 96-well plate. At 0, 2, 4, and 6 h, D-luciferin (Gold-Bio) was added to each well for measurement. For the preparation of the working assay solution for D-luciferin, a stock aliquot was mixed 1:4 in assay medium, and 50 µL was added to each assay well. The samples were directly evaluated for their cytotoxicity with the luminescence measurement in a TECAN Spark reader after adding D-luciferin.
To calculate the specific lysis by effector cells, the following formula was used:
4.8. Statistical Analysis
All statistical analyses were conducted using Prism 8.0 (GraphPad Software, La Jolla, CA, USA) on at least biological and experimental triplicate datasets. Two-way ANOVA analyses and non-parametric t-tests were performed for, respectively, Gaussian-distributed datasets and the normally distributed data (analyzed with the Shapiro–Wilk test). The FlowJo-10 software (FlowJo LLC, Ashland, OR, USA) was used for the analysis of flow cytometry data.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms242216065/s1.
Author Contributions
Conceptualization, T.M.G. and M.M.; methodology, T.M.G., G.U.-B., M.K., R.S., S.S. and P.G.G.; software, T.M.G. and M.K.; validation, T.M.G. and M.M.; formal analysis, T.M.G.; investigation, T.M.G., G.U.-B., M.K., R.S., S.S. and P.G.G.; resources, A.R.-M. and M.M.; data curation, T.M.G.; writing—original draft preparation, T.M.G. writing—review and editing, T.M.G., G.U.-B., F.Z., J.S.A. and M.K.; visualization, T.M.G., G.U.-B. and M.M.; supervision, T.M.G., M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Stefan Morsch Stiftung, the Clinician Scientist Program (N°. 440-0-0), Förderverein für krebskranke Kinder Tübingen e.V., MaxCyte Inc., and the University Children’s Hospital of Tübingen. We acknowledge support from the Open Access Publishing Fund of the University of Tübingen.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and the approval of the Institutional Review Board of the University of Tübingen Ethics Committee (No. 928/2020BO2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding authors upon reasonable request.
Acknowledgments
We are grateful to all healthy donors for providing blood for in vitro experiments. We acknowledge Christian Seitz and his team at the Children’s Hospital of Tübingen for providing the U937 CD19tag/Luc and Nalm-6 GFP/Luc cell lines. We also acknowledge the support of Evi Schmid’s group at the Children’s Hospital of Tübingen with the performance of standard Bradford assays for protein quantification.
Conflicts of Interest
Alicia Roig-Merino is employed in MaxCyte company that sells the GTX electroporator system used in this project. The other authors declare no conflict of interest.
References
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef] [PubMed]
- Lamers-Kok, N.; Panella, D.; Georgoudaki, A.M.; Liu, H.; Özkazanc, D.; Kučerová, L.; Duru, A.D.; Spanholtz, J.; Raimo, M. Natural killer cells in clinical development as non-engineered, engineered, and combination therapies. J. Hematol. Oncol. 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Maddineni, S.; Silberstein, J.L.; Sunwoo, J.B. Emerging NK cell therapies for cancer and the promise of next generation engineering of iPSC-derived NK cells. J. ImmunoTherapy Cancer 2022, 10, e004693. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Kang, S.; Gao, X.; Zhang, L.; Yang, E.; Li, Y.; Yu, L. The Advances and Challenges of NK Cell-Based Cancer Immunotherapy. Curr. Oncol. 2021, 28, 1077–1093. [Google Scholar] [CrossRef]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Seliger, B.; Koehl, U. Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front. Immunol. 2022, 13, 910595. [Google Scholar] [CrossRef]
- Allison, M.; Mathews, J.; Gilliland, T.; Mathew, S.O. Natural Killer Cell-Mediated Immunotherapy for Leukemia. Cancers 2022, 14, 843. [Google Scholar] [CrossRef]
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018, 78, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Mousavi, M.J.; Keshavarz Shahbaz, S.; Jafarzadeh, L.; Tahmasebi, S.; Spoor, J.; Esmaeilzadeh, A. E3 ubiquitin ligase Casitas B lineage lymphoma-b and its potential therapeutic implications for immunotherapy. Clin. Exp. Immunol. 2021, 204, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.S.; Atfield, A.; Venuprasad, K.; Krawczyk, C.; Sarao, R.; Elly, C.; Yang, C.; Arya, S.; Bachmaier, K.; Su, L.; et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 2004, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Reddi, A.L.; Ghosh, A.; Dimri, M.; Band, H. The Cbl family and other ubiquitin ligases: Destructive forces in control of antigen receptor signaling. Immunity 2004, 21, 7–17. [Google Scholar] [CrossRef]
- Lu, T.; Chen, L.; Mansour, A.G.; Yu, M.J.; Brooks, N.; Teng, K.Y.; Li, Z.; Zhang, J.; Barr, T.; Yu, J.; et al. Cbl-b Is Upregulated and Plays a Negative Role in Activated Human NK Cells. J. Immunol. 2021, 206, 677–685. [Google Scholar] [CrossRef]
- Linger, R.M.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar] [CrossRef]
- Chirino, L.M.; Kumar, S.; Okumura, M.; Sterner, D.E.; Mattern, M.; Butt, T.R.; Kambayashi, T. TAM receptors attenuate murine NK-cell responses via E3 ubiquitin ligase Cbl-b. Eur. J. Immunol. 2020, 50, 48–55. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; He, F.; Ma, X.; Fan, F.; Meng, M.; Zhuo, Y.; Zhang, L. CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma. Sci. Rep. 2020, 10, 10768. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, Z.; Fu, R.; Wang, H.; Li, L.; Liu, H. Expressions of CD96 and CD123 in Bone Marrow Cells of Patients with Myelodysplastic Syndromes. Clin. Lab. 2015, 61, 1429–1434. [Google Scholar] [CrossRef]
- Blake, S.J.; Dougall, W.C.; Miles, J.J.; Teng, M.W.; Smyth, M.J. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 5183–5188. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731.e1713–1743.e1713. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Invest. 2019, 129, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Bailén, G.; Dobrowolski, J.M.; Hou, Y.; Dirlam, A.; Roig-Merino, A.; Schleicher, S.; Atar, D.; Seitz, C.; Feucht, J.; Antony, J.S.; et al. Preclinical Evaluation of CRISPR-Edited CAR-NK-92 Cells for Off-the-Shelf Treatment of AML and B-ALL. Int. J. Mol. Sci. 2022, 23, 12828. [Google Scholar] [CrossRef]
- Seth, R.; Singh, A. Leukemias in Children. Indian J. Pediatr. 2015, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Arachchige, A.S.P.M. Human NK cells: From development to effector functions. Innate Immun. 2021, 27, 212–229. [Google Scholar] [CrossRef]
- Lapteva, N.; Szmania, S.M.; van Rhee, F.; Rooney, C.M. Clinical grade purification and expansion of natural killer cells. Crit. Rev. Oncog. 2014, 19, 121–132. [Google Scholar] [CrossRef]
- Grote, S.; Ureña-Bailén, G.; Chan, K.C.; Baden, C.; Mezger, M.; Handgretinger, R.; Schleicher, S. In Vitro Evaluation of CD276-CAR NK-92 Functionality, Migration and Invasion Potential in the Presence of Immune Inhibitory Factors of the Tumor Microenvironment. Cells 2021, 10, 1020. [Google Scholar] [CrossRef]
- Guo, X.; Mahlakõiv, T.; Ye, Q.; Somanchi, S.; He, S.; Rana, H.; DiFiglia, A.; Gleason, J.; van der Touw, W.; Hariri, R.; et al. CBLB ablation with CRISPR/Cas9 enhances cytotoxicity of human placental stem cell-derived NK cells for cancer immunotherapy. J. Immunother. Cancer 2021, 9, e001975. [Google Scholar] [CrossRef]
- Sundström, C.; Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H.J. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Childs, R.W.; Berg, M. Bringing natural killer cells to the clinic: Ex vivo manipulation. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; van Hoef, V.; Zhang, X.; Wennerberg, E.; Lorent, J.; Witt, K.; Masvidal, L.; Liang, S.; Murray, S.; Larsson, O.; et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 2016, 128, 1475–1489. [Google Scholar] [CrossRef]
- Sportoletti, P.; De Falco, F.; Del Papa, B.; Baldoni, S.; Guarente, V.; Marra, A.; Dorillo, E.; Rompietti, C.; Adamo, F.M.; Ruggeri, L.; et al. NK Cells in Chronic Lymphocytic Leukemia and Their Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6665. [Google Scholar] [CrossRef]
- Khaldoyanidi, S.; Nagorsen, D.; Stein, A.; Ossenkoppele, G.; Subklewe, M. Immune Biology of Acute Myeloid Leukemia: Implications for Immunotherapy. J. Clin. Oncol. 2021, 39, 419–432. [Google Scholar] [CrossRef]
- Bexte, T.; Alzubi, J.; Reindl, L.M.; Wendel, P.; Schubert, R.; Salzmann-Manrique, E.; von Metzler, I.; Cathomen, T.; Ullrich, E. CRISPR-Cas9-based gene editing of the immune checkpoint NKG2A enhances NK cell mediated cytotoxicity against multiple myeloma. Oncoimmunology 2022, 11, 2081415. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.J.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.C.; Mittal, D.; Aguilera, A.R.; Miles, J.J.; Lutzky, V.P.; de Andrade, L.F.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Faroudi, M.; Utzny, C.; Salio, M.; Cerundolo, V.; Guiraud, M.; Müller, S.; Valitutti, S. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: Manifestation of a dual activation threshold. Proc. Natl. Acad. Sci. USA 2003, 100, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Emens, L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 2018, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Serra, S.; Vitale, N.; Guerra, G.; Papait, A.; Baffour Gyau, B.; Tito, F.; Efremov, D.; Vaisitti, T.; Deaglio, S. Targeting of the A2A adenosine receptor counteracts immunosuppression in vivo in a mouse model of chronic lymphocytic leukemia. Haematologica 2021, 106, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.S.; Laursen, L.G.; Schuster, M.B.; Pundhir, S.; Schoof, E.; Ge, Y.; d’Altri, T.; Vitting-Seerup, K.; Rapin, N.; Gentil, C.; et al. Mutant CEBPA directly drives the expression of the targetable tumor-promoting factor CD73 in AML. Sci. Adv. 2019, 5, eaaw4304. [Google Scholar] [CrossRef]
- Brauneck, F.; Seubert, E.; Wellbrock, J.; Wiesch, J.S.Z.; Duan, Y.; Magnus, T.; Bokemeyer, C.; Koch-Nolte, F.; Menzel, S.; Fiedler, W. Combined Blockade of TIGIT and CD39 or A2AR Enhances NK-92 Cell-Mediated Cytotoxicity in AML. Int. J. Mol. Sci. 2021, 22, 12919. [Google Scholar] [CrossRef]
- Giuffrida, L.; Sek, K.; Henderson, M.A.; Lai, J.; Chen, A.X.Y.; Meyran, D.; Todd, K.L.; Petley, E.V.; Mardiana, S.; Mølck, C.; et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat. Commun. 2021, 12, 3236. [Google Scholar] [CrossRef]
- Arnold, D.P.; Xu, Y.; Takatori, S.C. Antibody binding reports spatial heterogeneities in cell membrane organization. Nat. Commun. 2023, 14, 2884. [Google Scholar] [CrossRef]
- Jiang, N.; Chen, W.; Jothikumar, P.; Patel, J.M.; Shashidharamurthy, R.; Selvaraj, P.; Zhu, C. Effects of anchor structure and glycosylation of Fcγ receptor III on ligand binding affinity. Mol. Biol. Cell 2016, 27, 3449–3458. [Google Scholar] [CrossRef]
- Van Audenaerde, J.R.M.; De Waele, J.; Marcq, E.; Van Loenhout, J.; Lion, E.; Van den Bergh, J.M.J.; Jesenofsky, R.; Masamune, A.; Roeyen, G.; Pauwels, P.; et al. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget 2017, 8, 56968–56979. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, R.; Kumar Singh, A.; Tsakem, E.L.; Kathania, M.; Riese, M.J.; Theiss, A.L.; Davila, M.L.; Venuprasad, K. Deletion of Cbl-b inhibits CD8(+) T-cell exhaustion and promotes CAR T-cell function. J. Immunother. Cancer 2021, 9, e001688. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ureña-Bailén, G.; Mohammadian Gol, T.; Gratz, P.G.; Gratz, H.P.; Roig-Merino, A.; Antony, J.S.; Lamsfus-Calle, A.; Daniel-Moreno, A.; Handgretinger, R.; et al. Challenges in Gene Therapy for Somatic Reverted Mosaicism in X-Linked Combined Immunodeficiency by CRISPR/Cas9 and Prime Editing. Genes 2022, 13, 2348. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Choi, U.; Gao, J.-L.; Thompson, R.D.; Rodman, L.E.; Malech, H.L.; Kang, E.M. A Novel Method for Screening Adenosine Receptor Specific Agonists for Use in Adenosine Drug Development. Sci. Rep. 2017, 7, 44816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).