VEGF and Other Gene Therapies Improve Flap Survival—A Systematic Review and Meta-Analysis of Preclinical Studies
Abstract
1. Introduction
2. Results
2.1. Primary Outcome—Flap Survival
2.1.1. Vascular Endothelial Growth Factor (VEGF)
2.1.2. Fibroblast Growth Factor (FGF)
2.1.3. Platelet-Derived Growth Factor (PDGF)
2.1.4. Hepatocyte Growth Factor (HGF) and HGF + Prostacyclin Synthase (PGIS)
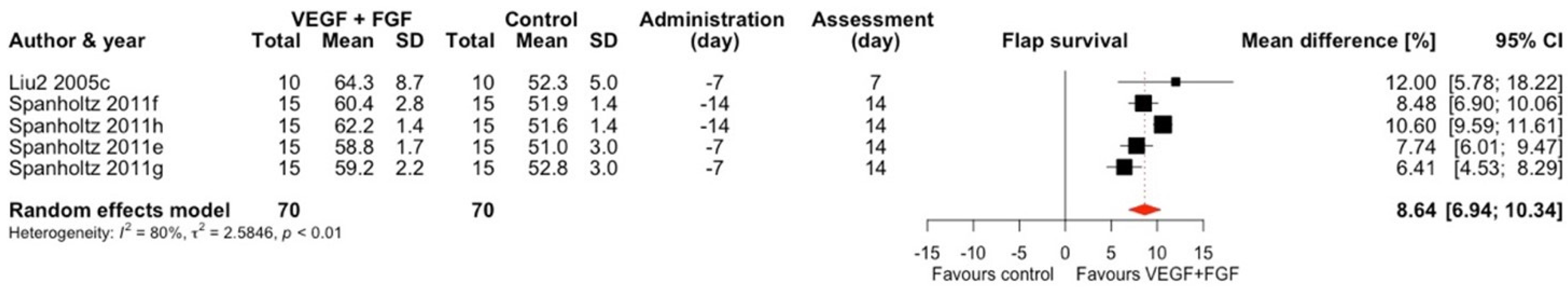
2.1.5. VEGF + FGF
2.1.6. Other Interventions
2.2. Secondary Outcome–Vessel Density
2.3. Risk of Bias
2.4. Certainty of Evidence
3. Discussion
3.1. Main Findings
3.2. Limitations of the Study
4. Methods
4.1. Search Strategy
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Primary Outcome
4.5. Secondary Outcomes
4.6. Data Extraction, Collection and Synthesis
4.7. Statistical Analysis
4.8. Risk of Bias (Quality) Assessment
4.9. Certainty of Evidence Assessment
5. Conclusions
- VEGF gene delivery to the flap increases its survival regardless of flap type, gene method, or timing in an in vivo model.
- Other proteins, such as FGF, HGF, and PDGF, may also be beneficial, but to a lesser extent.
- The quality of animal studies was predominantly low, which likely led to a high level of unexplained heterogeneity; however, general quantitative analysis is feasible.
- Translational studies appear to be sufficiently founded, yet any new interventions should be preceded by high-quality preclinical analysis.
- Additional, targeted, and detailed reviews and original studies should be performed to summarize the knowledge of pharmacodynamics and pharmacokinetics of gene therapies in flaps.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moellhoff, N.; Taha, S.; Wachtel, N.; Hirschmann, M.; Hellweg, M.; Giunta, R.E.; Ehrl, D. Analysis of Factors Determining Patient Survival after Receiving Free-Flap Reconstruction at a Single Center-A Retrospective Cohort Study. Diagnostics 2022, 12, 2877. [Google Scholar] [CrossRef]
- Harder, Y.; Amon, M.; Laschke, M.W.; Schramm, R.; Rücker, M.; Wettstein, R.; Bastiaanse, J.; Frick, A.; Machens, H.G.; Küntscher, M.; et al. An old dream revitalised: Preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 503–511. [Google Scholar] [CrossRef]
- Lee, J.-H.; You, H.-J.; Lee, T.-Y.; Kang, H.J. Current Status of Experimental Animal Skin Flap Models: Ischemic Preconditioning and Molecular Factors. Int. J. Mol. Sci. 2022, 23, 5234. [Google Scholar] [CrossRef] [PubMed]
- Afrooghe, A.; Damavandi, A.R.; Ahmadi, E.; Jafari, R.M.; Dehpour, A.R. The current state of knowledge on how to improve skin flap survival: A review. J. Plast. Reconstr. Aesthet. Surg. 2023, 82, 48–57. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle in Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Weir, L.; Chen, D.; Zheng, L.P.; Riessen, R.; Bauters, C.; Symes, J.F.; Ferrara, N.; Isner, J.M. Therapeutic angiogenesis following arterial gene transfer of vascular endothelial growth factor in a rabbit model of hindlimb ischemia. Biochem. Biophys. Res. Commun. 1996, 227, 628–635. [Google Scholar] [CrossRef]
- Neumeister, M.W.; Song, Y.H.; Mowlavi, A.; Suchy, H.; Mathur, A. Effects of liposome-mediated gene transfer of VEGF in ischemic rat gracilis muscle. Microsurgery 2001, 21, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Michlits, W.; Mittermayr, R.; Schafer, R.; Redl, H.; Aharinejad, S. Fibrin-embedded administration of VEGF plasmid enhances skin flap survival. Wound Repair Regen. 2007, 15, 360–367. [Google Scholar] [CrossRef]
- Meirer, R.; Huemer, G.M.; Oehlbauer, M.; Wanner, S.; Piza-Katzer, H.; Kamelger, F.S. Comparison of the effectiveness of gene therapy with vascular endothelial growth factor or shock wave therapy to reduce ischaemic necrosis in an epigastric skin flap model in rats. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 266–271. [Google Scholar] [CrossRef]
- Lubiatowski, P.; Goldman, C.K.; Gurunluoglu, R.; Carnevale, K.; Siemionow, M. Enhancement of epigastric skin flap survival by adenovirus-mediated VEGF gene therapy. Plast. Reconst. Surg. 2002, 109, 1986–1993. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wang, X.T.; Badiavas, E.; Rieger-Christ, K.; Tang, J.B.; Summerhayes, I. Enhancement of ischemic flap survival by prefabrication with transfer of exogenous PDGF gene. J. Reconstr. Microsurg. 2005, 21, 273–279. [Google Scholar] [CrossRef]
- Liu, P.Y.; Tong, W.; Liu, K.; Han, S.H.; Wang, X.T.; Badiavas, E.; Rieger-Christ, K.; Summerhayes, I. Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen. 2004, 12, 80–85. [Google Scholar] [CrossRef]
- Liu, P.Y.; Liu, K.; Wang, X.T.; Badiavas, E.; Rieger-Christ, K.M.; Tang, J.B.; Summerhayes, I.C. Efficacy of combination gene therapy with multiple growth factor cDNAs to enhance skin flap survival in a rat model. DNA Cell Biol. 2005, 24, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Lasso, J.M.; Del Río, M.; García, M.; Martínez Calleja, V.; Nava, P.; Muñoz-Fernández, M.A.; Pérez Cano, R. Improving flap survival by transplantation of a VEGF-secreting endothelised scaffold during distal pedicle flap creation. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 279–286. [Google Scholar] [CrossRef]
- Huang, N.; Khan, A.; Ashrafpour, H.; Neligan, P.C.; Forrest, C.R.; Kontos, C.D.; Pang, C.Y. Efficacy and mechanism of adenovirus-mediated VEGF-165 gene therapy for augmentation of skin flap viability. Am. J. Physiol. Heart Circ. 2006, 291, H127–H137. [Google Scholar] [CrossRef] [PubMed]
- Holzbach, T.; Vlaskou, D.; Neshkova, I.; Konerding, M.A.; Wörtler, K.; Mykhaylyk, O.; Gänsbacher, B.; Machens, H.G.; Plank, C.; Giunta, R.E. Non-viral VEGF165 gene therapy-magnetofection of acoustically active magnetic lipospheres (‘magnetobubbles’) increases tissue survival in an oversized skin flap model. J. Cell. Mol. Med. 2010, 14, 587–599. [Google Scholar] [CrossRef]
- Hijjawi, J.; Mogford, J.E.; Chandler, L.A.; Cross, K.J.; Said, H.; Sosnowski, B.A.; Mustoe, T.A. Platelet-derived growth factor B, but not fibroblast growth factor 2, plasmid DNA improves survival of ischemic myocutaneous flaps. Arch. Surg. 2004, 139, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Machens, H.G.; Morgan, J.R.; Berthiaume, F.; Stefanovich, P.; Siemers, F.; Krapohl, B.; Berger, A.; Mailänder, P. Platelet-derived growth factor-AA-mediated functional angiogenesis in the rat epigastric island flap after genetic modification of fibroblasts is ischemia dependent. Surgery 2002, 131, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Makino, H.; Aoki, M.; Miyake, T.; Shiraya, S.; Nakamura, T.; Ogihara, T.; Kimata, Y.; Morishita, R. Improvement of survival of skin flaps by combined gene transfer of hepatocyte growth factor and prostacyclin synthase. J. Gene Med. 2007, 9, 1087–1094. [Google Scholar] [CrossRef]
- Rah, D.K.; Yun, I.S.; Yun, C.O.; Lee, S.B.; Lee, W.J. Gene therapy using hepatocyte growth factor expressing adenovirus improves skin flap survival in a rat model. J. Korean Med. Sci. 2014, 29 (Suppl. 3), S228–S236. [Google Scholar] [CrossRef]
- Huemer, G.M.; Shafighi, M.; Meirer, R.; Debagge, P.; Piza-Katzer, H.; Gurunluoglu, R. Adenovirus-mediated transforming growth factor-β ameliorates ischemic necrosis of epigastric skin flaps in a rat model. J. Surg. Res. 2004, 121, 101–107. [Google Scholar] [CrossRef]
- Huemer, G.M.; Meirer, R.; Gurunluoglu, R.; Kamelger, F.S.; Dunst, K.M.; Wanner, S.; Piza-Katzer, H. Comparison of the effectiveness of gene therapy with transforming growth factor-β or extracorporal shock wave therapy to reduce ischemic necrosis in an epigastric skin flap model in rats. Wound Repair Regen. 2005, 13, 262–268. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wang, X.T.; Xin, K.Q.; Chen, C.H.; Rieger-Christ, K.; Summerhayes, I.C.; Fang Wu, Y.; Tang, J.B. Application of AAV2-mediated bFGF gene therapy on survival of ischemic flaps: Effects of timing of gene transfer. Ann. Plast. Surg. 2009, 62, 87–91. [Google Scholar] [CrossRef]
- Lee, W.J.; Yun, C.O.; Yun, I.S.; Kim, Y.O.; Choi, I.K.; Yun, T.J.; Rah, D.K. Augmentation of rat skin flap viability by relaxin-expressing adenovirus. Wound Repair Regen. 2011, 19, 709–717. [Google Scholar] [CrossRef]
- Jung, H.; Gurunluoglu, R.; Scharpf, J.; Siemionow, M. Adenovirus-mediated angiopoietin-1 gene therapy enhances skin flap survival. Microsurgery 2003, 23, 374–380. [Google Scholar] [CrossRef]
- Choi, S.W.; Jeon, Y.R.; Baek, W.; Yun, C.O.; Roh, T.S.; Lee, W.J. Dickkopf 2-Expressing Adenovirus Increases the Survival of Random-Pattern Flaps and Promotes Vasculogenesis in a Rat Model. Ann. Plast. Surg. 2020, 84, 588–594. [Google Scholar] [CrossRef]
- Lou, J.; Wang, X.; Zhang, H.; Yu, G.; Ding, J.; Zhu, X.; Li, Y.; Wu, Y.; Xu, H.; Gao, W.; et al. Inhibition of PLA2G4E/cPLA2 promotes survival of random skin flaps by alleviating Lysosomal membrane permeabilization-Induced necroptosis. Autophagy 2021, 18, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Gurunluoglu, R.; Ozer, K.; Skugor, B.; Lubiatowski, P.; Carnevale, K.; Siemionow, M. Effect of transfection time on the survival of epigastric skin flaps pretreated with adenovirus encoding the VEGF gene. Ann. Plast. Surg. 2002, 49, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gurunluoglu, R.; Meirer, R.; Shafighi, M.; Huemer, G.M.; Yilmaz, B.; Piza-Katzer, H. Gene therapy with adenovirus-mediated VEGF enhances skin flap prefabrication. Microsurgery 2005, 25, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Giunta, R.E.; Holzbach, T.; Taskov, C.; Holm, P.S.; Konerding, M.A.; Schams, D.; Biemer, E.; Gänsbacher, B. AdVEGF165 gene transfer increases surviral in overdimensioned skin flaps. J. Gene Med. 2005, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, Y.; Koyama, H.; Nishiyama, N.; Eguchi, T.; Takato, T. Gene transfer of bFGF to recipient bed improves survival of ischemic skin flap. Br. J. Surg. 2005, 58, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Cruz, Y.L.; Coppola, D.; Heller, R. Intradermal delivery of plasmid VEGF-165 by electroporation promotes wound healing. Mol. Ther. 2009, 17, 651–657. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, A.L.P.; Gomes, H.C.; Lisboa, B.C.G.; Arias, V.; Han, S.W.; Ferreira, L.M. Effect of gene therapy with vascular endothelial growth factor after abdominoplasty on TRAM flap viability in a rat model. Plast. Reconst. Surg. 2010, 125, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, D.; Wang, P.; Gao, S.; Zhang, X.; Wang, T. Microencapsulated myoblasts transduced by the vascular endothelial growth factor (VEGF) gene for the ischemic skin flap. Aesthetic Plast. Surg. 2011, 35, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Shoureshi, P.; Lay, F.; Sebastian, R.; Alikhassy Habibabady, Z.; Born, L.J.; Marti, G.P.; Meltzer, S.J.; Abraham, J.M.; Harmon, J.W. Preconditioning of surgical pedicle flaps with DNA plasmid expressing hypoxia-inducible factor-1α (HIF-1α) promotes tissue viability. Gene Ther. 2021, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Basu, G.; Downey, H.; Guo, S.; Israel, A.; Asmar, A.; Hargrave, B.; Heller, R. Prevention of distal flap necrosis in a rat random skin flap model by gene electrotransfer delivering VEGF-165 plasmid. J. Gene Med. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Zacchigna, S.; Papa, G.; Novati, F.; Pascone, M.; Giacca, M. Improved survival of rat ischemic cutaneous and musculocutaneous flaps after VEGF gene transfer. Microsurgery 2007, 27, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.J.; Marmur, J.D.; Zhang, W.X.; Senderoff, D.; Urken, M.L.; Silver, L.; Weinberg, H. Effect of time on the viability of ischemic skin flaps treated with vascular endothelial growth factor (VEGF) cDNA. J. Reconstr. Microsurg. 1998, 14, 387–390. [Google Scholar] [CrossRef]
- Rinsch, C.; Quinodoz, P.; Pittet, B.; Alizadeh, N.; Baetens, D.; Montandon, D.; Aebischer, P.; Pepper, M.S. Delivery of FGF-2 but not VEGF by encapsulated genetically engineered myoblasts improves survival and vascularization in a model of acute skin flap ischemia. Gene Ther. 2001, 8, 523–533. [Google Scholar] [CrossRef]
- O’Toole, G.; MacKenzie, D.; Lindeman, R.; Buckley, M.F.; Marucci, D.; McCarthy, N.; Poole, M. Vascular endothelial growth factor gene therapy in ischaemic rat skin flaps. Br. J. Plast. Surg. 2002, 55, 55–58. [Google Scholar] [CrossRef]
- Yang, L.W.; Zhang, J.X.; Zeng, L.; Xu, J.J.; Du, F.T.; Luo, W.; Luo, Z.J.; Jiang, J.H. Vascular endothelial growth factor gene therapy with intramuscular injections of plasmid DNA enhances the survival of random pattern flaps in a rat model. Br. J. Plast. Surg. 2005, 58, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Papa, G.; Antonini, A.; Novati, F.; Moimas, S.; Carrer, A.; Arsic, N.; Zentilin, L.; Visintini, V.; Pascone, M.; et al. Improved survival of ischemic cutaneous and musculocutaneous flaps after vascular endothelial growth factor gene transfer using adeno-associated virus vectors. Am. J. Pathol. 2005, 167, 981–991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, F.; Yang, F.; Hu, E.C.; Sones, W.; Lei, M.; Lineaweaver, W.C. Vascular endothelial growth factor gene therapy in improvement of skin paddle survival in a rat TRAM flap model. J. Reconstr. Microsurg. 2005, 21, 391–396. [Google Scholar] [CrossRef]
- Wang, X.T.; Liu, P.Y.; Tang, J.B. PDGF gene therapy enhances expression of VEGF and bFGF genes and activates the NF-κB gene in signal pathways in ischemic flaps. Plast. Reconst. Surg. 2006, 117, 129–137. [Google Scholar] [CrossRef]
- Yi, C.; Xia, W.; Zheng, Y.; Zhang, L.; Shu, M.; Liang, J.; Han, Y.; Guo, S. Transplantation of endothelial progenitor cells transferred by vascular endothelial growth factor gene for vascular regeneration of ischemic flaps. J. Surg. Res. 2006, 135, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yi, C.; Xia, W.; Ding, T.; Zhou, Z.; Han, Y.; Guo, S. Mesenchymal stem cells transduced by vascular endothelial growth factor gene for ischemic random skin flaps. Plast. Reconst. Surg. 2008, 121, 59–69. [Google Scholar] [CrossRef]
- Spanholtz, T.; Maichle, A.; Niedworok, C.; Stoeckelhuber, B.M.; Krüger, S.; Wedel, T.; Aach, T.; Middeler, G.; Hellwig-Bürgel, T.; Bader, A.; et al. Timing and Targeting of Cell-Based VEGF165 Gene Expression in Ischemic Tissue. J. Surg. Res. 2009, 151, 153–162. [Google Scholar] [CrossRef]
- Rezende, F.C.; Gomes, H.C.; Lisboa, B.; Lucca, A.F.; Han, S.W.; Ferreira, L.M. Electroporation of vascular endothelial growth factor gene in a unipedicle transverse rectus abdominis myocutaneous flap reduces necrosis. Ann. Plast. Surg. 2010, 64, 242–246. [Google Scholar] [CrossRef]
- Spanholtz, T.A.; Theodorou, P.; Holzbach, T.; Wutzler, S.; Giunta, R.E.; MacHens, H.G. Vascular endothelial growth factor (VEGF 165) plus basic fibroblast growth factor (bFGF) producing cells induce a mature and stable vascular network-A future therapy for ischemically challenged tissue. J. Surg. Res. 2011, 171, 329–338. [Google Scholar] [CrossRef]
- Wang, X.T.; Avanessian, B.; Ma, Q.; Durfee, H.; Tang, Y.Q.; Liu, P.Y. Enhancement of flap survival and changes in angiogenic gene expression after AAV2-mediated VEGF gene transfer to rat ischemic flaps. Wound Repair Regen. 2011, 19, 498–504. [Google Scholar] [CrossRef]
- Uemura, T.; Tsujii, M.; Akeda, K.; Iino, T.; Satonaka, H.; Hasegawa, M.; Sudo, A. Transfection of nuclear factor-kappaB decoy oligodeoxynucleotide protects against ischemia/reperfusion injury in a rat epigastric flap model. J. Gene Med. 2012, 14, 623–631. [Google Scholar] [CrossRef]
- Wang, X.; Yu, M.; Zhu, W.; Bao, T.; Zhu, L.; Zhao, W.; Zhao, F.; Wang, H. Adenovirus-mediated expression of keratinocyte growth factor promotes secondary flap necrotic wound healing in an extended animal model. Aesthetic Plast. Surg. 2013, 37, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Seyed Jafari, S.M.; Shafighi, M.; Beltraminelli, H.; Geiser, T.; Hunger, R.E.; Gazdhar, A. Improvement of Flap Necrosis in a Rat Random Skin Flap Model by In Vivo Electroporation-Mediated HGF Gene Transfer. Plast. Reconst. Surg. 2017, 139, 1116e–1127e. [Google Scholar] [CrossRef] [PubMed]
- Seyed Jafari, S.M.; Shafighi, M.; Beltraminelli, H.; Weber, B.; Schmid, R.A.; Geiser, T.; Gazdhar, A.; Hunger, R.E. Efficacy of In Vivo Electroporation-Mediated IL-10 Gene Delivery on Survival of Skin Flaps. J. Membr. Biol. 2018, 251, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Seyed Jafari, S.M.; Blank, F.; Ramser, H.E.; Woessner, A.E.; Shafighi, M.; Geiser, T.; Quinn, K.P.; Hunger, R.E.; Gazdhar, A. Efficacy of Combined in-vivo Electroporation-Mediated Gene Transfer of VEGF, HGF, and IL-10 on Skin Flap Survival, Monitored by Label-Free Optical Imaging: A Feasibility Study. Front. Surg. 2021, 8, 639661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-G.; Yao, Y.; Feng, Y.; Hua, C.-G.; Tang, X.-F. Mesenchymal Stem Cells Transduced by Stromal Cell–Derived Factor-1α Augment Ischemic Free Flaps’ Survival. Ann. Plast. Surg. 2011, 66, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Bian, Y.; Zheng, G.; Wang, H.; Yan, B.; Su, W.; Dong, W.; Hu, Z.; Ding, J.; Wang, A.; et al. Chemically Modified SDF-1α mRNA Promotes Random Flap Survival by Activating the SDF-1α/CXCR4 Axis in Rats. Front. Cell Dev. Biol. 2021, 9, 623959. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Abdou, S.A.; Daar, D.A.; Anzai, L.; Stranix, J.T.; Thanik, V.; Levine, J.P.; Saadeh, P.B. Comparing Outcomes for Fasciocutaneous versus Muscle Flaps in Foot and Ankle Free Flap Reconstruction. J. Reconstr. Microsurg. 2019, 35, 646–651. [Google Scholar] [CrossRef]
- Sara, C.; David, M.; Simon, R.; Garrett, C.; Martina, S.; Patrick, H.; Gerald, O.S.; Mark, T. Comparison of DNA Delivery and Expression Using Frequently Used Delivery Methods. In Gene Ther.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Li, X. VEGF-B: A thing of beauty. Cell Res. 2010, 20, 741–744. [Google Scholar] [CrossRef]
- Murakami, M.; Nguyen, L.T.; Hatanaka, K.; Schachterle, W.; Chen, P.-Y.; Zhuang, Z.W.; Black, B.L.; Simons, M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J. Clin. Investig. 2011, 121, 2668–2678. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 27 August 2023).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes Text with EEA relevance. Off. J. Eur. Union 2010, L 276, 33–79.
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]

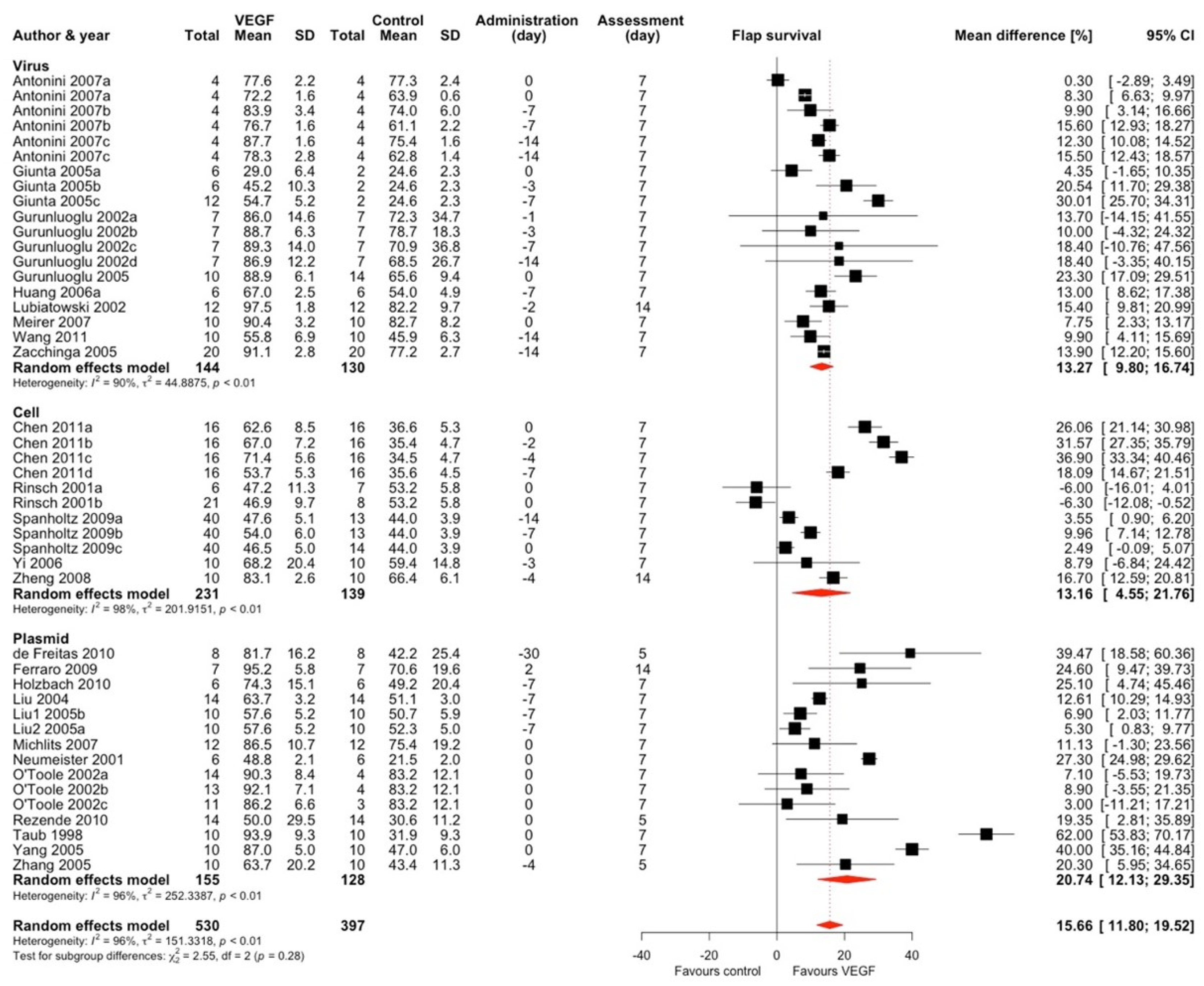
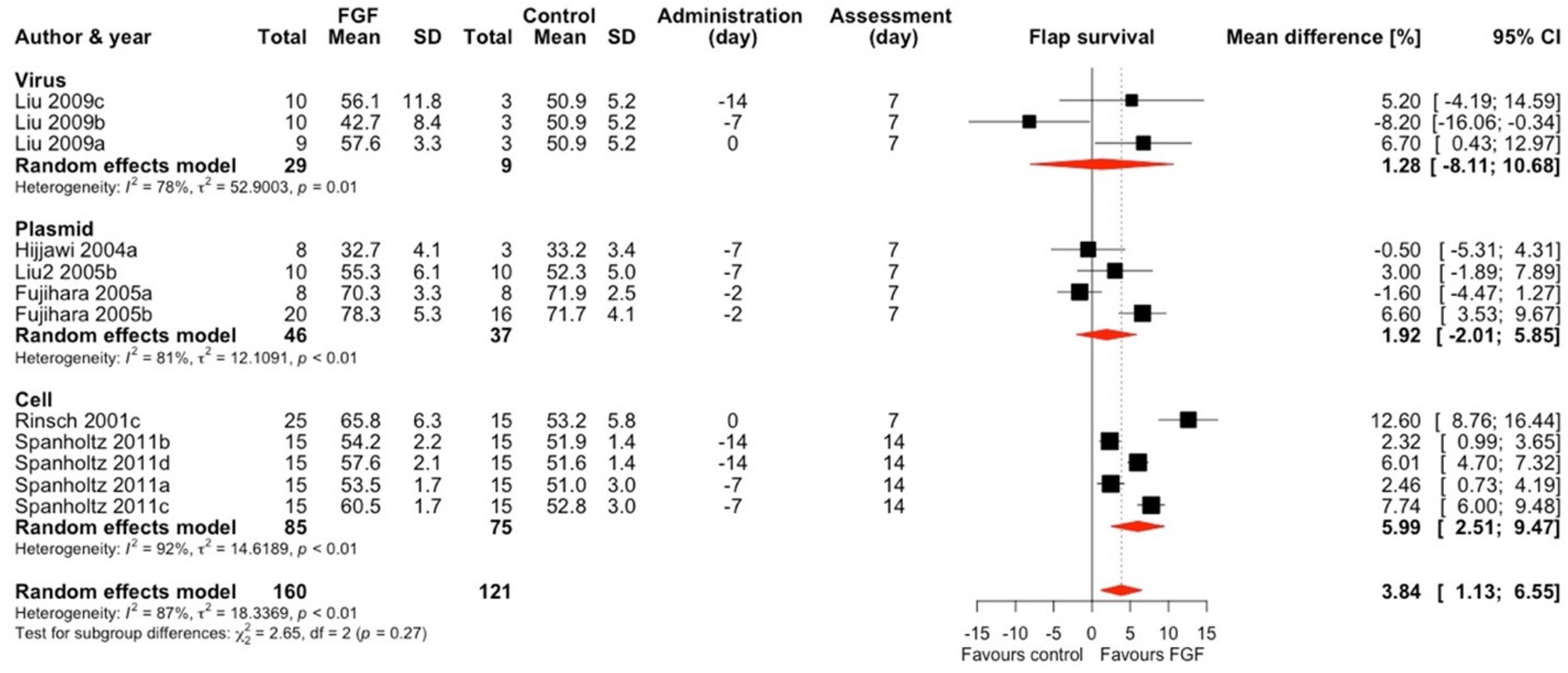
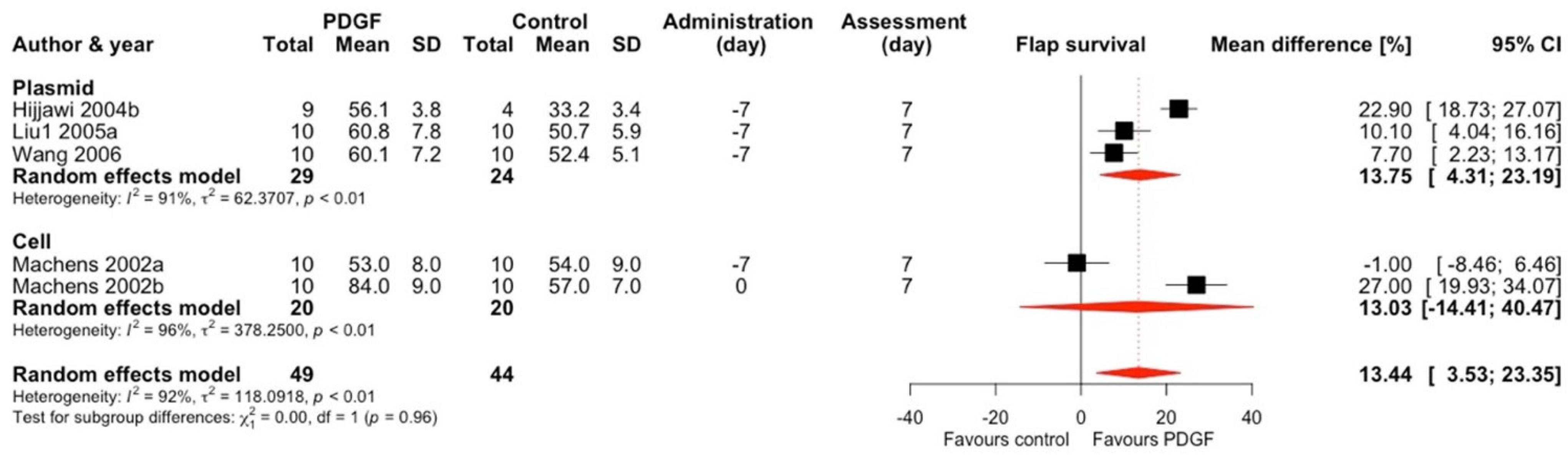
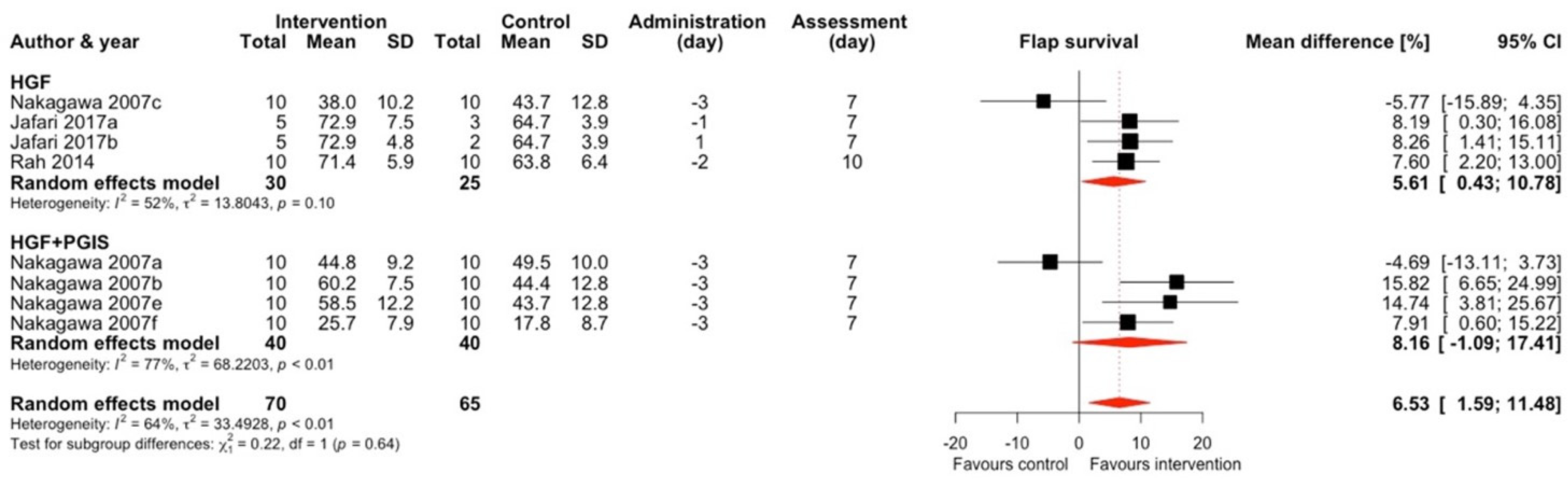

| First Author; Year (*)—Included In Meta-Analysis | Target | Vector | Mode of Expression * | Vector Quantity | Type of Control Group | Follow-Up | Flap Type | Animal Characteristics | Surgical Technique | Control Group Flap Survival (%, SD) ** | Treatment Group Flap Survival (%, SD) ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neumeister; 2001 (*) [7] | VEGF-165 | Plasmid | Unspecified | 50 μL | Lipofectamine only | 7 days | Muscle | 12 Wistar male rats (300–325 g) | Intramuscular injection after 4-h ischemia | 21.5 ± 2.0 | 48.8 ± 2.1 |
| Michlits; 2007 (*) [8] | VEGF-165 | Plasmid | Unspecified | 100 μg | No treatment (A) Fibrin-sealant (B) Fibrin-sealant and sham plasmid (C) | 7 days | Epigastric | 48 Sprague–Dawley male rats (300–350 g) | Topical administration after induced ischemia (not specified) | A—60.76 ± 10.20 B—74.30 ± 18.15 C—75.41 ± 19.19 | 86.54 ± 10.71 |
| Meirer; 2007 (*) [9] | VEGF (I) Shockwave (II) | Virus | Unspecified | 108 PFU | No treatment | 7 days | Epigastric | 30 Sprague–Dawley male rats (300–500 g) | Subdermal injection | 82.67 ± 8.15 | I—90.42 ± 3.19 II—97.77 ± 4.34 |
| Lubiatowski; 2002 (*) [10] | VEGF | Virus | Unspecified | 108 PFU | Sham virus (A) No treatment (B) | 14 days | Epigastric | 30 Sprague–Dawley male rats (300–350 g) | Subdermal injection two days before elevation | A—82.15 ± 9.7 B—79.9 ± 3.3 | 97.55 ± 1.84 |
| Liu; 2005 (*) [11] | PDGF-B (I) VEGF (II) | Plasmid | Unspecified | 50 μg | Saline (A) Sham plasmid (B) | 7 days | McFarlane | 45 Sprague–Dawley female rats (250–300 g) | Intradermal injection seven days before elevation | A—52.3 ± 5.0 B—50.7 ± 5.9 | I—60.8 ± 7.8 II—57.6 ± 5.2 |
| Liu; 2004 (*) [12] | VEGF-165 | Plasmid | Unspecified | 50 μg | Saline (A) Sham plasmid (B) | 7 days | McFarlane | 32 Sprague–Dawley female rats (250 g) | Intradermal injection seven days before elevation | A—51.3 ± 2.45 B—51.1 ± 3.05 | 63.71 ± 3.2 |
| Liu; 2005 (*) [13] | VEGF-165 (I) FGF-β (II) PDGF-B (III) | Plasmid | Unspecified | 50 μg | Sham plasmid | 7 days | McFarlane | 60 Sprague–Dawley female rats (250–300 g) | Intradermal injection 7 days before flap elevation | 52.3 ± 5.0 | I—57.6 ± 5.2 II—55.3 ± 6.1 I + II—64.3 ± 8.7 I + III—57.6 ± 9.5 I + II + III—44.9 ± 2.7 |
| Lasso; 2007 [14] | VEGF-165 | Cells (transduction) | Unspecified | 5 × 105 cells | Non-transduced cells | 6 days | Axial ear | 32 New Zealand white male rabbits (2500–3000 g) | Topical administration onto the scaffold with pedicle divided after five or two days (1, 2) | 1—2.50 ± 7.07 2—51.25 ± 45.88 | 1—55.62 ± 38.95 2—95.62 ± 4.95 |
| Huang; 2006 (*) [15] | VEGF-165 (I) eNOS (II) | Virus | Unspecified | 5 × 108 PFU | PBS (A) Sham virus (B) | 7 days | McFarlane | 24 Sprague–Dawley male rats (350–375 g) | Subdermal injection seven days before flap elevation | A—56.0 ± 3.0 B—54.0 ± 2.0 | I—67.0 ± 1.0 II—70.0 ± 3.0 |
| Holzbach; 2010 (*) [16] | VEGF-165 | Plasmid (magnetized) | Unspecified | 200 µl | Sham plasmid | 7 days | McFarlane | 46 Sprague–Dawley male rats (350 g) | Subcutaneous injection with additional ultrasounds seven days before flap elevation | 49.2 ± 0.08 | 74.3 ± 0.05 |
| Hijjawi; 2004 (*) [17] | PDGF-B (I) FGF-β (II) | Plasmid (collagen matrix) | Unspecified | 4.8 mg | No treatment | 7 days | Muscle | 24 Sprague–Dawley male rats (250–300 g) | Subcutaneous injection seven days before flap elevation | 33.2 ± 1.3 | I—56.1 ± 1.25 II—32.7 ± 1.44 |
| Machens; 2002 (*) [18] | PDGF-A | Cells (transduction) | Constant (up to 96 h) | 1 × 107 cells | Saline (A) Cell medium (B) Non-modified cells (C) | 0 or 7 days (1,2) | Epigastric | 80 Lewis inbred female rats (200–225 g) | Intramuscular at the time of flap elevation | A1—50.0 ± 9.0 A2—56.0± 8.0 B1—49.0 ± 11.0 B2—52.0 ± 10.0 C1—54.0 ± 9.0 C2—57.0 ± 7.0 | 1—53.0 ± 8.0 2—84.0 ± 9.0 |
| Nakagawa; 2007 (*) [19] | HGF (I) PGIS (II) | Plasmid (jet injector) | Unspecified | 400 µg | Sham plasmid | 7 days | McFarlane | 40 Sprague–Dawley male rats and 40 GK/Jcl male rats | Intracutaneous injection − 8 or 4 sites (1, 2) three days before flap elevation | 1—43.73 ± 12.78 2—44.42 ± 10.02 | I + II1—44.76 ± 9.17 I + II2—60.24 ± 7.45 I—37.96 ± 10.15 II—32.2 ± 11.64 I + II—58.47 ± 12.15 |
| Rah; 2014 (*) [20] | HGF | Virus | Constant (up to 10 days) | 1 × 107 PFU | PBS (A) Sham protein (B) | 10 days | McFarlane | 30 Sprague–Dawley male rats (300–350 g) | Subdermal injection two days and immediately after flap elevation | A—39.2 ± 13.0 B—63.8 ± 6.4 | 71.4 ± 5.9 |
| Huemer; 2004 [21] | TGF-β | Virus | Constant (up to 7 days) | 1 × 108 PFU | Saline (A) Sham virus (B) | 7 days | Epigastric | 30 Sprague–Dawley male rats (300–400 g) | Subdermal injection just prior to flap elevation | A—82.6 ± 4.6 B—82.2 ± 8.7 | 90.3 ± 4.0 |
| Huemer; 2005 [22] | TGF-β (I) Shockwave (II) | Virus | Constant (up to 7 days) | 1 × 108 PFU | No treatment | 7 days | Epigastric | 30 Sprague–Dawley male rats (300–400 g) | Subdermal injection just prior to flap elevation | 82.6 ± 4.3 | I—90.3 ± 4.0 II—97.7 ± 1.8 |
| Liu; 2009 (*) [23] | FGF-β | Virus | Unspecified | 7.5 × 1010 PFU | Saline | 7 days | McFarlane | 38 Sprague–Dawley female rats (250–300 g) | Intradermal injection 14 days, 7 days, or at the time of flap elevation (1, 2, 3) | 50.9 ± 5.2 | 1—56.1 ± 11.8 2—42.7 ± 8.4 3—57.6 ± 3.3 |
| Lee; 2011 [24] | Relaxin | Virus | Constant (up to 10 days) | 1 × 107 PFU | PBS (A) Sham virus (B) | 10 days | McFarlane | 30 Sprague–Dawley male rats (300–350 g) | Subdermal injection two days before and immediately after flap elevation | A—37.0 ± 7.0 B—43.0 ± 3.0 | 55.0 ± 5.0 |
| Jung; 2003 [25] | Ang1 | Virus | Unspecified | 1 × 108 PFU | No treatment (A) Sham virus (B) | 7 days | Epigastric | 19 Sprague–Dawley male rats (200–250 g) | Subdermal injection two days before flap elevation | A—67.76 ± 5.98 B—78.65 ± 6.44 | 84.24 ± 6.54 |
| Choi; 2020 [26] | DKK2 | Virus | Constant (up to 10 days) | 1 × 107 PFU | No treatment (A) Sham virus (B) | 10 days | McFarlane | 30 Sprague–Dawley male rats (300–350 g) | Subdermal injection two days before and immediately after flap elevation | A—no results B—57.5 ± 4.21 | 80.0 ± 4.49 |
| Lou; 2021 [27] | PLA2G4E | Virus | Constant (up to 7 days) | 5 × 109 PFU | Sham virus (measured here) | 7 days | McFarlane | 191 C57BL/6 J male mice (20–30g) | Subcutaneous injection of viral particles in 3 areas, 28 days prior to flap elevation | 56.81 ± 8.03 | 81.57 ± 4.87 |
| Gurunluoglu; 2002 (*) [28] | VEGF-164 | Virus | Unspecified | 108 PFU | Saline (A) Sham virus (B) | 7 days | Epigastric | 84 Sprague–Dawley male rats (300–350 g) | Subdermal injections, 12 h, 3 days, 7 days, and 14 days prior to flap elevation (1, 2, 3, 4) | A1—68.3 ± 4.3 B1—72.3 ± 13.1 A2—65.7 ± 3.2 B2—78.7 ± 6.9 A3—63.8 ± 7.0 B3—70.9 ± 13.9 A4—69.6 ± 5.3 B4—68.5 ± 10.1 | 1—86.0 ± 5.5 2—88.7 ± 2.4 3—89.3 ± 5.3 4—86.9 ± 4.6 |
| Gurunluoglu; 2005 (*) [29] | VEGF-121 | Virus | Constant (up to 21 days) | 108 PFU | Saline (A) Sham virus (B) | 7 days | Peninsular abdominal flap | 34 Sprague–Dawley male rats (300–500 g) | Subdermal injection at the time of flap elevation | A—65.6 ± 9.4 B—56.0 ± 3.4 | 88.9 ± 6.1 |
| Giunta; 2005 (*) [30] | VEGF-165 | Virus | Constant (up to 7 days) | 5 × 108 PFU 1 × 109 PFU(X) | Saline | 7 days | Abdominal random-pattern | 50 Sprague–Dawley male rats (350 g) | Subcutaneous injection at the time of flap elevation, 3 or 7 days before (1, 2, 3) | 1—24.65 ± 2.27 | 1—29.0 ± 6.39 2—45.19 ± 10.32 3—48.5 ± 4.12 3 X—60.81 ± 6.25 |
| Fujihara; 2005 (*) [31] | FGF-β | Plasmid | Constant (up to 3 days) | 300 µg | Sham plasmid | 7 days | Dorsal island skin | 52 Sprague–Dawley male rats (200–250 g) | Intramuscular injection with additional electroporation (E) two days before flap elevation | 71.9 ± 2.5 E—71.7 ± 4.1 | 70.3 ± 3.3 E—78.3 ± 5.3 |
| Ferraro; 2009 (*) [32] | VEGF-165 | Plasmid | Transient (at 12 days baseline expression) | 100 µg | No treatment (A) Sham plasmid (B) | 14 days | Single pedicle random | 51 Sprague–Dawley male rats (275–300 g) | Intradermal injection (group with additional electroporation, E) two days before flap elevation | A—73.5 ± 4.1 BE—70.6 ± 7.4 | 65.6 ± 9.3 E—95.2 ± 2.2 |
| De Freitas; 2010 (*) [33] | VEGF-165 | Plasmid | Unspecified | 100 µg | No treatment (A) PBS (B) Sham plasmid (C) | 5 days | Muscle | 32 Wistar male rats (350–400 g) | Intrafascial injection 30 days before flap elevation (during abdominoplasty) with electroporation in plasmid presence | A—75.35 ± 18.13 B—37.51 ± 28.06 C—42.2 ± 25.43 | 81.67 ± 16.20 |
| Chen; 2011 (*) [34] | VEGF-165 | Cells (transfection) | Transient (at 14 days baseline expression) | 5 × 106 cells | Non-transfected cells | 7 days | Rectangular full-thickness | 64 Wistar male and female rats (250–300 g) | Subdermal injection with internal control at different times before flap elevation (0, 2, 4, and 7 days—1, 2, 3, 4) | 1—36.57 ± 5.28 2—35.43 ± 4.73 3—34.54 ± 4.68 4—35.62 ± 4.51 | 1—62.63 ± 8.53 2—67.00 ± 7.19 3—71.44 ± 5.56 4—53.71 ± 5.34 |
| Chang; 2021 [35] | HIF1α | Plasmid | Transient (decrease after 48 h) | 50 µg | Sham plasmid | 7 days (1) 14 days (2) | McFarlane | 20 Sprague–Dawley male rats | Intradermal injection seven days before flap elevation | 1—68.75 ± 36.07 2—80.31 ± 21.25 | 1—92.97 ± 40.02 2—92.58 ± 15.07 |
| Basu; 2014 [36] | VEGF-165 | Plasmid | Unspecified | 50 µg | No treatment | 7 days (1) 14 days (2) | McFarlane | 109 Sprague–Dawley male rats (275–300 g) | Intradermal injection at the time of flap elevation. Additional electrode array (E) during procedure. | 1—84.75 ± 6.8 1E—84.75 ± 6.8 2E—79.8 ± 5.2 | 1—88.7 ± 4.84 1E—98.48 ± 1.87 2E—98.22 ± 2.82 |
| Antonini; 2007 (*) [37] | VEGF-165 | Virus | Unspecified | 150 µL | Sham virus | 14 days | Epigastric (Epi) Muscle (M) | 48 Wistar male rats (260–290 g) | Subcutaneous/intramuscular injection at the time of flap elevation, 7 or 14 days prior (1, 2, 3) | Epi1—63.9 ± 0.6 Epi2—61.1 ± 2.2 Epi3—62.8 ± 1.4 M1—77.3 ± 1.2 M2—74 ± 3.0 M3—75.4 ± 0.8 | Epi1—72.2 ± 1.6 Epi2—76.7 ± 1.5 Epi3—78.3 ± 2.8 M1—77.6 ± 1.1 M2—83.9 ± 1.7 M3—87.7 ± 0.8 |
| Taub;1998 (*) [38] | VEGF-121 | Plasmid | Unspecified | 7 µg | Saline (A) Sham plasmid (B) | 7 days | Epigastric | 30 Sprague–Dawley female rats (250–300 g) | Intra-arterial injection during flap elevation | A—31.9 ± 12.64 B—28.1 ± 10.13 | 93.9 ± 4.91 |
| Rinsch; 2001 (*) [39] | VEGF-121 (I) VEGF-165 (II) FGF-β (III) | Cells (transfection) | Constant | 5 × 105 cells | No treatment (A) Capsule only (B) Capsule containing non-modified cells (C) | 7 days | McFarlane | 86 Wistar female rats (250–300 g) | Administration of capsule on the subcutaneous tissue of flap during elevation | A—50.0 ± 3.8 B—44.9 ± 7.8 C—53.2 ± 5.8 | I—47.2 ± 11.3 II—46.9 ± 9.7 III—65.8 ± 6.3 |
| O’Toole; 2002 (*) [40] | VEGF-165 (I) VEGF-167 (II) VEGF-186 (III) | Plasmid | Constant (up to 2 days) | 50 µg | Sham plasmid (A) Saline (B) | 7 days | Epigastric | 60 Sprague–Dawley male rats (400–550 g) | Subcutaneous injection at the time of flap elevation | A—83.2 ± 12.08 B—81.2 ± 12.08 | I—90.3 ± 8.4 II—92.1 ± 7.09 III—86.2 ± 6.57 |
| Yang; 2005 (*) [41] | VEGF-121 | Plasmid | Unspecified | 80 µg | Sham plasmid (A) Saline (B) | 7 days | McFarlane | 30 Sprague–Dawley female rats (280–320 g) | Intramuscular injection at the time of flap elevation | A—47.0 ± 6.0 B—46.0 ± 5.0 | 87.0 ± 5.0 |
| Zacchigna; 2005 (*) [42] | VEGF-165 | Virus | Unspecified | 1.5 × 1011 PFU | Sham plasmid | 7 days | Epigastric (Epi) Muscle (M) | 88 Wistar male rats (250–300 g) | Subcutaneous (E) or intramuscular (M) injection at the time of flap elevation (1), 7 days before (2) or 14 days before (3) | 1E—62.6 ± 1.8 2E—61.0 3E—63.0 1M—78.0 2M—75.0 3M—77.2 ± 2.7 | 1E—71.0 2E—77.0 3E—79.0 1M—78.0 2M—85.0 3M—91.1 ± 2.8 |
| Zhang; 2005 (*) [43] | VEGF-165 | Plasmid | Unspecified | 50 µg | Sham plasmid (A) Saline (B) | 4 days | Muscle | 52 Sprague–Dawley rats (380–420 g) | Subcutaneous injection 4 days before flap elevation | A—36.3 ±13.1 B—43.4 ± 11.3 | 63.7 ± 20.2 |
| Wang; 2006 (*) [44] | PDGF-B | Plasmid | Unspecified | 50 µg | Saline | 7 days | McFarlane | 20 Sprague–Dawley female rats (250–300 g) | Intradermal injection at the time of flap elevation | 52.4 ± 5.1 | 60.1 ± 7.2 |
| Yi; 2006 (*) [45] | VEGF-165 | Cells (transfection) | Transient (peak at day 4) | 5 × 105 cells | Non-transfected cells (A) culture medium (B) | 28 days | Cranially based | 30 athymic nude mice | Subcutaneous injection three days before flap elevation | A—59.37 ± 14.83 B—40.0 ± 16.65 | 68.16 ± 20.4 |
| Zheng; 2008 (*) [46] | VEGF-165 | Cells (transfection) | Transient (baseline expression at day 14) | 5 × 106 cells | Non-transfected cells (A) culture medium (B) | 14 days | McFarlane | 30 Sprague–Dawley rats (100–120 g) | Subcutaneous injection four days before flap elevation | A—66.4 ± 6.1 B—51.5 ± 7.5 | 83.1 ± 2.6 |
| Spanholtz; 2009 (*) [47] | VEGF-165 | Cells (transduction) | Transient (baseline expression at day 5) | 1 × 107 cells | Sham transduced cells (A) Non-transduced cells (B) saline (C) | 7 days | McFarlane | 80 Sprague–Dawley female rats (200–225 g) | Intradermal injection at different times before flap elevation: at the same time (1), 7 days (2), or 14 days before (3). Injection within the flap and into the surroundings | A—44.05 ± 3.92 B—45.0 C—47.0 | 1—47.60 ± 5.11 2—54.01 ± 5.95 3—46.54 ± 5.02 |
| Rezende; 2010 (*) [48] | VEGF-165 | Plasmid | Unspecified | 50 µg | Empty plasmid in two different areas (A, B), sham plasmid (C) Saline (D) | 5 days | Muscle | 49 Wistar male rats (300 g) | Intradermal injection at the time of flap elevation with electroporation in different areas (* or **) | A—77.20 ± 9.79 B—61.38 ± 12.50 C—no data D—75.0 ± 1.0 | *—60.77 ± 27.10 **—33.06 ± 31.76 |
| Spanholtz; 2011 (*) [49] | FGF-β (I) VEGF-165 (II) | Cells (transfection) | Transient (baseline expression at day 14) | 5 x106 cells (*) 1 × 107 cells(**) | Sham-modified fibroblasts (compared here) Non-modified fibroblast Cell medium only | 14 days | McFarlane | 320 Sprague–Dawley female rats (200–225 g) | Subdermal injections 7 days or 14 days (1, 2) before flap elevation within flap alone (*) and in flap surrounding and flap (**) | 1 *—51.02 ± 2.96 2 *—51.90 ± 1.43 1 **—52.79 ± 3.01 2 **—51.56 ± 1.43 | I + II * 1—58.76 ± 1.70 I + II * 2—60.38 ± 2.77 I + II ** 1—59.20 ± 2.17 I + II ** 2—62.16 ± 1.38 I * 1—53.48 ± 1.72 I * 2—54.22 ± 2.21 I ** 1—60.53 ± 1.68 I ** 2—57.57 ± 2.15 |
| Wang; 2011 (*) [50] | VEGF-165 | Virus | Unspecified | 3 × 1010 PFU | Sham virus (A) Saline (B) | 7 days | McFarlane | 30 Sprague–Dawley female rats (250–300 g) | Intradermal injection 14 days before flap elevation | A—45.9 ± 6.3 B—48.7 ± 4.9 | 55.8 ± 6.9 |
| Uemura; 2012 [51] | NF-kb | Oligonucleotide | Transient | 1–2 mg | Sham oligonucleotide (A) No treatment (B) | 5 days | Epigastric | 36 Sprague–Dawley male rats (350–400 g) | Intra-arterial injection at the time of flap elevation | A—31.1 ± 3.7 B—31.7 ± 4.8 | 57.9 ± 8.4 |
| Wang; 2013 [52] | KGF | Virus | Unspecified | 1 × 109 PFU | Sham virus with dexamethasone (A) Dexamethasone (B) PBS (C) | 35 days | McFarlane | 60 Sprague–Dawley male rats (300–350 g) | Subdermal injection in wound margin five days after flap elevation | A—86.19 ± 1.63 B—85.33 ± 1.98 C—84.20 ± 2.55 | 88.63 ± 0.69 |
| Jafari; 2017 (*) [53] | HGF | Plasmid | Unspecified | 25 µg | No treatment | 7 days | McFarlane | 15 Wistar male rats (290–320 g) | Intradermal injection 24 h before (1) or 24 h after (2) flap elevation followed by electroporation | 64.67 ± 3.90 | 1—72.86 ± 7.46 2—2.93 ± 4.81 |
| Jafari; 2018 [54] | IL-10 | Plasmid | Unspecified | 100 µg | No treatment | 7 days | McFarlane | 9 Wistar male rats (300–330 g) | Intradermal injection 24 h before flap elevation followed by electroporation | 64.77 ± 3.90 | 81.26 ± 4.70 |
| Jafari; 2021 [55] | IL-10 (I) HGF (II) VEGF-165 (III) | Plasmid | Unspecified | 100 µg | No treatment | 7 days | McFarlane | 15 Wistar male rats (290–320 g) | Intradermal injection 24 h before flap (1) or 24 h (2) after flap elevation followed by electroporation | 64.77 ± 3.90 | I1 + III2—81.66 ± 9.70 I1 + II2—74.06 ± 1.65 |
| Zhang; 2011 [56] | SDF-1 | Cells (transduction) (I) Virus (II) | Unspecified | 1 × 106 cells (I) 6.25 × 109 PFU (II) | Non-transduced MSC (A) saline (B) | 10 days | Epigastric | 24 Lewis rats (200–300 g) | Intravascular injection proximal to the anastomosis of femoral artery at the time of flap elevation | A—88.56 ± 3.72 B—79.12 ± 3.34 | I—96.82 ± 2.35 II—86.86 ± 4.09 |
| Luo; 2021 [57] | SDF-1α | Cells (modRNA transfection) | Constant (up to 14 days) | 5.4 × 107 cells | Sham transfected fibroblasts (A) PBS (B) | 10 days (extended to 28 days for the treatment group) | McFarlane | 60 Sprague–Dawley male rats (250–300 g) | Intradermal injection at the time of flap elevation | A—animals died B—animals died | after 28 days—92.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskal, W.; Gotowiec, M.; Stachura, A.; Kopka, M.; Włodarski, P. VEGF and Other Gene Therapies Improve Flap Survival—A Systematic Review and Meta-Analysis of Preclinical Studies. Int. J. Mol. Sci. 2024, 25, 2622. https://doi.org/10.3390/ijms25052622
Paskal W, Gotowiec M, Stachura A, Kopka M, Włodarski P. VEGF and Other Gene Therapies Improve Flap Survival—A Systematic Review and Meta-Analysis of Preclinical Studies. International Journal of Molecular Sciences. 2024; 25(5):2622. https://doi.org/10.3390/ijms25052622
Chicago/Turabian StylePaskal, Wiktor, Mateusz Gotowiec, Albert Stachura, Michał Kopka, and Paweł Włodarski. 2024. "VEGF and Other Gene Therapies Improve Flap Survival—A Systematic Review and Meta-Analysis of Preclinical Studies" International Journal of Molecular Sciences 25, no. 5: 2622. https://doi.org/10.3390/ijms25052622
APA StylePaskal, W., Gotowiec, M., Stachura, A., Kopka, M., & Włodarski, P. (2024). VEGF and Other Gene Therapies Improve Flap Survival—A Systematic Review and Meta-Analysis of Preclinical Studies. International Journal of Molecular Sciences, 25(5), 2622. https://doi.org/10.3390/ijms25052622








