Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas
Abstract
:1. Introduction
2. Results
2.1. The ieTILs Create an Interesting Phenomenon of Intracordal Tumor Colonization, Which Is Associated with the Neoadjuvant Therapy
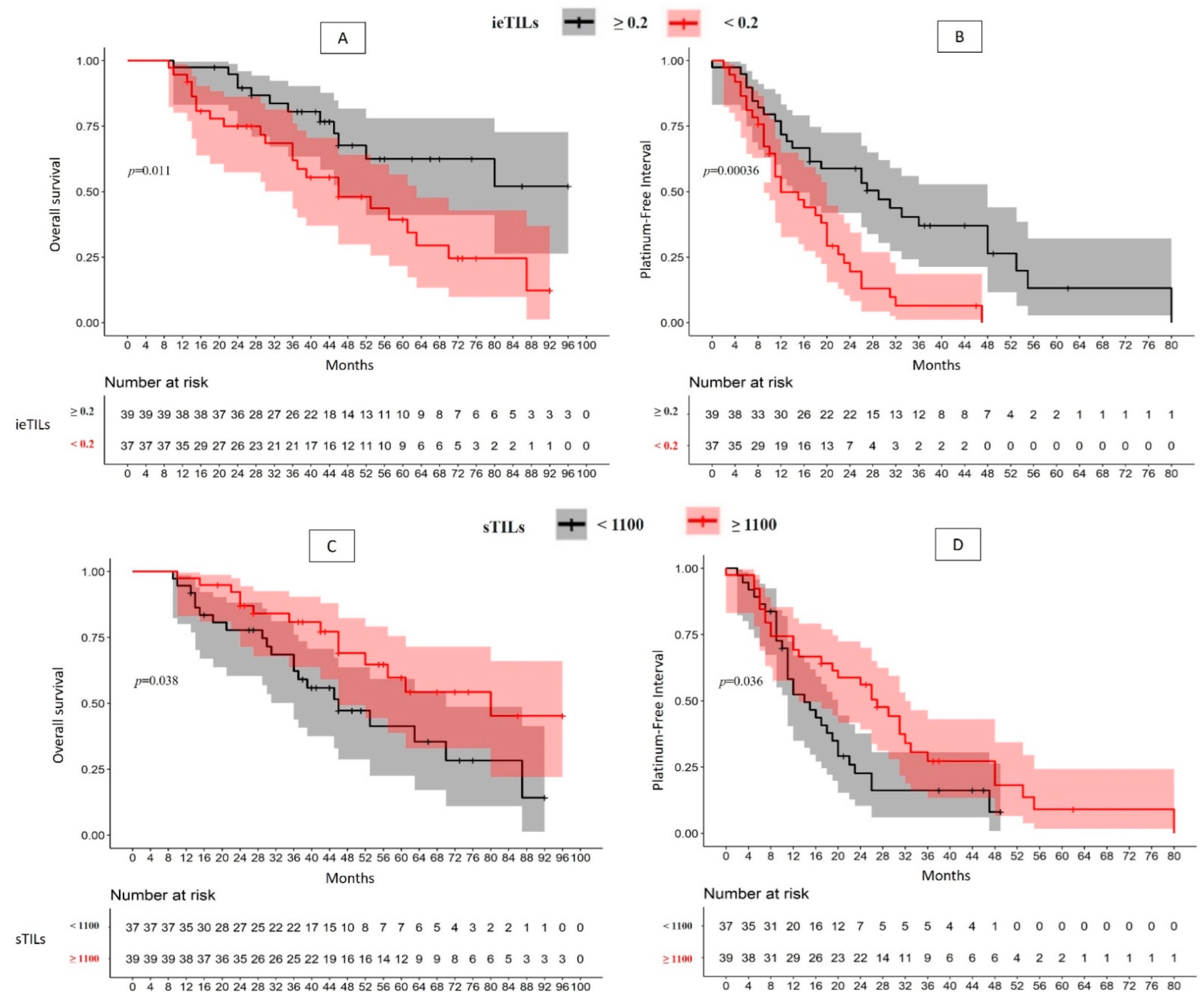
2.2. There Is a Significant Association between High ieTILs and sTILs Values with OS and PFI
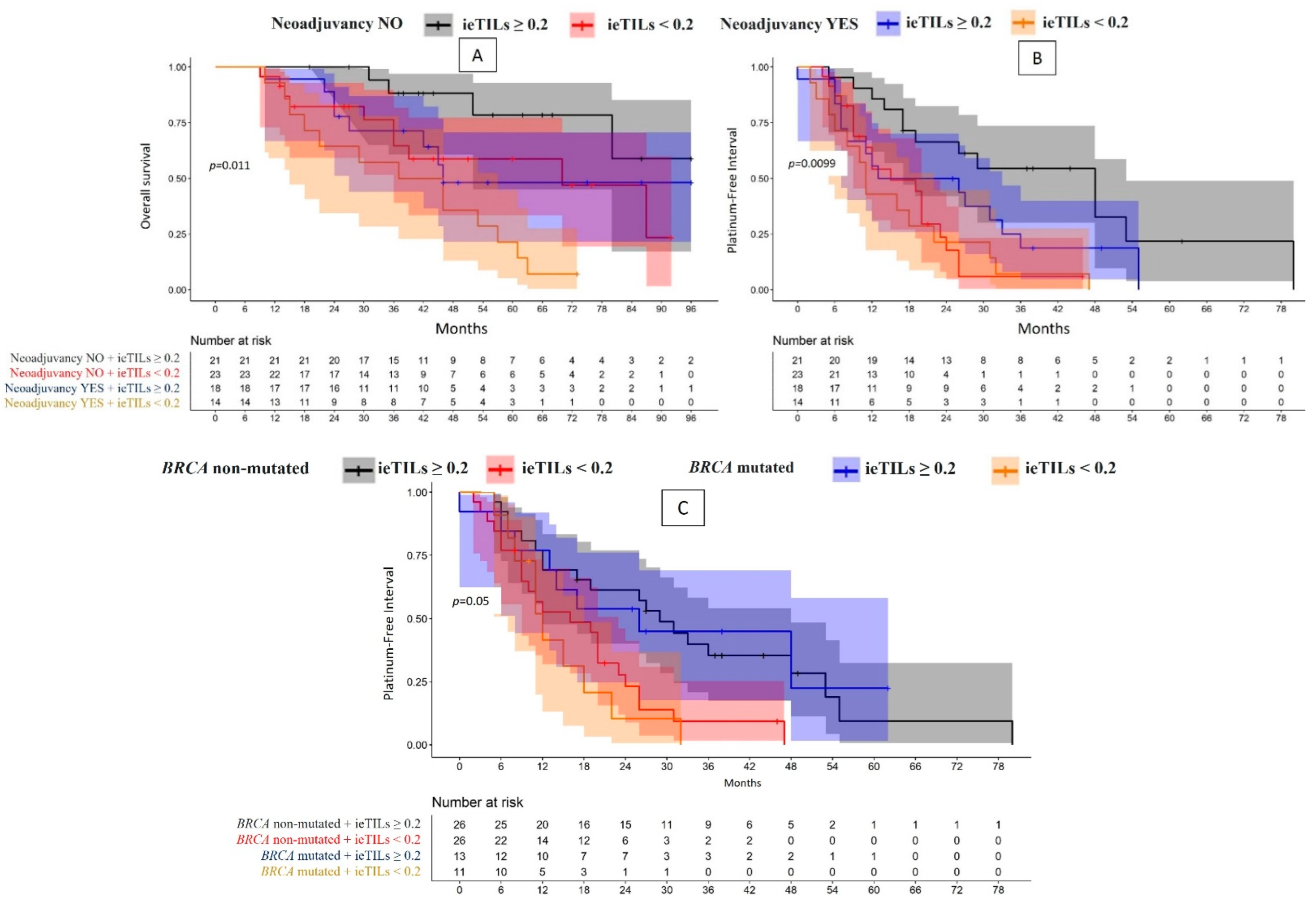
2.3. The Role of TILs as Prognostic Biomarkers Is Especially Relevant for Patients Who Have Not Undergone Neoadjuvant Therapy
2.4. Although the ieTIL Concentration Was Not Higher in the Tumors Mutated for Gene BRCA, This Immune Component Appeared to Be More Efficient in the Tumors in Which the Mutation Was Present
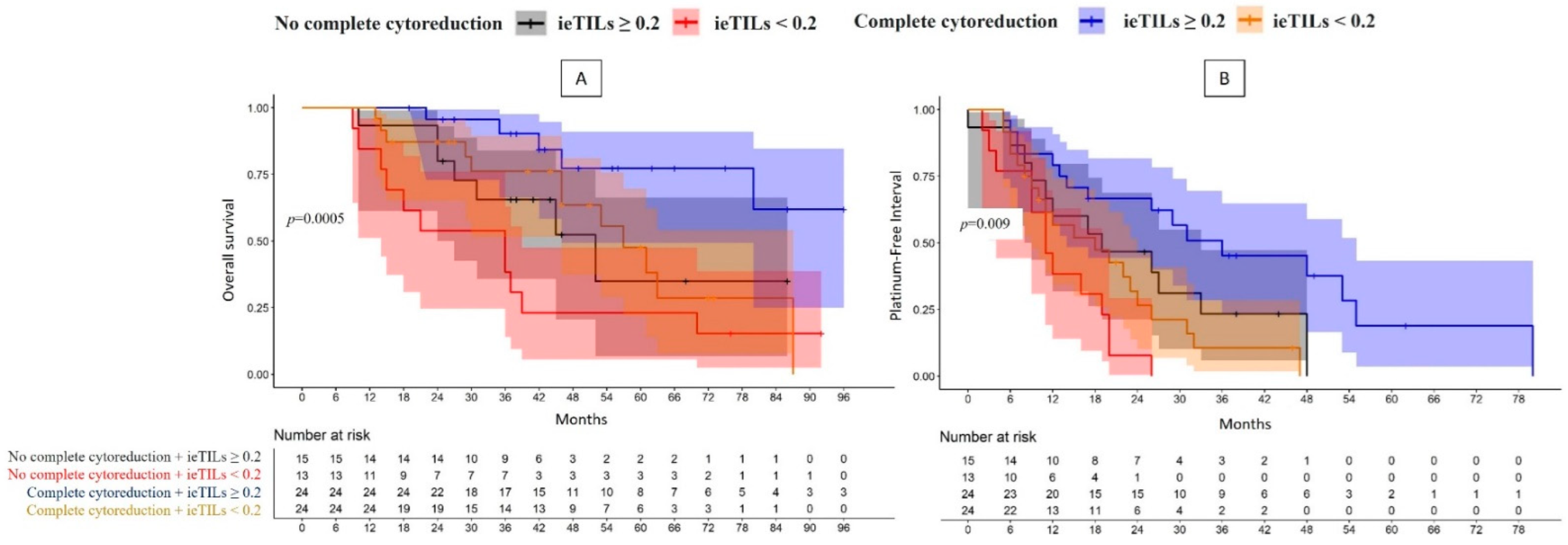
2.5. The Patients with Both a Complete Surgical Resection and High Tumor ieTIL Concentration Had an Especially Favorable Prognosis
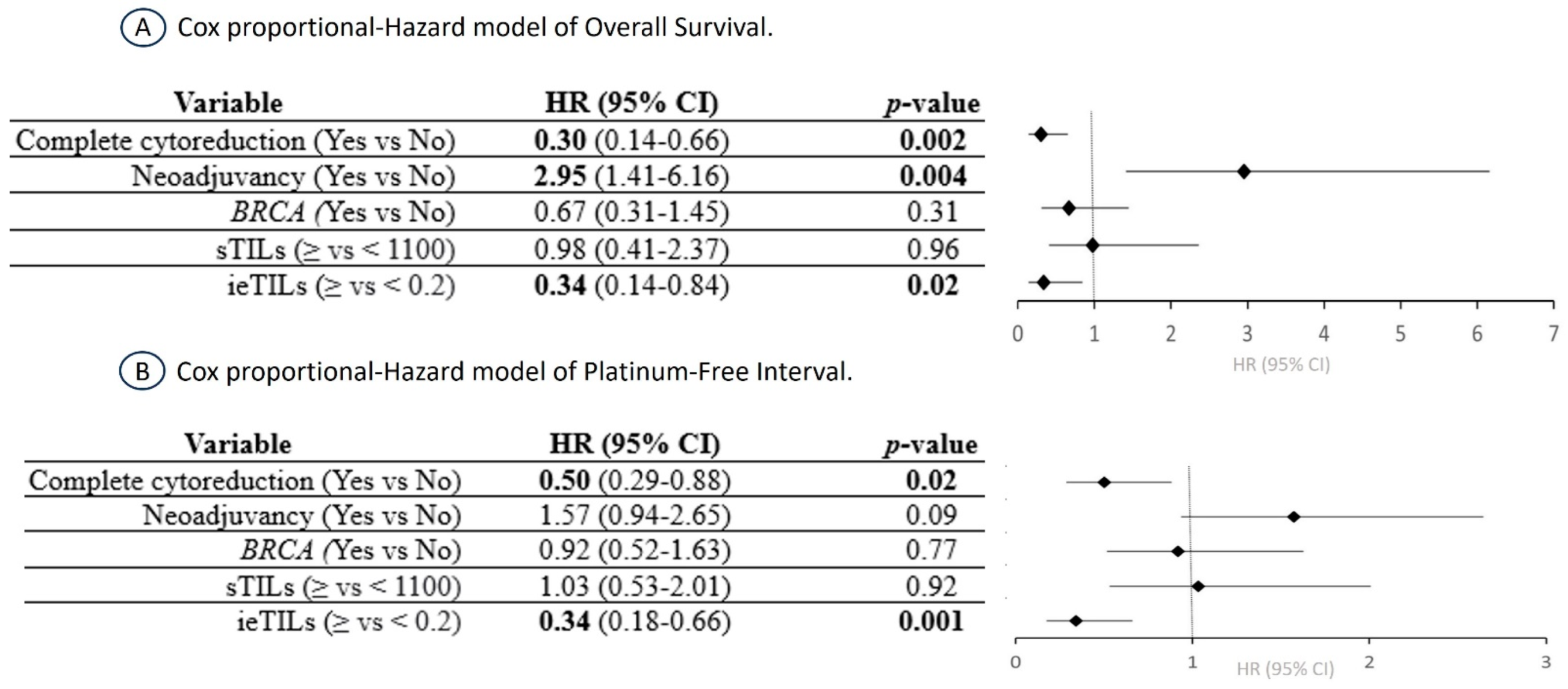
2.6. The ieTIL Concentration Was an Independent Prognostic Factor Both for OS and PFI in the Multivariate Analysis
3. Discussion
3.1. The Importance of TIL Quantification According to Its Relationship with Tumor Cords
3.2. The Importance of ieTILs as an Independent Prognostic Factor
3.3. Neoadjuvant Therapy and BRCA Gene Mutation Appear to Influence the Prognostic Effect of TILs
3.4. The ieTILs as Prognostic Modifier in Tumor Resection Groups
3.5. The Predictive Role of TILs, Especially ieTILs, and the Potential Application as an Immunotherapy Biomarker
3.6. Considerations of the Study’s Methodological Limitations
4. Material and Methods
- -
- Intraepithelial TILs (ieTILs) = ratio of the total number of immune cells in close contact with the tumor epithelium/total number of tumor cells.
- -
- Stromal TILs (sTILs) = total number of immune cells in the stromal compartment/surface area of the tumor stroma (mm2).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCO | American Society of Clinical Oncology |
| BRCA | breast cancer gene |
| H&E | hematoxylin-eosin |
| HGSOC | high-grade serous ovarian carcinoma |
| HR | hazard ratio |
| ieTILs | intraepithelial tumor-infiltrating lymphocytes |
| iPARP | inhibitors of the poly-ADP-ribose polymerase |
| OS | overall survival |
| PD-L1 | programmed death ligand 1 |
| PFI | platinum-free interval |
| sTILs | stromal tumor-infiltrating lymphocytes |
| TILs | tumor-infiltrating lymphocytes |
References
- Redondo, A.; Guerra, E.; Manso, L.; Martin-Lorente, C.; Martinez-Garcia, J.; Perez-Fidalgo, J.A.; Varela, M.Q.; Rubio, M.J.; Barretina-Ginesta, M.P.; González-Martín, A. SEOM clinical guideline in ovarian cancer. Clin. Transl. Oncol. 2021, 23, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Herrington, C.S. (Ed.) WHO Classification of Tumours Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020.
- Tossetta, G.; Fantone, S.; Goteri, G.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D. The Role of NQO1 in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 7839. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.; Morand, S.; Staats, H.; Royfman, R.; Devanaboyina, M.; Einloth, K.; Dever, D.; Stanbery, L.; Aaron, P.; Manning, L.; et al. Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J. Cancer 2021, 12, 38–53. [Google Scholar] [CrossRef]
- Loizzi, V.; Ranieri, G.; Laforgia, M.; Gadaleta, C.D.; Gargano, G.; Kardhashi, A.; De Liso, M.; Naglieri, E.; Del Vecchio, V.; Cicinelli, E.; et al. PARP inhibitors and epithelial ovarian cancer: Molecular mechanisms, clinical development and future prospective. Oncol. Lett. 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Mouret-Reynier, M.A.; Pignata, S.; Cropet, C.; González-Martín, A.; Bogner, G.; Fujiwara, K.; Vergote, I.; Colombo, N.; Nottrup, T.J.; et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol. Oncol. 2022, 164, 254–264. [Google Scholar] [CrossRef]
- Awada, A.; Ahmad, S.; McKenzie, N.D.; Holloway, R.W. Immunotherapy in the Treatment of Platinum-Resistant Ovarian Cancer: Current Perspectives. OncoTargets Ther. 2022, 15, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, A.; Jang, H. Immunotherapeutic Approaches in Ovarian Cancer. Curr. Issues Mol. Biol. 2023, 45, 1233–1249. [Google Scholar] [CrossRef]
- Tancoš, V.; Blichárová, A. Predictive biomarkers of response to immunotherapy in triple-negative breast cancer-state of the art and future perspectives. Klin. Onkol. 2023, 36, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Magraner, L.; Gumuzio, J.; Miles, J.; Quimi, N.; Martínez Del Prado, P.; Abad-Villar, M.T.; Pikabea, F.; Ortega, L.; Etxezarraga, C.; Martín-Algarra, S.; et al. Functional Engagement of the PD-1/PD-L1 Complex But Not PD-L1 Expression Is Highly Predictive of Patient Response to Immunotherapy in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Rakaee, M.; Adib, E.; Ricciuti, B.; Sholl, L.M.; Shi, W.; Alessi, J.V.; Cortellini, A.; Flugenzi, C.A.M.; Viola, P.; Pinato, D.J.; et al. Association of Machine Learning-Based Assessment of Tumor-Infiltrating Lymphocytes on Standard Histologic Images With Outcomes of Immunotherapy in Patients with NSCLC. JAMA Oncol. 2023, 9, 51–60. [Google Scholar] [CrossRef]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and Prognostic Significance of BRCA1/2-Mutation Status with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes and Expression of PD-1/PD-L1 in High Grade Serous Ovarian Cancer. Oncotarget 2016, 7, 13587–13598. [Google Scholar] [CrossRef] [PubMed]
- Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; Bouligny, A.L.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef]
- James, F.; Jiminez-Linan, M.; Alsop, J.; Mack, M.; Song, H.; Brenton, J.D.; Paul, D.P.; Pharoah, H.; Raza, A. Association between Tumour Infiltrating Lymphocytes, Histotype and Clinical Outcome in Epithelial Ovarian Cancer. BMC Cancer 2017, 17, 657. [Google Scholar] [CrossRef]
- Hwang, C.; Lee, S.; Lee, J.; Kim, K.; Suh, D.; Kwon, B.; Choi, K. Stromal Tumor-Infiltrating Lymphocytes Evaluated on H&E-Stained Slides Are an Independent Prognostic Factor in Epithelial Ovarian Cancer and Ovarian Serous Carcinoma. Oncol. Lett. 2019, 17, 4557–4565. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, S.J.L.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C.; et al. Neoadjuvant Chemotherapy (NACT) Increases Immune Infiltration and Programmed Death-Ligand 1 (PD-L1) Expression in Epithelial Ovarian Cancer (EOC). Ann. Oncol. 2017, 28, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, J.P.; Mai Chan, L.; Haruki, K.; Dias Costa, A.; Borowsky, J.; Zhao, M.; Fujiyoshi, K.; Arima, K.; Twombly, T.S.; Kishikawa, J.; et al. Prognostic Significance of Immune Cell Populations Identified by Machine Learning in Colorectal Cancer Using Routine Hematoxylin and Eosin–Stained Sections. Clin. Cancer Res. 2020, 26, 4326–4338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van de Vijver, K.; Estrada, M.V.; González-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Leary, A.; Genestie, C.; Blanc-Durand, F.; Gouy, S.; Dunant, A.; Maulard, A.; Drusch, F.; Cheaib, B.; Michels, J.; Bentivegna, E.; et al. Neoadjuvant Chemotherapy Alters the Balance of Effector to Suppressor Immune Cells in Advanced Ovarian Cancer. Cancer Immunol. Immunother. 2020, 70, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-Associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.S.; Sanii, S.; Kroeger, D.R.; Milne, K.; Talhouk, A.; Chiu, D.S.; Rahimi, K.; Shaw, P.; Clarke, B.A.; Nelson, B.H. Neoadjuvant Chemotherapy of Ovarian Cancer Results in Three Patterns of Tumor-Infiltrating Lymphocyte Response with Distinct Implications for Immunotherapy. Clin. Cancer Res. 2017, 23, 925–934. [Google Scholar] [CrossRef]
- Wouters, M.A.C.; Komdeur, F.L.; Workel, H.H.; Klip, H.G.; Plat, A.; Kooi, N.M.; Wisman, B.A.; Mourits, M.J.E.; Arts, H.J.G.; Oonk, M.H.; et al. Treatment Regimen, Surgical Outcome, and T-Cell Differentiation Influence Prognostic Benefit of Tumor-Infiltrating Lymphocytes in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2016, 22, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, A.S.; Genestie, C.; Auguste, A.; Leary, A. Impact of Neoadjuvant Chemotherapy on the Immune Microenvironment in Advanced Epithelial Ovarian Cancer: Prognostic and Therapeutic Implications. Int. J. Cancer 2017, 143, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Scurry, J.; van Zyl, B.; Gulliver, D.; Otton, G.; Jaaback, K.; Lombard, J.; Vilain, R.E.; Bowden, N.A. Nucleotide Excision Repair Protein ERCC1 and Tumour-Infiltrating Lymphocytes Are Potential Biomarkers of Neoadjuvant Platinum Resistance in High Grade Serous Ovarian Cancer. Gynecol. Oncol. 2018, 151, 306–310. [Google Scholar] [CrossRef]
- Wieser, V.; Gaugg, I.; Fleischer, M.; Shivalingaiah, G.; Wenzel, S.; Sprung, S.; Lax, S.F.; Zeimet, A.G.; Fiegl, H.; Marth, C. BRCA1/2 and TP53 Mutation Status Associates with PD-1 and PD-L1 Expression in Ovarian Cancer. Oncotarget 2018, 9, 17501–17511. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Porter, H.; Köbel, M.; Nelson, B.H.; Prentice, L.M.; Kalloger, S.E.; Senz, J.; Milne, K.; Ding, J.; Shah, S.P.; et al. BRCA1 and BRCA2 Mutations Correlate with TP53 Abnormalities and Presence of Immune Cell Infiltrates in Ovarian High-Grade Serous Carcinoma. Mod. Pathol. 2012, 25, 740–750. [Google Scholar] [CrossRef]
- Fujiwara, M.; McGuire, V.A.; Felberg, A.; Sieh, W.; Whittemore, A.S.; Longacre, T.A. Prediction of BRCA1 Germline Mutation Status in Women with Ovarian Cancer Using Morphology-Based Criteria. Am. J. Surg. Pathol. 2012, 36, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.B.; Toukatly, M.N.; Kilgore, M.R.; Agnew, K.J.; Bernards, S.S.; Norquist, B.M.; Pennington, K.P.; Garcia, R.L.; Liao, J.B.; Swisher, E.M. Tumor Infiltrating Lymphocytes and Homologous Recombination Deficiency Are Independently Associated with Improved Survival in Ovarian Carcinoma. Gynecol. Oncol. 2019, 153, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Trillsch, F.; Okamoto, A.; Reuss, A.; Kim, J.W.; Rubio-Pérez, M.J.; Vardar, M.A.; Scambia, G.; Tredan, O.; Nyvang, G.B.; et al. Durvalumab with paclitaxel/carboplatin (PC) and bevacizumab (bev), followed by maintenance durvalumab, bev, and olaparib in patients (pts) with newly diagnosed advanced ovarian cancer (AOC) without a tumor BRCA1/2 mutation (non-tBRCAm): Results from the randomized, placebo (pbo)-controlled phase III DUO-O trial. J. Clin. Oncol. 2023, 41 (Suppl. 17), LBA5506. [Google Scholar]
- Zhang, W.; Li, S.; Zhang, C.; Mu, Z.; Chen, K.; Xu, Z. Tumor-infiltrating lymphocytes predict efficacy of immunotherapy in advanced non-small cell lung cancer: A single-center retrospective cohort study. Acta Oncol. 2023, 62, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Fanale, D.; Dimino, A.; Pedone, E.; Brando, C.; Corsini, L.R.; Filorizzo, C.; Fiorino, A.; Lisanti, M.C.; Magrin, L.; Randazzo, U.; et al. Prognostic and Predictive Role of Tumor-Infiltrating Lymphocytes (TILs) in Ovarian Cancer. Cancers 2022, 14, 4344. [Google Scholar] [CrossRef] [PubMed]

| Overall Survival | Platinum-Free Interval | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % survivors (median) | p | HR (95% CI) | Median | p | HR (95% CI) | ||||
| Overall (76) | ieTILs | High | 51.1% | p = 0.01 | 2.40 (1.19–4.84); p = 0.01 | 29 m | p = 0.0004 | 2.59 (1.50–4.50); p = 0.0007 | |
| Low | 12.3% (46 m) | 12 m | |||||||
| sTILs | High | 45.3% (80 m) | p = 0.04 | 2.02 (1.02–3.99); p = 0.04 | 27 m | p = 0.04 | 1.73 (1.02–2.93); p = 0.04 | ||
| Low | 14.2% (46 m) | 14 m | |||||||
| Neoadjuvant therapy | YES (32) | ieTILs | High | 48.1% (46 m) | p = 0.01 | 2.88 (0.86–9.58); p = 0.1 | 13 m | p = 0.01 | 2.09 (0.97–4.48); p = 0.06 |
| Low | 7.14% (37 m) | 6.67 (2.15–20.76); p = 0.001 | 11 m | 4.09 (1.83–9.16); p = 0.0006 | |||||
| NO (44) | High | 58.8% | 1 | 48 m | 1 | ||||
| Low | 23.5% (70 m) | 2.93 (0.92–9.37); p = 0.07 | 15 m | 3.45 (1.60–7.45); p = 0.002 | |||||
| YES (32) | sTILs | High | 27.4% (46 m) | p = 0.02 | 4.99 (1.39–17.91); p = 0.01 | 13 m | p = 0.053 | 1.78 (0.91–5.03) p = 0.09 | |
| Low | 12.8% (45 m) | 7.53 (2.05–27.70); p = 0.002 | 11 m | 2.89 (1.78–9.83); p = 0.01 | |||||
| NO (44) | High | 63% | 1 | 29 m | 1 | ||||
| Low | 21.2% (70 m) | 4.38 (1.22–15.75); p = 0.02 | 15 m | 2.68 (1.22–5.91); p = 0.01 | |||||
| Somatic BRCA | Mutated (24) | ieTILs | High | 68.8% | p = 0.1 | 1 | 26 m | p = 0.05 | 1 |
| Low | 0% (53 m) | 4.16 (1.09–15.88); p = 0.04 | 12 m | 3.08 (1.37–6.92); p = 0.006 | |||||
| Non-mutated (52) | High | 45.2% (80 m) | 1..09 (0.33–3.05); p = 0.8 | 29 m | 1.01 (0.46–2.32); p = 0.99 | ||||
| Low | 17.7% (46 m) | 1.93 (0.84–4.42); p = 0.1 | 16 m | 2.44 (1.28–4.63); p = 0.007 | |||||
| Mutated (24) | sTILs | High | 63% | p = 0.2 | 1.40 (0.47–4.23); p = 0.6 | 26 m | p = 0.22 | 0.83 (0.38–1.81); p = 0.6 | |
| Low | 21.2% (70 m) | 2.75 (0.96–7.84); p = 0.6 | 12 m | 2.69 (1.17–6.20); p = 0.02 | |||||
| Non-mutated (52) | High | 55.9% | 1 | 27 m | 1 | ||||
| Low | 22% (46 m) | 2.10 (0.92–4.82); p = 0.08 | 17 m | 1.42 (0.76–2.64); p = 0.3 | |||||
| Complete cytorreduction | YES (48) | ieTILs | High | 61.8% | p = 0.0005 | 1 | 36 m | p = 0.009 | 1 |
| Low | 0% (57 m) | 3.05 (1.07–8.71); p = 0.04 | 18 m | 2.99 (1.44–6.21); p = 0.003 | |||||
| NO (28) | High | 34.9% (52 m) | 3.27 (1.03–10.42); p = 0.04 | 19 m | 2.10 (0.95–4.65); p = 0.07 | ||||
| Low | 15.4% (36 m) | 6.27 (2.17–18.09); p = 0.0007 | 11 m | 5.29 (2.31–12.11); p = 0.00008 | |||||
| YES (48) | sTILS | High | 47.5% (80 m) | p = 0.009 | 1 | 29 m | p = 0.1 | 1 | |
| Low | 0% (63 m) | 1.78 (0.68–4.63); p = 0.2 | 15 m | 1.41 (0.70–2.86); p = 0.3 | |||||
| NO (28) | High | 38.9% (52 m) | 2.35 (0.78–7.07); p = 0.1 | 19 m | 1.35 (0.59–3.06); p = 0.5 | ||||
| Low | 20.7% (36 m) | 3.48 (1.48–7.07); p = 0.004 | 12 m | 2.55 (1.30–4.99); p = 0.006 | |||||
| Age (Years) | 36–83 (Median 59.2) |
|---|---|
| FIGO stage | IIIA: 7; IIIB: 14; IIIC: 35 IVA: 8; IVB: 12 |
| Neoadyuvancy treatment | 32/76 (42%) |
| BRCA mutation | 24/76 (31.6%) |
| Overall survival (months) | 10–134 (median 61) |
| Platinum-free interval (months) | 0–80 (median 19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machuca-Aguado, J.; Conde-Martín, A.F.; Alvarez-Muñoz, A.; Rodríguez-Zarco, E.; Polo-Velasco, A.; Rueda-Ramos, A.; Rendón-García, R.; Ríos-Martin, J.J.; Idoate, M.A. Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas. Int. J. Mol. Sci. 2023, 24, 16060. https://doi.org/10.3390/ijms242216060
Machuca-Aguado J, Conde-Martín AF, Alvarez-Muñoz A, Rodríguez-Zarco E, Polo-Velasco A, Rueda-Ramos A, Rendón-García R, Ríos-Martin JJ, Idoate MA. Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas. International Journal of Molecular Sciences. 2023; 24(22):16060. https://doi.org/10.3390/ijms242216060
Chicago/Turabian StyleMachuca-Aguado, Jesús, Antonio Félix Conde-Martín, Alejandro Alvarez-Muñoz, Enrique Rodríguez-Zarco, Alfredo Polo-Velasco, Antonio Rueda-Ramos, Rosa Rendón-García, Juan José Ríos-Martin, and Miguel A. Idoate. 2023. "Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas" International Journal of Molecular Sciences 24, no. 22: 16060. https://doi.org/10.3390/ijms242216060
APA StyleMachuca-Aguado, J., Conde-Martín, A. F., Alvarez-Muñoz, A., Rodríguez-Zarco, E., Polo-Velasco, A., Rueda-Ramos, A., Rendón-García, R., Ríos-Martin, J. J., & Idoate, M. A. (2023). Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas. International Journal of Molecular Sciences, 24(22), 16060. https://doi.org/10.3390/ijms242216060






