HCV and HCC Tango—Deciphering the Intricate Dance of Disease: A Review Article
Abstract
1. Introduction
2. HCV Infection and Development of HCC—Key Aspects of Pathogenesis
2.1. Chronic Inflammation
2.2. Liver Fibrosis and Cirrhosis
2.3. Immune Response Dysfunction
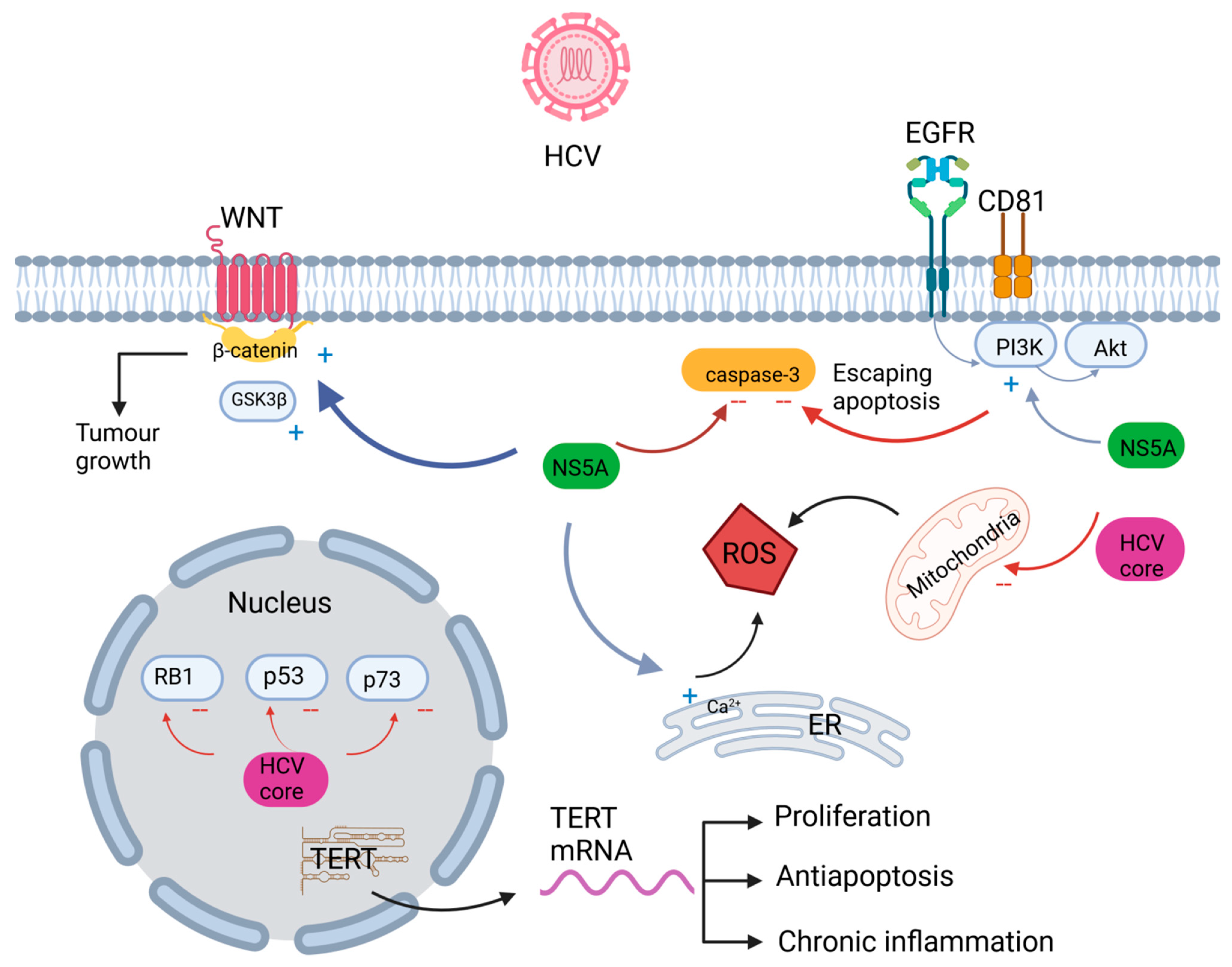
2.4. Oncogenic Signaling Pathways
2.5. Epigenetic Changes
2.6. MicroRNA Dysregulation
3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primer. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.P. Hepatocellular carcinoma: An overview: Hepatocellular carcinoma. Histopathology 2001, 39, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jinjuvadia, R.; Patel, S.; Liangpunsakul, S. The Association between Metabolic Syndrome and Hepatocellular Carcinoma: Systemic Review and Meta-analysis. J. Clin. Gastroenterol. 2014, 48, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Mahale, P.; Torres, H.A.; Kramer, J.R.; Hwang, L.Y.; Li, R.; Brown, E.L.; Engels, E.A. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study: HCV Infection and the Risk of Cancers. Cancer 2017, 123, 1202–1211. [Google Scholar] [CrossRef]
- Huang, Y.T.; Jen, C.L.; Yang, H.I.; Lee, M.H.; Su, J.; Lu, S.N.; Iloeje, U.H.; Chen, C.J. Lifetime Risk and Sex Difference of Hepatocellular Carcinoma among Patients with Chronic Hepatitis B and C. J. Clin. Oncol. 2011, 29, 3643–3650. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273.e1. [Google Scholar] [CrossRef]
- Sapena, V.; Enea, M.; Torres, F.; Celsa, C.; Rios, J.; Rizzo, G.E.M.; Nahon, P.; Mariño, Z.; Tateishi, R.; Minami, T.; et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: An individual patient data meta-analysis. Gut 2022, 71, 593–604. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pocino, K.; Stefanile, A.; Basile, V.; Napodano, C.; D’Ambrosio, F.; Di Santo, R.; Callà, C.A.M.; Gulli, F.; Saporito, R.; Ciasca, G.; et al. Cytokines and Hepatocellular Carcinoma: Biomarkers of a Deadly Embrace. J. Pers. Med. 2022, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Vujovic, A.; Isakovic, A.M.; Misirlic-Dencic, S.; Juloski, J.; Mirkovic, M.; Cirkovic, A.; Djelic, M.; Milosevic, I. IL-23/IL-17 Axis in Chronic Hepatitis C and Non-Alcoholic Steatohepatitis—New Insight into Immunohepatotoxicity of Different Chronic Liver Diseases. Int. J. Mol. Sci. 2023, 24, 12483. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, M.H.; Jiang, J.D.; Peng, Z.G. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J. Gastroenterol. 2018, 24, 5297–5311. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Capone, F.; Guerriero, E.; Maio, P.; Colonna, G.; Castello, G. Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur. Cytokine Netw. 2010, 21, 251–256. [Google Scholar]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef]
- Marino, M.; Scuderi, F.; Mannella, F.; Bartoccioni, E. TGF-β1 and IL-10 modulate IL-1β-induced membrane and soluble ICAM-1 in human myoblasts. J. Neuroimmunol. 2003, 134, 151–157. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Syed, V. TGF-β Signaling in Cancer. J. Cell Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Aroucha, D.C.; Carmo, R.F.; Vasconcelos, L.R.S.; Lima, R.E.; Mendonça, T.F.; Arnez, L.E.; Cavalcanti, M.d.o.S.; Muniz, M.T.; Aroucha, M.L.; Siqueira, E.R.; et al. TNF-α and IL-10 polymorphisms increase the risk to hepatocellular carcinoma in HCV infected individuals: TNF-α and IL-10 Polymorphisms and HCC. J. Med. Virol. 2016, 88, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Hu, M.H.; Xu, F.G.; Xu, H.J.; She, J.J.; Xia, H.P. Understanding the inflammation-cancer transformation in the development of primary liver cancer. Hepatoma Res. 2018, 4, 29. [Google Scholar] [CrossRef]
- Wungu, C.D.K.; Ariyanto, F.C.; Prabowo, G.I.; Soetjipto; Handajani, R. Association between five types of Tumor Necrosis Factor-α gene polymorphism and hepatocellular carcinoma risk: A meta-analysis. BMC Cancer 2020, 20, 1134. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Sun, K.; Liu, W.; Sheng, D.; Zhao, S.; Gao, L.; Wei, L. Tumor necrosis factor-α promotes hepatocellular carcinogenesis through the activation of hepatic progenitor cells. Cancer Lett. 2018, 434, 22–32. [Google Scholar] [CrossRef]
- Medvedev, R.; Ploen, D.; Hildt, E. HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis. Oxid. Med. Cell. Longev. 2016, 2016, 9012580. [Google Scholar] [CrossRef]
- Schank, M.; Zhao, J.; Wang, L.; Nguyen, L.N.T.; Cao, D.; Dang, X.; Khanal, S.; Zhang, J.; Zhang, Y.; Wu, X.Y.; et al. Oxidative Stress Induces Mitochondrial Compromise in CD4 T Cells from Chronically HCV-Infected Individuals. Front. Immunol. 2021, 12, 760707. [Google Scholar] [CrossRef]
- Wang, T.; Weinman, S.A. Interactions Between Hepatitis C Virus and Mitochondria: Impact on Pathogenesis and Innate Immunity. Curr. Pathobiol. Rep. 2013, 1, 179–187. [Google Scholar] [CrossRef]
- Dang, X.; Ogbu, S.C.; Zhao, J.; Nguyen, L.N.T.; Cao, D.; Nguyen, L.N.; Khanal, S.; Schank, M.; Thakuri, B.K.C.; Wu, X.Y.; et al. Inhibition of topoisomerase IIA (Top2α) induces telomeric DNA damage and T cell dysfunction during chronic viral infection. Cell Death Dis. 2020, 11, 196. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A.; Karin, M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Beyer, T.A.; Xu, W.; Teupser, D.; Auf Dem Keller, U.; Bugnon, P.; Hildt, E.; Thiery, J.; Kan, Y.W.; Werner, S. Impaired liver regeneration in Nrf2 knockout mice: Role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008, 27, 212–223. [Google Scholar] [CrossRef]
- Rico Montanari, N.; Anugwom, C.M.; Boonstra, A.; Debes, J.D. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers 2021, 13, 4876. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From Cirrhosis to Hepatocellular Carcinoma: New Molecular Insights on Inflammation and Cellular Senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Omland, L.H.; Krarup, H.; Jepsen, P.; Georgsen, J.; Harritshøj, L.H.; Riisom, K.; Jacobsen, S.E.; Schouenborg, P.; Christensen, P.B.; Sørensen, H.T.; et al. Mortality in patients with chronic and cleared hepatitis C viral infection: A nationwide cohort study. J. Hepatol. 2010, 53, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Axley, P.; Ahmed, Z.; Ravi, S.; Singal, A.K. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. J Clin. Transl. Hepatol. 2018, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef]
- Patel, K.; Bedossa, P.; Castera, L. Diagnosis of Liver Fibrosis: Present and Future. Semin. Liver Dis. 2015, 35, 166–183. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.; Uribe, M.; Mendez-Sanchez, N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Liang, S.; Kisseleva, T.; Brenner, D.A. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front Physiol. 2016, 7, 17. Available online: http://journal.frontiersin.org/Article/10.3389/fphys.2016.00017/abstract (accessed on 1 September 2023). [CrossRef]
- Baglieri, J.; Brenner, D.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Lekakis, V.; Davakis, S.; Christodoulou, M.I.; Germanidis, G.; Theocharis, S. The Role of TLR4 in the Immunotherapy of Hepatocellular Carcinoma: Can We Teach an Old Dog New Tricks? Cancers 2023, 15, 2795. [Google Scholar] [CrossRef]
- Ramirez, M.; Fernandez, M. Pathological Angiogenesis: The New Culprit behind Chronic Liver Disease. OBM Hepatol. Gastroenterol. 2019, 3, 15. [Google Scholar] [CrossRef]
- Allegretti, A.S.; Vela Parada, X.; Ortiz, G.A.; Long, J.; Krinsky, S.; Zhao, S.; Fuchs, B.C.; Sojoodi, M.; Zhang, D.; Karumanchi, S.A.; et al. Serum Angiopoietin-2 Predicts Mortality and Kidney Outcomes in Decompensated Cirrhosis. Hepatology 2019, 69, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Vanderborght, B.; Lefere, S.; Vlierberghe, H.V.; Devisscher, L. The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma. Cells 2020, 9, 2382. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.A.; Casciato, P.C.; Caldirola, M.S.; Gaillard, M.I.; Giadans, C.; Ameigeiras, B.; De Matteo, E.N.; Preciado, M.V.; Valva, P. Chronic Hepatitis C Pathogenesis: Immune Response in the Liver Microenvironment and Peripheral Compartment. Front. Cell. Infect. Microbiol. 2021, 11, 712105. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host–Virus Interaction and Mechanisms of Viral Persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef]
- Li, K.; Foy, E.; Ferreon, J.C.; Nakamura, M.; Ferreon, A.C.M.; Ikeda, M.; Ray, S.C.; Gale, M., Jr.; Lemon, S.M. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 2005, 102, 2992–2997. [Google Scholar] [CrossRef]
- Amjad, M.; Abdel-Haq, N.; Faisal, M.; Kamal, M.; Moudgal, V. Decreased interferon-α production and impaired regulatory function of plasmacytoid dendritic cells induced by the hepatitis C virus NS 5 protein. Microbiol. Immunol. 2008, 52, 499–507. [Google Scholar] [CrossRef]
- Song, X.; Yao, Z.; Yang, J.; Zhang, Z.; Deng, Y.; Li, M.; Ma, C.; Yang, L.; Gao, X.; Li, W.; et al. HCV core protein binds to gC1qR to induce A20 expression and inhibit cytokine production through MAPKs and NF-κB signaling pathways. Oncotarget 2016, 7, 33796–33808. [Google Scholar] [CrossRef]
- Zaki, M.Y.W.; Fathi, A.M.; Samir, S.; Eldafashi, N.; William, K.Y.; Nazmy, M.H.; Fathy, M.; Gill, U.S.; Shetty, S. Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma. Cancers 2022, 14, 1255. [Google Scholar] [CrossRef]
- Siavoshian, S.; Abraham, J.D.; Thumann, C.; PauleKieny, M.; Schuster, C. Hepatitis C virus core, NS3, NS5A, NS5B proteins induce apoptosis in mature dendritic cells. J. Med. Virol. 2005, 75, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; Jang, J.W. Natural Killer Cell Dysfunction in Hepatocellular Carcinoma: Pathogenesis and Clinical Implications. Int. J. Mol. Sci. 2018, 19, 3648. [Google Scholar] [CrossRef] [PubMed]
- Chu-sung, P.; Nakamoto, N.; Taniki, N.; Ojiro, K.; Amiya, T.; Makita, Y.; Murata, H.; Yamaguchi, A.; Shiba, S.; Miyake, R.; et al. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS ONE 2017, 12, e0179096. [Google Scholar]
- Oliviero, B.; Varchetta, S.; Paudice, E.; Michelone, G.; Zaramella, M.; Mavilio, D.; De Filippi, F.; Bruno, S.; Mondelli, M.U. Natural Killer Cell Functional Dichotomy in Chronic Hepatitis B and Chronic Hepatitis C Virus Infections. Gastroenterology 2009, 137, 1151–1160.e7. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.C.; Sung, P.S.; Park, S.H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016, 16, 509–523. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, B.; Chen, D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell. Mol. Life Sci. 2022, 79, 191. [Google Scholar] [CrossRef]
- Lasfar, A.; Gogas, H.; Zloza, A.; Kaufman, H.L.; Kirkwood, J.M. IFN-λ cancer immunotherapy: New kid on the block. Immunotherapy 2016, 8, 877–888. [Google Scholar] [CrossRef]
- Hofmann, M.; Tauber, C.; Hensel, N.; Thimme, R. CD8+ T Cell Responses during HCV Infection and HCC. J. Clin. Med. 2021, 10, 991. [Google Scholar] [CrossRef]
- Wieland, D.; Kemming, J.; Schuch, A.; Emmerich, F.; Knolle, P.; Neumann-Haefelin, C.; Held, W.; Zehn, D.; Hofmann, M.; Thimme, R. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat. Commun. 2017, 8, 15050. [Google Scholar] [CrossRef]
- Negash, A.A.; Olson, R.M.; Griffin, S.; Gale, M. Modulation of calcium signaling pathway by hepatitis C virus core protein stimulates NLRP3 inflammasome activation. Walker, C.M.; editor. PLoS Pathog. 2019, 15, e1007593. [Google Scholar] [CrossRef]
- Bolte, F.J.; O’Keefe, A.C.; Webb, L.M.; Serti, E.; Rivera, E.; Liang, T.J.; Ghany, M.; Rehermann, B. Intra-Hepatic Depletion of Mucosal-Associated Invariant T Cells in Hepatitis C Virus-Induced Liver Inflammation. Gastroenterology 2017, 153, 1392–1403.e2. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze ZurWiesch, J.; Von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate with Development of Hepatocellular Carcinoma in Patients with HCV Infection Treated with Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, T.; Zhou, Y.; Kawai, S.; Eguchi, H.; Wands, J.R.; Li, J. Hepatitis C virus core protein stimulates hepatocyte growth: Correlation with upregulation of wnt-1 expression. Hepatology 2005, 41, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Zemel, R.; Gerechet, S.; Greif, H.; Bachmatove, L.; Birk, Y.; Golan-Goldhirsh, A.; Kunin, M.; Berdichevsky, Y.; Benhar, I.; Tur-Kaspa, R. Cell transformation induced by hepatitis C virus NS3 serine protease. J. Viral Hepat. 2001, 8, 96–102. [Google Scholar] [CrossRef]
- Arima, N.; Kao, C.Y.; Licht, T.; Padmanabhan, R.; Sasaguri, Y.; Padmanabhan, R. Modulation of Cell Growth by the Hepatitis C Virus Nonstructural Protein NS5A. J Biol Chem. 2001, 276, 12675–12684. [Google Scholar] [CrossRef]
- Moriya, K.; Fujie, H.; Shintani, Y.; Yotsuyanagi, H.; Tsutsumi, T.; Ishibashi, K.; Matsuura, Y.; Kimura, S.; Miyamura, T.; Koike, K.; et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat. Med. 1998, 4, 1065–1067. [Google Scholar] [CrossRef]
- Shibata, T.; Aburatani, H. Exploration of liver cancer genomes. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 340–349. [Google Scholar] [CrossRef]
- Bressac, B.; Galvin, K.M.; Liang, T.J.; Isselbacher, K.J.; Wands, J.R.; Ozturk, M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1990, 87, 1973–1977. [Google Scholar] [CrossRef]
- Kao, C.F.; Chen, S.Y.; Chen, J.Y.; Wu Lee, Y.H. Modulation of p53 transcription regulatory activity and post-translational modification by hepatitis C virus core protein. Oncogene 2004, 23, 2472–2483. [Google Scholar] [CrossRef]
- Alisi, A.; Giambartolomei, S.; Cupelli, F.; Merlo, P.; Fontemaggi, G.; Spaziani, A.; Balsano, C. Physical and functional interaction between HCV core protein and the different p73 isoforms. Oncogene 2003, 22, 2573–2580. [Google Scholar] [CrossRef]
- Deng, L.; Nagano-Fujii, M.; Tanaka, M.; Nomura-Takigawa, Y.; Ikeda, M.; Kato, N.; Sada, K.; Hotta, H. NS3 protein of Hepatitis C virus associates with the tumour suppressor p53 and inhibits its function in an NS3 sequence-dependent manner. J. Gen. Virol. 2006, 87, 1703–1713. [Google Scholar] [CrossRef]
- Majumder, M.; Ghosh, A.K.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C Virus NS5A Physically Associates with p53 and Regulates p21/waf1 Gene Expression in a p53-Dependent Manner. J. Virol. 2001, 75, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; Jung, E.Y.; Ahn, J.Y.; Lee, M.N.; Jang, K.L. p53-dependent transcriptional repression of p21waf1 by hepatitis C virus NS3. J. Gen Virol. 2001, 82, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Munakata, T.; Nakamura, M.; Liang, Y.; Li, K.; Lemon, S.M. Down-regulation of the retinoblastoma tumor suppressor by the hepatitis C virus NS5B RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 2005, 102, 18159–18164. [Google Scholar] [CrossRef] [PubMed]
- Munakata, T.; Liang, Y.; Kim, S.; McGivern, D.R.; Huibregtse, J.; Nomoto, A.; Lemon, S.M. Hepatitis C Virus Induces E6AP-Dependent Degradation of the Retinoblastoma Protein. Young, J.A.T.; editor. PLoS Pathog. 2007, 3, e139. [Google Scholar] [CrossRef]
- Mileo, A.M.; Mattarocci, S.; Matarrese, P.; Anticoli, S.; Abbruzzese, C.; Catone, S.; Sacco, R.; Paggi, M.G.; Ruggieri, A. Hepatitis C virus core protein modulates pRb2/p130 expression in human hepatocellular carcinoma cell lines through promoter methylation. J. Exp. Clin. Cancer Res. 2015, 34, 140. [Google Scholar] [CrossRef]
- Kim, D.W.; Suzuki, R.; Harada, T.; Saito, L.; Miyamura, T. Trans-suppression of gene expression by hepatitis C viral core protein. Jpn. J. Med. Sci. Biol. 1994, 47, 211–220. [Google Scholar] [CrossRef][Green Version]
- Satyanarayana, A.; Manns, M.P.; Rudolph, K.L. Telomeres and telomerase: A dual role in hepatocarcinogenesis. Hepatology 2004, 40, 276–283. [Google Scholar] [CrossRef]
- Chen, Y.L.; Jeng, Y.M.; Chang, C.N.; Lee, H.J.; Hsu, H.C.; Lai, P.L.; Yuan, R.H. TERT promoter mutation in resectable hepatocellular carcinomas: A strong association with hepatitis C infection and absence of hepatitis B infection. Int. J. Surg. 2014, 12, 659–665. [Google Scholar] [CrossRef]
- Cho, J.; Baek, W.; Yang, S.; Chang, J.; Sung, Y.C.; Suh, M. HCV core protein modulates Rb pathway through pRb down-regulation and E2F-1 up-regulation. Biochim. Biophys. Acta 2001, 1538, 59–66. [Google Scholar] [CrossRef]
- Nault, J.C.; Calderaro, J.; Di Tommaso, L.; Balabaud, C.; Zafrani, E.S.; Bioulac-Sage, P.; Roncalli, M.; Zucman-Rossi, J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 2014, 60, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Li, K.; Beard, M.R.; Showalter, L.A.; Scholle, F.; Lemon, S.M.; Weinmann, S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002, 122, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Waris, G.; Tanveer, R.; Siddiqui, A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-κB. Proc. Natl. Acad. Sci. USA 2001, 98, 9599–9604. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Quarato, G.; Ripoli, M.; D’Aprile, A.; Scrima, R.; Cela, O.; Boffoli, D.; Moradpour, D.; Capitanio, N. HCV infection induces mitochondrial bioenergetic unbalance: Causes and effects. Biochim. Biophys. Acta BBA Bioenerg. 2009, 1787, 539–546. [Google Scholar] [CrossRef]
- Xing, L.; Tang, Y.; Li, L.; Tao, X. ROS in hepatocellular carcinoma: What we know. Arch. Biochem. Biophys. 2023, 744, 109699. [Google Scholar] [CrossRef]
- Wu, J.; Guo, L.; Qiu, X.; Ren, Y.; Li, F.; Cui, W.; Song, S. Genkwadaphnin inhibits growth and invasion in hepatocellular carcinoma by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation. Br. J. Cancer 2020, 123, 1673–1685. [Google Scholar] [CrossRef]
- Mahmoudvand, S.; Shokri, S.; Taherkhani, R.; Farshadpour, F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 42–58. [Google Scholar] [CrossRef]
- Taniguchi, H.; Kato, N.; Otsuka, M.; Goto, T.; Yoshida, H.; Shiratori, Y.; Omata, M. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J. Med. Virol. 2004, 72, 52–59. [Google Scholar] [CrossRef]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef]
- Battaglia, S.; Benzoubir, N.; Nobilet, S.; Charneau, P.; Samuel, D.; Zignego, A.L.; Atfi, A.; Bréchot, C.; Bourgeade, M.F. Liver cancer-derived hepatitis C virus core proteins shift TGF-beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS ONE 2009, 4, e4355. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Battaglia, S.; Boucreux, D.; Arnulf, B.; Sobesky, R.; Hermine, O.; Brechot, C. Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene 2005, 24, 6119–6132. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, Y.; Lam, K.S.L.; Yau, M.H.; Cheng, K.K.Y.; Zhang, J.; Zhu, W.; Wu, D.; Xu, A. Suppression of the Raf/MEK/ERK Signaling Cascade and Inhibition of Angiogenesis by the Carboxyl Terminus of Angiopoietin-Like Protein 4. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 835–840. [Google Scholar] [CrossRef]
- Kotsiri, I.; Hadziyannis, E.; Georgiou, A.; Papageorgiou, M.V.; Vlachogiannakos, I.; Papatheodoridis, G. Changes in serum transforming growth factor-β1 levels in chronic hepatitis C patients under antiviral therapy. Ann. Gastroenterol. 2016, 29, 79–84. [Google Scholar]
- Nunez, O. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: Role of viral core and NS5A proteins. Gut 2004, 53, 1665–1672. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Hsieh, M.S.; Wang, H.Y.; Li, Y.S.; Lin, H.; Hsu, H.W.; Huang, C.Y.; Hsu, C.H.; Cheng, A.L. Hepatitis C virus core protein potentiates proangiogenic activity of hepatocellular carcinoma cells. Oncotarget 2017, 8, 86681–86692. [Google Scholar] [CrossRef][Green Version]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C Virus Core Protein Augments Androgen Receptor-Mediated Signaling. J. Virol. 2008, 82, 11066–11072. [Google Scholar] [CrossRef]
- Hassan, M.; Selimovic, D.; Ghozlan, H.; Abdel-kader, O. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology 2009, 49, 1469–1482. [Google Scholar] [CrossRef]
- Presser, L.D.; McRae, S.; Waris, G. Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: Role of TGF-β1 in hepatic stellate cell activation and invasion. PLoS ONE 2013, 8, e56367. [Google Scholar] [CrossRef]
- Choi, S.H.; Hwang, S.B. Modulation of the Transforming Growth Factor-β Signal Transduction Pathway by Hepatitis C Virus Nonstructural 5A Protein. J. Biol. Chem. 2006, 281, 7468–7478. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Gao, Y.; Hu, W.; Qu, Y.; Lou, N.; Zhu, Y.; Zhang, X.; Yang, H. Hepatitis C virus NS3 protein enhances hepatocellular carcinoma cell invasion by promoting PPM1A ubiquitination and degradation. J. Exp. Clin. Cancer Res. 2017, 36, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Duan, X.; Liang, Y.Y.; Su, Y.; Wrighton, K.H.; Long, J.; Hu, M.; Davis, C.M.; Wang, J.; Brunicardi, F.C.; et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 2006, 125, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, A.K.; Prasad, S.R.; Takahashi, N.; Vikram, R.; Sahani, D.V. Recent Advances in Cytogenetics and Molecular Biology of Adult Hepatocellular Tumors: Implications for Imaging and Management. Radiology 2011, 258, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sheng, Y.Y.; Gao, X.M.; Wang, C.Q.; Wang, X.Y.; Lu, X.; Wei, J.W.; Zhang, K.L.; Dong, Q.Z.; Qin, L.X. β-catenin mutation is correlated with a favorable prognosis in patients with hepatocellular carcinoma. Mol. Clin. Oncol. 2015, 3, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Xie, S.X.; Chen, Y.T.; Xue, J.L.; Zhang, C.J.; Zhu, F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7486. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Feng, W.; Zhang, S.; Liang, H.; Wang, Y.; Zheng, Q.; Li, Z. PIN1 gene overexpression and β-catenin gene mutation/expression in hepatocellular carcinoma and their significance. J. Huazhong Univ. Sci. Technol. 2007, 27, 54–57. [Google Scholar] [CrossRef]
- Street, A.; Macdonald, A.; McCormick, C.; Harris, M. Hepatitis C Virus NS5A-Mediated Activation of Phosphoinositide 3-Kinase Results in Stabilization of Cellular β-Catenin and Stimulation of β-Catenin-Responsive Transcription. J. Virol. 2005, 79, 5006–5016. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F.; et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Fujimoto, A.; Totoki, Y.; Abe, T.; Boroevich, K.A.; Hosoda, F.; Nguyen, H.H.; Aoki, M.; Hosono, N.; Kubo, M.; Miya, F.; et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 2012, 44, 760–764. [Google Scholar] [CrossRef]
- Park, C.Y.; Choi, S.H.; Kang, S.M.; Kang, J.I.; Ahn, B.Y.; Kim, H.; Jung, G.; Choi, K.Y.; Hwang, S.B. Nonstructural 5A protein activates β-catenin signaling cascades: Implication of hepatitis C virus-induced liver pathogenesis. J. Hepatol. 2009, 51, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.; Marais, R.; Zhu, A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010, 29, 4989–5005. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Ruiz De Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhinaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti–PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Tian, Y.; Mok, M.; Yang, P.; Cheng, A. Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers 2016, 8, 76. [Google Scholar] [CrossRef]
- Stella, L.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Viral hepatitis and hepatocellular carcinoma: From molecular pathways to the role of clinical surveillance and antiviral treatment. World J. Gastroenterol. 2022, 28, 2251–2281. [Google Scholar] [CrossRef]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef]
- Lim, J.S.; Park, S.H.; Jang, K.L. Hepatitis C virus Core protein overcomes stress-induced premature senescence by down-regulating p16 expression via DNA methylation. Cancer Lett. 2012, 321, 154–161. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, C.; Feng, M.; Liu, W.; Xie, H.; Qin, Q.; Zhao, E.; Wan, L. Methylation analysis of p16, SLIT2, SCARA5, and Runx3 genes in hepatocellular carcinoma. Medicine 2017, 96, e8279. [Google Scholar] [CrossRef]
- Quan, H.; Zhou, F.; Nie, D.; Chen, Q.; Cai, X.; Shan, X.; Zhou, Z.; Chen, K.; Huang, A.; Li, S.; et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial–mesenchymal transition. Oncogene 2014, 33, 2826–2835. [Google Scholar] [CrossRef]
- Sun, S.; Li, Y.; Han, S.; Jia, H.; Li, X.; Li, X. A comprehensive genome-wide profiling comparison between HBV and HCV infected hepatocellular carcinoma. BMC Med. Genom. 2019, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Wijetunga, N.A.; Pascual, M.; Tozour, J.; Delahaye, F.; Alani, M.; Adeyeye, M.; Wolkoff, A.W.; Werma, A.; Greally, J.M. A pre-neoplastic epigenetic field defect in HCV-infected liver at transcription factor binding sites and polycomb targets. Oncogene 2017, 36, 2030–2044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, H.; Liu, W.; Wu, Y.; Chen, W.; Jiang, B.; Zhou, Y.; Xue, R.; Luo, C.; Wang, L.; et al. Abnormal activation of the synuclein-gamma gene in hepatocellular carcinomas by epigenetic alteration. Int. J. Oncol. 2006, 28, 1081–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calvisi, D.F.; Ladu, S.; Conner, E.A.; Seo, D.; Hsieh, J.T.; Factor, V.M.; Thorgerirsson, S.S. Inactivation of RasGTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J. Hepatol. 2011, 54, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Q.; Liang, Q.; Zhang, H.; Ou, W.; Yuan, G. Association of PHD3 and HIF2α gene expression with clinicopathological characteristics in human hepatocellular carcinoma. Oncol. Lett. 2017, 15, 545–551. [Google Scholar] [CrossRef]
- Erez, N.; Milyavsky, M.; Eilam, R.; Shats, I.; Goldfinger, N.; Rotter, V. Expression of prolyl-hydroxylase-1 (PHD1/EGLN2) suppresses hypoxia inducible factor-1alpha activation and inhibits tumor growth. Cancer Res. 2003, 63, 8777–8783. [Google Scholar]
- Liu, J.; Xu, R.; Mai, S.J.; Ma, Y.S.; Zhang, M.Y.; Cao, P.S.; Weng, P.S.; Wang, R.Q.; Cao, D.; Wei, W.; et al. LncRNA CSMD1-1 promotes the progression of Hepatocellular Carcinoma by activating MYC signaling. Theranostics 2020, 10, 7527–7544. [Google Scholar] [CrossRef]
- Zhu, Q.; Gong, L.; Wang, J.; Tu, Q.; Yao, L.; Zhang, J.R.; Han, X.J.; Zhu, S.J.; Wang, S.M.; Li, Y.H.; et al. miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer 2016, 16, 806. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Yin, G.; Liu, Z.; Wang, Y.; Dou, C.; Li, C.; Yang, W.; Yao, Y.; Liu, Q.; Tu, K. Retraction Note: BCORL1 is an independent prognostic marker and contributes to cell migration and invasion in human hepatocellular carcinoma. BMC Cancer 2023, 23, 542. [Google Scholar] [CrossRef]
- Dang, S.; Zhou, J.; Chen, Y.; Chen, P.; Ji, M.; Shi, B.; Yang, Q.; Hou, P. Dynamic expression of ZNF382 and its tumor-suppressor role in hepatitis B virus-related hepatocellular carcinogenesis. Oncogene 2019, 38, 4804–4819. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Cho, Y.G.; Kim, C.J.; Song, J.H.; Lee, Y.S.; Kim, S.Y.; NAM, S.W.; Lee, S.H.; Yoo, N.J.; Lee, J.Y. Hypermethylation of the RUNX3 gene in hepatocellular carcinoma. Exp. Mol. Med. 2005, 37, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.; Kitano, Y.; Arima, K.; Miyata, T.; Hyuoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl oxidase induces epithelial-mesenchymal transition and predicts intrahepatic metastasis of hepatocellular carcinoma. Cancer Sci. 2019, 110, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Transcriptional control by the retinoblastoma protein. Semin. Cancer Biol. 1995, 6, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tannapfel, A.; Wasner, M.; Krause, K.; Geissler, F.; Katalinic, A.; Hauss, J.; Mössner, J.; Engeland, K.; Wittekind, C. Expression of p73 and Its Relation to Histopathology and Prognosis in Hepatocellular Carcinoma. JNCI J. Natl. Cancer Inst. 1999, 91, 1154–1158. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, L.; Wang, J.P.; Li, J.Q.; Zhang, C.Q.; Zheng, L.; Yuan, Y.F. Correlations of CpG island methylator phenotype and OPCML gene methylation to carcinogenesis of hepatocellular carcinoma. Ai Zheng Aizheng Chin. J. Cancer. 2006, 25, 696–700. [Google Scholar]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ahsan, H.; Chen, Y.; Lunn, R.M.; Wang, L.Y.; Chen, S.Y.; Lee, P.H.; Chen, C.J.; Santella, R.M. High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol. Carcinog. 2002, 35, 85–92. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, S.B.; Lee, W.S.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Choi, S.K.; Rew, J.S.; Lee, J.H.; Kim, S.J. Methylation pattern of DNA repair genes and microsatellite instability in hepatocelluar carcinoma. Korean J. Gastroenterol. 2006, 48, 327–336. [Google Scholar]
- Matsukura, S.; Soejima, H.; Nakagawachi, T.; Yakushiji, H.; Ogawa, A.; Fukuhara, M.; Miyazaki, K.; Nakabeppu, Y.; Sekiguchi, M.; Mukai, T. CpG methylation of MGMT and hMLH1 promoter in hepatocellular carcinoma associated with hepatitis viral infection. Br. J. Cancer 2003, 88, 521–529. [Google Scholar] [CrossRef]
- Tischoff, I.; Tannapfe, A. DNA methylation in hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P. Epigenetic mechanisms involved in the pathogenesis of hepatobiliary malignancies. Epigenomics 2010, 2, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.T.; Li, H.-M.; Lo, K.W.; Leow, C.K.; Chan, J.Y.; Hin, L.-Y.; Lau, W.Y.; Lai, P.B.-S.; Lim, B.K.; Huang, J.; et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene 1999, 18, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, M.; Qie, J.; Ye, T. FOXA1 Promotes Cell Proliferation and Suppresses Apoptosis in HCC by Directly Regulating miR-212-3p/FOXA1/AGR2 Signaling Pathway. Onco Targets Ther. 2020, 13, 5231–5240. [Google Scholar] [CrossRef]
- Ning, B.F.; Ding, J.; Liu, J.; Yin, C.; Xu, W.P.; Cong, W.M.; Zhang, Q.; Chen, F.; Han, T.; Deng, X.; et al. Hepatocyte nuclear factor 4α-nuclear factor-κB feedback circuit modulates liver cancer progression. Hepatology 2014, 60, 1607–1619. [Google Scholar] [CrossRef]
- Lu, G.; Leung, C.H.; Yan, B.; Tan, C.M.; Low, S.Y.; Aung, M.O.; Salto-Tellez, M.; Lim, S.G.; Hooi, S.C. C/EBPα Is Up-regulated in a Subset of Hepatocellular Carcinomas and Plays a Role in Cell Growth and Proliferation. Gastroenterology 2010, 139, 632–643.e4. [Google Scholar] [CrossRef]
- Zheng, Y.; Hlady, R.A.; Joyce, B.T.; Robertson, K.D.; He, C.; Nannini, D.R.; Kibbe, W.A.; Achenbach, C.J.; Murphy, R.L.; Roberts, L.R.; et al. DNA methylation of individual repetitive elements in hepatitis C virus infection-induced hepatocellular carcinoma. Clin. Epigenetics 2019, 11, 145. [Google Scholar] [CrossRef]
- Shukla, R.; Upton, K.R.; Munoz-Lopez, M.; Gerhardt, D.J.; Fisher, M.E.; Nguyen, T.; Brennan, P.M.; Baillie, J.K.; Collino, A.; Ghislleti, S.; et al. Endogenous retrotransposition activates oncogenic pathways in hepatocellular carcinoma. Cell 2013, 153, 101–111. [Google Scholar] [CrossRef]
- Belancio, V.P.; Deininger, P.L.; Roy-Engel, A.M. LINE dancing in the human genome: Transposable elements and disease. Genome Med. 2009, 1, 97. [Google Scholar] [CrossRef]
- Chung, Y.L.; Sheu, M.L.; Yen, S.H. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int. J. Cancer 2003, 107, 65–73. [Google Scholar] [CrossRef]
- Isoyama, T.; Kuge, S.; Nomoto, A. The Core Protein of Hepatitis C Virus Is Imported into the Nucleus by Transport Receptor Kap123p but Inhibits Kap121p-dependent Nuclear Import of Yeast AP1-like Transcription Factor in Yeast Cells. J. Biol. Chem. 2002, 277, 39634–39641. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Neufeldt, C.J.; Pang, D.; Wilson, K.; Loewen-Dobler, D.; Joyce, M.A.; Wozniak, R.W.; Tyrrel, D.L.J. Functional Characterization of Nuclear Localization and Export Signals in Hepatitis C Virus Proteins and Their Role in the Membranous Web. PLoS ONE 2014, 9, e114629. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.N.; Ul Haq, A.; Siddiqui, O.A.; Khan, R. DNA methyltransferase 1, 3a, and 3b expression in hepatitis C associated human hepatocellular carcinoma and their clinicopathological association. Tumor Biol. 2016, 37, 10487–10497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P. Proportions of acetyl-histone-positive hepatocytes indicate the functional status and prognosis of cirrhotic patients. World J. Gastroenterol. 2015, 21, 6665. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Li, S.; et al. HCV-Induced Epigenetic Changes Associated with Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Acetylation/Deacetylation Modulates the Stability of DNA Replication Licensing Factor Cdt1. J. Biol. Chem. 2009, 284, 11446–11453. [Google Scholar] [CrossRef]
- Perez, S.; Kaspi, A.; Domovitz, T.; Davidovich, A.; Lavi-Itzkovitz, A.; Meirson, T.; Nimer, A.; Yaari, G.; Huang, C.F.; Haviv, I. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genet. 2019, 15, e1008181. [Google Scholar] [CrossRef]
- Halley-Stott, R.P.; Gurdon, J.B. Epigenetic memory in the context of nuclear reprogramming and cancer. Brief. Funct. Genomics. 2013, 12, 164–173. [Google Scholar] [CrossRef]
- Jühling, F.; Hamdane, N.; Crouchet, E.; Li, S.; El Saghire, H.; Mukherji, A.; Fujiwara, N.; Oudot, M.A.; Thumann, C.; Goto, K.; et al. Targeting clinical epigenetic reprogramming for chemoprevention of metabolic and viral hepatocellular carcinoma. Gut 2021, 70, 157–169. [Google Scholar] [CrossRef]
- Unfried, J.P.; Fortes, P. LncRNAs in HCV Infection and HCV-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 2255. [Google Scholar] [CrossRef]
- Zhang, Q.; Matsuura, K.; Kleiner, D.E.; Zamboni, F.; Alter, H.J.; Farci, P. Analysis of long noncoding RNA expression in hepatocellular carcinoma of different viral etiology. J. Transl. Med. 2016, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, C.; Zhao, Y.; Li, M.; Wu, L.; Yang, X.; Wan, X.; Wang, A.; Zhang, M.Q.; Sang, X.; et al. Long non-coding RNA expression profiles of hepatitis C virus-related dysplasia and hepatocellular carcinoma. Oncotarget 2015, 6, 43770–43778. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Oura, K.; Tadokoro, T.; Fujita, K.; Tani, J.; Masaki, T. MicroRNAs in the Pathogenesis of Hepatocellular Carcinoma: A Review. Cancers 2021, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Varnholt, H.; Drebber, U.; Schulze, F.; Wedemeyer, I.; Schirmacher, P.; Dienes, H.P.; Odhental, M. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology 2007, 47, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Steele, R.; Ray, R.; Ray, R.B. MicroRNAs: Role in hepatitis C virus pathogenesis. Genes. Dis. 2015, 2, 35–45. [Google Scholar] [CrossRef]
- Khan, S.; Ayub, H.; Khan, T.; Wahid, F. MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie 2019, 167, 12–24. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe–Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Wong, Q.W.-L.; Lung, R.W.-M.; Law, P.T.-Y.; Lai, P.B.-S.; Chan, K.Y.-Y.; To, K.; Wong, N. MicroRNA-223 Is Commonly Repressed in Hepatocellular Carcinoma and Potentiates Expression of Stathmin1. Gastroenterology 2008, 135, 257–269. [Google Scholar] [CrossRef]
- Gramantieri, L.; Ferracin, M.; Fornari, F.; Veronese, A.; Sabbioni, S.; Liu, C.G.; Callin, G.A.; Giovanini, C.; Ferazzi, E.; Grazi, G.L.; et al. Cyclin G1 Is a Target of miR-122a, a MicroRNA Frequently Down-regulated in Human Hepatocellular Carcinoma. Cancer Res. 2007, 67, 6092–6099. [Google Scholar] [CrossRef]
- Murakami, Y.; Yasuda, T.; Saigo, K.; Urashima, T.; Toyoda, H.; Okanoue, T.; Shimotohno, K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 2006, 25, 2537–2545. [Google Scholar] [CrossRef]
- Wei, J.; Feng, L.; Li, Z.; Xu, G.; Fan, X. MicroRNA-21 activates hepatic stellate cells via PTEN/Akt signaling. Biomed. Pharmacother. 2013, 67, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Bandyopadhyay, S.; Wendlandt, E.B.; Keck, K.; Hoffer, B.A.; Icardi, M.S.; Christensen, R.N.; Schmidt, W.N.; McCaffey, A.P. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab. Investig. 2010, 90, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum -infected mice via transforming growth factor-β/Smad signaling. World J. Gastroenterol. 2013, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, D.; Xie, J.; Su, Q.; He, X.; Bai, R.; Gao, G.; Pan, W. MicroRNA-96 Promotes Schistosomiasis Hepatic Fibrosis in Mice by Suppressing Smad7. Mol. Ther. Methods Clin. Dev. 2018, 11, 73–82. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Kusumanchi, P.; Han, S.; Liangpunsakul, S. Critical Role of microRNA-21 in the Pathogenesis of Liver Diseases. Front. Med. 2020, 7, 7. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Guo, X.; Lv, X.; Lv, X.; Ma, Y.; Chen, L.; Chen, Y. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget 2017, 8, 44050–44058. [Google Scholar] [CrossRef]
- Wang, X.W.; Heegaard, N.H.H.; Ørum, H. MicroRNAs in Liver Disease. Gastroenterology 2012, 142, 1431–1443. [Google Scholar] [CrossRef]
- Chang, J.; Nicolas, E.; Marks, D.; Sander, C.; Lerro, A.; Buendia, M.A.; Xu, C.; Mason, W.S.; Moloshok, T.; Bort, R.; et al. miR-122, a Mammalian Liver-Specific microRNA, is Processed from hcr mRNA and MayDownregulate the High Affinity Cationic Amino Acid Transporter CAT-1. RNA Biol. 2004, 1, 106–113. [Google Scholar] [CrossRef]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of Hepatitis C Virus RNA Abundance by a Liver-Specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, M.; Li, F.; Qian, L.; Zhang, P.; Lv, F.; Cheng, W.; Hou, R. MiR-221 Promotes Hepatocellular Carcinoma Cells Migration via Targeting PHF2. BioMed Res. Int. 2019, 2019, 4371405. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.W.L.; Ching, A.K.K.; Chan, A.W.H.; Choy, K.W.; To, K.F.; Lai, P.B.S.; Wong, N. MiR-222 Overexpression Confers Cell Migratory Advantages in Hepatocellular Carcinoma through Enhancing AKT Signaling. Clin. Cancer Res. 2010, 16, 867–875. [Google Scholar] [CrossRef]
- Qi, X.; Yang, L. (Eds.) Hepatitis C—Recent Advances; IntechOpen: London, UK, 2023; Available online: https://www.intechopen.com/books/1002335 (accessed on 2 September 2023).
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J. On “Biological effects of the superoxide radical” by Irwin Fridovich. Arch. Biochem. Biophys. 2022, 726, 109117. [Google Scholar] [CrossRef] [PubMed]
- Oneyama, C.; Hikita, T.; Nada, S.; Okada, M. Functional dissection of transformation by c-Src and v-Src: C-Src vs. v-Src transformation. Genes. Cells 2007, 13, 1–12. [Google Scholar] [CrossRef]
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Karlo, J.C.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and Erk2 Regulate Endothelial Cell Proliferation and Migration during Mouse Embryonic Angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Nakagawa, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Fushimi, K.; Kobayashi, E.; Kishi, H.; Muraguchi, A.; et al. Immunological features of T cells induced by human telomerase reverse transcriptase-derived peptides in patients with hepatocellular carcinoma. Cancer Lett. 2015, 364, 98–105. [Google Scholar] [CrossRef]
- Cossío, F.P.; Esteller, M.; Berdasco, M. Towards a more precise therapy in cancer: Exploring epigenetic complexity. Curr. Opin. Chem. Biol. 2020, 57, 41–49. [Google Scholar] [CrossRef]
- Saleh, M.H.; Wang, L.; Goldberg, M.S. Improving cancer immunotherapy with DNA methyltransferase inhibitors. Cancer Immunol. Immunother. 2016, 65, 787–796. [Google Scholar] [CrossRef]
- Zhu, S.; Denman, C.J.; Cobanoglu, Z.S.; Kiany, S.; Lau, C.C.; Gottschalk, S.M.; Hughes, D.P.M.; Kleinerman, E.S.; Lee, D.A. The Narrow-Spectrum HDAC Inhibitor Entinostat Enhances NKG2D Expression Without NK Cell Toxicity, Leading to Enhanced Recognition of Cancer Cells. Pharm. Res. 2015, 32, 779–792. [Google Scholar] [CrossRef]
- Costantini, B.; Kordasti, S.Y.; Kulasekararaj, A.G.; Jiang, J.; Seidl, T.; Abellan, P.P.; Mohamedali, A.; Thomas, N.S.B.; Farzaneh, F.; Mufti, G.J. The effects of 5-azacytidine on the function and number of regulatory T cells and T-effectors in myelodysplastic syndrome. Haematologica 2013, 98, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Gailhouste, L.; Liew, L.C.; Yasukawa, K.; Hatada, I.; Tanaka, Y.; Nakagama, H.; Ochiya, T. Differentiation Therapy by Epigenetic Reconditioning Exerts Antitumor Effects on Liver Cancer Cells. Mol. Ther. 2018, 26, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Meng, L.; Liu, K.; Ji, B. Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol. Rep. 2017, 38, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Raynal, N.J.M.; Issa, J.P.J. DNA Methyltransferase Inhibitors. In Drug Discovery in Cancer Epigenetics; Elsevier, 2016; pp. 169–190. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128022085000072 (accessed on 2 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milosevic, I.; Todorovic, N.; Filipovic, A.; Simic, J.; Markovic, M.; Stevanovic, O.; Malinic, J.; Katanic, N.; Mitrovic, N.; Nikolic, N. HCV and HCC Tango—Deciphering the Intricate Dance of Disease: A Review Article. Int. J. Mol. Sci. 2023, 24, 16048. https://doi.org/10.3390/ijms242216048
Milosevic I, Todorovic N, Filipovic A, Simic J, Markovic M, Stevanovic O, Malinic J, Katanic N, Mitrovic N, Nikolic N. HCV and HCC Tango—Deciphering the Intricate Dance of Disease: A Review Article. International Journal of Molecular Sciences. 2023; 24(22):16048. https://doi.org/10.3390/ijms242216048
Chicago/Turabian StyleMilosevic, Ivana, Nevena Todorovic, Ana Filipovic, Jelena Simic, Marko Markovic, Olja Stevanovic, Jovan Malinic, Natasa Katanic, Nikola Mitrovic, and Natasa Nikolic. 2023. "HCV and HCC Tango—Deciphering the Intricate Dance of Disease: A Review Article" International Journal of Molecular Sciences 24, no. 22: 16048. https://doi.org/10.3390/ijms242216048
APA StyleMilosevic, I., Todorovic, N., Filipovic, A., Simic, J., Markovic, M., Stevanovic, O., Malinic, J., Katanic, N., Mitrovic, N., & Nikolic, N. (2023). HCV and HCC Tango—Deciphering the Intricate Dance of Disease: A Review Article. International Journal of Molecular Sciences, 24(22), 16048. https://doi.org/10.3390/ijms242216048






