Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products
Abstract
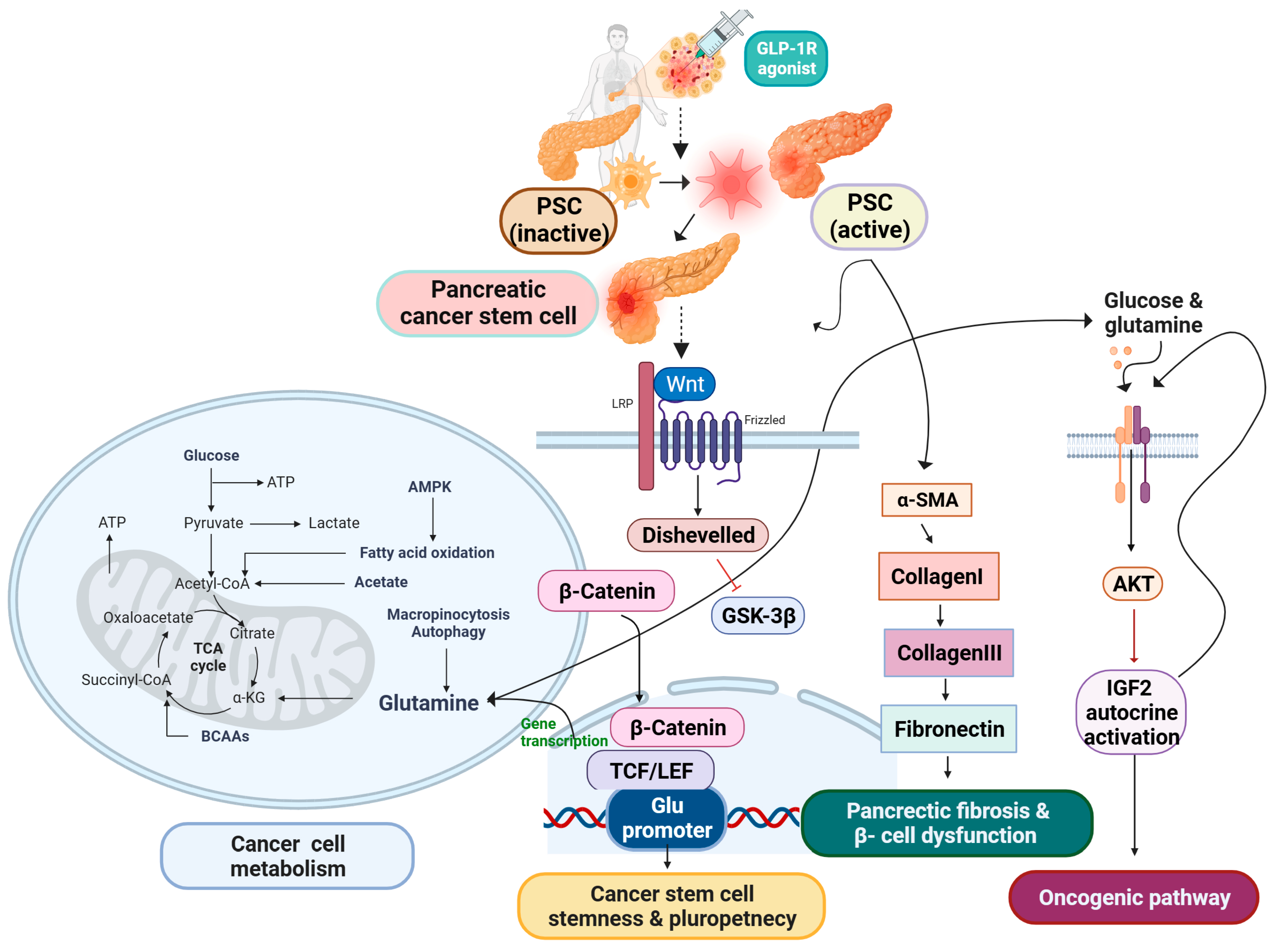
1. Introduction
1.1. PSC Activation Spurred Due to Type 2 Diabetes Has Become an Attractive Possibility for PDAC Stem Cells
1.2. Type 2 Diabetes Is a Significant Prognostic Indicator for Pancreatic Cancer
1.3. Diabetes Management Strategies May Reduce the Risk of Pancreatic Cancer
1.4. Herbal Medicines In Vitro Assay to Improve PDAC Therapeutic Strategies
1.5. Potential New Treatments Include Natural Compounds for Fighting PDAC
1.6. Tendency in FDA-Approved Therapies
2. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quante, A.S.; Ming, C.; Rottmann, M.; Engel, J.; Boeck, S.; Heinemann, V.; Westphalen, C.B.; Strauch, K. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016, 5, 2649–2656. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ng, J.; Allendorf, J.; Saif, M.W. Locally advanced pancreatic adenocarcinoma: Are we making progress? Highlights from the “2011 ASCO Annual Meeting”. Chicago, IL, USA; June 3–7, 2011. JOP J. Pancreas 2011, 12, 347–350. [Google Scholar]
- Thakur, G.; Kumar, R.; Kim, S.B.; Lee, S.Y.; Lee, S.L.; Rho, G.J. Therapeutic Status and Available Strategies in Pancreatic Ductal Adenocarcinoma. Biomedicines 2021, 9, 178. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; White, C.M.; Naito, Y.; Zhong, Y.; Brosnan, J.A.; Macgregor-Das, A.M.; Morgan, R.A.; Saunders, T.; Laheru, D.A.; Herman, J.M.; et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin. Cancer Res. 2012, 18, 6339–6347. [Google Scholar] [CrossRef]
- Lowery, M.A.; Jordan, E.J.; Basturk, O.; Ptashkin, R.N.; Zehir, A.; Berger, M.F.; Leach, T.; Herbst, B.; Askan, G.; Maynard, H.; et al. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin. Cancer Res. 2017, 23, 6094–6100. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Lito, P.; Solomon, M.; Li, L.S.; Hansen, R.; Rosen, N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 2016, 351, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; O’Reilly, E.M. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Shimosegawa, T. Signal transduction in pancreatic stellate cells. J. Gastroenterol. 2009, 44, 249–260. [Google Scholar] [CrossRef]
- Erkan, M.; Reiser-Erkan, C.; Michalski, C.W.; Deucker, S.; Sauliunaite, D.; Streit, S.; Esposito, I.; Friess, H.; Kleeff, J. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Bachem, M.G.; Schneider, E.; Gross, H.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Siech, M.; Beger, H.; Grünert, A.; Adler, G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115, 421–432. [Google Scholar] [CrossRef]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef]
- Shields, M.A.; Dangi-Garimella, S.; Redig, A.J.; Munshi, H.G. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem. J. 2012, 441, 541–552. [Google Scholar] [CrossRef]
- Shi, X.; Wang, M.; Zhang, Y.; Guo, X.; Liu, M.; Zhou, Z.; Zhao, Y.; He, R.; Gao, Y.; Liu, Y.; et al. Hypoxia activated HGF expression in pancreatic stellate cells confers resistance of pancreatic cancer cells to EGFR inhibition. EBioMedicine 2022, 86, 104352. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019, 569, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Neesse, A.; Algül, H.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef]
- Waghray, M.; Yalamanchili, M.; di Magliano, M.P.; Simeone, D.M. Deciphering the role of stroma in pancreatic cancer. Curr. Opin. Gastroenterol. 2013, 29, 537–543. [Google Scholar] [CrossRef]
- Apte, M.V.; Haber, P.S.; Darby, S.J.; Rodgers, S.C.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, Y.; Tashiro, M.; Yamaguchi, T.; Watanabe, S.; Taguchi, M.; Asaumi, H.; Nakamura, H.; Otsuki, M. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas 2007, 34, 364–372. [Google Scholar] [CrossRef]
- Ko, S.H.; Hong, O.K.; Kim, J.W.; Ahn, Y.B.; Song, K.H.; Cha, B.Y.; Son, H.Y.; Kim, M.J.; Jeong, I.K.; Yoon, K.H. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J. Cell Biochem. 2006, 98, 343–355. [Google Scholar] [CrossRef]
- Ryu, G.R.; Lee, E.; Chun, H.J.; Yoon, K.H.; Ko, S.H.; Ahn, Y.B.; Song, K.H. Oxidative stress plays a role in high glucose-induced activation of pancreatic stellate cells. Biochem. Biophys. Res. Commun. 2013, 439, 258–263. [Google Scholar] [CrossRef]
- Kiss, K.; Baghy, K.; Spisák, S.; Szanyi, S.; Tulassay, Z.; Zalatnai, A.; Löhr, J.M.; Jesenofsky, R.; Kovalszky, I.; Firneisz, G. Chronic hyperglycemia induces trans-differentiation of human pancreatic stellate cells and enhances the malignant molecular communication with human pancreatic cancer cells. PLoS ONE 2015, 10, e0128059. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Waldron, R.T.; Su, H.Y.; Moro, A.; Chang, H.H.; Eibl, G.; Ferreri, K.; Kandeel, F.R.; Lugea, A.; Li, L.; et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G675–G687. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, J.; Yuan, Z.; Zhang, H.; Chong, Y.; Huang, Y.; Wang, J.; Xiong, Q.; Wang, S.; Wu, Q.; et al. PSC-derived Galectin-1 inducing epithelial-mesenchymal transition of pancreatic ductal adenocarcinoma cells by activating the NF-κB pathway. Oncotarget 2017, 8, 86488–86502. [Google Scholar] [CrossRef]
- Augstein, P.; Loudovaris, T.; Bandala-Sanchez, E.; Heinke, P.; Naselli, G.; Lee, L.; Hawthorne, W.J.; Góñez, L.J.; Neale, A.M.; Vaillant, F.; et al. Characterization of the Human Pancreas Side Population as a Potential Reservoir of Adult Stem Cells. Pancreas 2018, 47, 25–34. [Google Scholar] [CrossRef]
- Murray, E.R.; Menezes, S.; Henry, J.C.; Williams, J.L.; Alba-Castellón, L.; Baskaran, P.; Quétier, I.; Desai, A.; Marshall, J.J.T.; Rosewell, I.; et al. Disruption of pancreatic stellate cell myofibroblast phenotype promotes pancreatic tumor invasion. Cell Rep. 2022, 38, 110227. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Y.; Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 2021, 172, 37–51. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Wang, L.; Zhang, H.; Lu, J.; Liang, Z.; Liu, T. Asporin promotes pancreatic cancer cell invasion and migration by regulating the epithelial-to-mesenchymal transition (EMT) through both autocrine and paracrine mechanisms. Cancer Lett. 2017, 398, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, H.; Ma, Q.; Huang, R.; Lu, J.; Liang, X.; Liu, X.; Zhang, Z.; Yu, L.; Pang, J.; et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett. 2019, 462, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Nofal, M.; Commisso, C.; Hackett, S.R.; Lu, W.; Grabocka, E.; Vander Heiden, M.G.; Miller, G.; Drebin, J.A.; Bar-Sagi, D.; et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015, 75, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Liu, X.; Guo, W.; Liu, Q.; Chen, L.; Pang, J.; Liu, X.; Li, R.; Tong, W.M.; et al. Pancreatic stellate cells exploit Wnt/β-catenin/TCF7-mediated glutamine metabolism to promote pancreatic cancer cells growth. Cancer Lett. 2023, 555, 216040. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Dai, C.; Martell, E.; Ghassemi-Rad, M.S.; Hanes, M.R.; Murphy, P.J.; Kennedy, B.E.; Venugopal, C.; Subapanditha, M.; Giacomantonio, C.A.; et al. TAp73 Modifies Metabolism and Positively Regulates Growth of Cancer Stem-Like Cells in a Redox-Sensitive Manner. Clin. Cancer Res. 2019, 25, 2001–2017. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Ly, S.; Nguyen, K.; Anand, V.; Yuan, B.; El-Dana, F.; Yan, Y.; Arvanitis, Z.; Piyarathna, D.W.B.; Putluri, N.; et al. Metabolic stress induces GD2(+) cancer stem cell-like phenotype in triple-negative breast cancer. Br. J. Cancer 2022, 126, 615–627. [Google Scholar] [CrossRef]
- Mukha, A.; Kahya, U.; Linge, A.; Chen, O.; Löck, S.; Lukiyanchuk, V.; Richter, S.; Alves, T.C.; Peitzsch, M.; Telychko, V.; et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics 2021, 11, 7844–7868. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tsuchihashi, K.; Ishimoto, T.; Yae, T.; Motohara, T.; Sugihara, E.; Onishi, N.; Masuko, T.; Yoshizawa, K.; Kawashiri, S.; et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fu, Z.; Chen, R.; Zhao, X.; Zhou, Y.; Zeng, B.; Yu, M.; Zhou, Q.; Lin, Q.; Gao, W.; et al. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget 2015, 6, 31151–31163. [Google Scholar] [CrossRef]
- Modi, H.; Cornu, M.; Thorens, B. Glutamine stimulates biosynthesis and secretion of insulin-like growth factor 2 (IGF2), an autocrine regulator of beta cell mass and function. J. Biol. Chem. 2014, 289, 31972–31982. [Google Scholar] [CrossRef]
- Bouwens, L.; Houbracken, I.; Mfopou, J.K. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Jiang, F.X.; Morahan, G. Pancreatic stem cells remain unresolved. Stem Cells Dev. 2014, 23, 2803–2812. [Google Scholar] [CrossRef]
- Kuninty, P.R.; Bansal, R.; De Geus, S.W.L.; Mardhian, D.F.; Schnittert, J.; van Baarlen, J.; Storm, G.; Bijlsma, M.F.; van Laarhoven, H.W.; Metselaar, J.M.; et al. ITGA5 inhibition in pancreatic stellate cells attenuates desmoplasia and potentiates efficacy of chemotherapy in pancreatic cancer. Sci. Adv. 2019, 5, eaax2770. [Google Scholar] [CrossRef]
- Erkan, M.; Kleeff, J.; Gorbachevski, A.; Reiser, C.; Mitkus, T.; Esposito, I.; Giese, T.; Büchler, M.W.; Giese, N.A.; Friess, H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 2007, 132, 1447–1464. [Google Scholar] [CrossRef]
- Apte, M.; Haber, P.; Applegate, T.; Norton, I.; McCaughan, G.; Korsten, M.; Pirola, R.; Wilson, J. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut 1998, 43, 128–133. [Google Scholar] [CrossRef]
- Phillips, P. Pancreatic stellate cells and fibrosis. In Pancreatic Cancer and Tumor Microenvironment; Grippo, P.J., Munshi, H.G., Eds.; Transworld Research Network: Trivandrum, India, 2012. [Google Scholar]
- Nielsen, M.F.B.; Mortensen, M.B.; Detlefsen, S. Identification of markers for quiescent pancreatic stellate cells in the normal human pancreas. Histochem. Cell Biol. 2017, 148, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Li, F.; Xu, W.; Chen, B.; Sun, Z. Isolation and characterization of islet stellate cells in rat. Islets 2014, 6, e28701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zang, G.; Sandberg, M.; Carlsson, P.O.; Welsh, N.; Jansson, L.; Barbu, A. Activated pancreatic stellate cells can impair pancreatic islet function in mice. Ups. J. Med. Sci. 2015, 120, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ryu, G.R.; Ko, S.H.; Ahn, Y.B.; Song, K.H. A role of pancreatic stellate cells in islet fibrosis and β-cell dysfunction in type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 2017, 485, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Utsunomiya, T.; Inoue, H.; Tanaka, F.; Mimori, K.; Barnard, G.F.; Mori, M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006, 24, 506–513. [Google Scholar] [CrossRef]
- Feuring-Buske, M.; Hogge, D.E. Hoechst 33342 efflux identifies a subpopulation of cytogenetically normal CD34+CD38− progenitor cells from patients with acute myeloid leukemia. Blood 2001, 97, 3882–3889. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, B.; Li, W.; Zhou, J.; Gao, F.; Wang, X.; Cai, M.; Sun, Z. Pancreatic Stellate Cells: A Rising Translational Physiology Star as a Potential Stem Cell Type for Beta Cell Neogenesis. Front. Physiol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Zulewski, H.; Abraham, E.J.; Gerlach, M.J.; Daniel, P.B.; Moritz, W.; Müller, B.; Vallejo, M.; Thomas, M.K.; Habener, J.F. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001, 50, 521–533. [Google Scholar] [CrossRef]
- Abraham, E.J.; Leech, C.A.; Lin, J.C.; Zulewski, H.; Habener, J.F. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology 2002, 143, 3152–3161. [Google Scholar] [CrossRef]
- Skoglund, G.; Hussain, M.A.; Holz, G.G. Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes 2000, 49, 1156–1164. [Google Scholar] [CrossRef]
- Buteau, J.; Roduit, R.; Susini, S.; Prentki, M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 1999, 42, 856–864. [Google Scholar] [CrossRef]
- Montrose-Rafizadeh, C.; Avdonin, P.; Garant, M.J.; Rodgers, B.D.; Kole, S.; Yang, H.; Levine, M.A.; Schwindinger, W.; Bernier, M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 1999, 140, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ito, T.; Uchida, M.; Hijioka, M.; Igarashi, H.; Oono, T.; Kato, M.; Nakamura, K.; Suzuki, K.; Jensen, R.T.; et al. PSCs and GLP-1R: Occurrence in normal pancreas, acute/chronic pancreatitis and effect of their activation by a GLP-1R agonist. Lab. Investig. 2014, 94, 63–78. [Google Scholar] [CrossRef]
- Homo-Delarche, F.; Calderari, S.; Irminger, J.C.; Gangnerau, M.N.; Coulaud, J.; Rickenbach, K.; Dolz, M.; Halban, P.; Portha, B.; Serradas, P. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes 2006, 55, 1625–1633. [Google Scholar] [CrossRef]
- Haber, P.S.; Keogh, G.W.; Apte, M.V.; Moran, C.S.; Stewart, N.L.; Crawford, D.H.; Pirola, R.C.; McCaughan, G.W.; Ramm, G.A.; Wilson, J.S. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999, 155, 1087–1095. [Google Scholar] [CrossRef]
- Landi, S. Genetic predisposition and environmental risk factors to pancreatic cancer: A review of the literature. Mutat. Res. 2009, 681, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Jura, N.; Archer, H.; Bar-Sagi, D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005, 15, 72–77. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Huang, L.; Yu, C. GLP-1R agonist may activate pancreatic stellate cells to induce rat pancreatic tissue lesion. Pancreatology 2013, 13, 498–501. [Google Scholar] [CrossRef]
- Bhagwandin, V.J.; Shay, J.W. Pancreatic cancer stem cells: Fact or fiction? Biochim. Biophys. Acta 2009, 1792, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, F.; Luo, B.; Wang, X.H.; Sun, H.C.; Liu, S.; Cui, Y.Q.; Xu, X.X. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 2009, 56, 371–378. [Google Scholar] [CrossRef]
- Ishiwata, T.; Matsuda, Y.; Yoshimura, H.; Sasaki, N.; Ishiwata, S.; Ishikawa, N.; Takubo, K.; Arai, T.; Aida, J. Pancreatic cancer stem cells: Features and detection methods. Pathol. Oncol. Res. 2018, 24, 797–805. [Google Scholar] [CrossRef]
- Mato, E.; Lucas, M.; Petriz, J.; Gomis, R.; Novials, A. Identification of a pancreatic stellate cell population with properties of progenitor cells: New role for stellate cells in the pancreas. Biochem. J. 2009, 421, 181–191. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Götze, S.; Häussinger, D. Stellate cells from rat pancreas are stem cells and can contribute to liver regeneration. PLoS ONE 2012, 7, e51878. [Google Scholar] [CrossRef]
- Zha, M.; Xu, W.; Jones, P.M.; Sun, Z. Isolation and characterization of human islet stellate cells. Exp. Cell Res. 2016, 341, 61–66. [Google Scholar] [CrossRef]
- Pang, T.C.Y.; Wilson, J.S.; Apte, M.V. Pancreatic stellate cells: What’s new? Curr. Opin. Gastroenterol. 2017, 33, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, F.; Leonardi, A.; Crescenzi, E. Glutamine Metabolism in Cancer Stem Cells: A Complex Liaison in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 2337. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Morganstein, D.L. Diabetes and cancer: Optimising glycaemic control. J. Hum. Nutr. Diet. 2023, 36, 504–513. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Coe, P.O.; Williams, S.R.; Morris, D.M.; Parkin, E.; Harvie, M.; Renehan, A.G.; O’Reilly, D.A. Development of MR quantified pancreatic fat deposition as a cancer risk biomarker. Pancreatology 2018, 18, 429–437. [Google Scholar] [CrossRef]
- Rebours, V.; Gaujoux, S.; d’Assignies, G.; Sauvanet, A.; Ruszniewski, P.; Lévy, P.; Paradis, V.; Bedossa, P.; Couvelard, A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin. Cancer Res. 2015, 21, 3522–3528. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Nguyen, N.N.; Cervantes, A.; Alarcon Ramos, G.C.; Cho, J.; Petrov, M.S. Associations between intra-pancreatic fat deposition and circulating levels of cytokines. Cytokine 2019, 120, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.W.; Glancy, C.; Jones, S.; Lewis, S.A.; McKeever, T.M.; Britton, J.R. A prospective study of weight change and systemic inflammation over 9 y. Am. J. Clin. Nutr. 2008, 87, 30–35. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Brehm, B.J.; Seeley, R.J.; Bean, J.; Wener, M.H.; Daniels, S.; D’Alessio, D.A. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 2244–2249. [Google Scholar] [CrossRef]
- Chang, H.H.; Moro, A.; Takakura, K.; Su, H.Y.; Mo, A.; Nakanishi, M.; Waldron, R.T.; French, S.W.; Dawson, D.W.; Hines, O.J.; et al. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS ONE 2017, 12, e0184455. [Google Scholar] [CrossRef]
- Li, Z.J.; Dai, H.Q.; Huang, X.W.; Feng, J.; Deng, J.H.; Wang, Z.X.; Yang, X.M.; Liu, Y.J.; Wu, Y.; Chen, P.H.; et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2021, 42, 301–310. [Google Scholar] [CrossRef]
- Turchi, R.; Tortolici, F.; Guidobaldi, G.; Iacovelli, F.; Falconi, M.; Rufini, S.; Faraonio, R.; Casagrande, V.; Federici, M.; De Angelis, L.; et al. Frataxin deficiency induces lipid accumulation and affects thermogenesis in brown adipose tissue. Cell Death Dis. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.J.; Gao, L.; Cheng, L.; Sun, B.; Wang, G. Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend? Antioxid. Redox Signal 2022, 37, 1206–1221. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Al-Shukaili, A.; Al-Ghafri, S.; Al-Marhoobi, S.; Al-Abri, S.; Al-Lawati, J.; Al-Maskari, M. Analysis of inflammatory mediators in type 2 diabetes patients. Int. J. Endocrinol. 2013, 2013, 976810. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet 1999, 353, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R., Jr.; Tracy, R.P.; Haffner, S.M. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2002, 51, 1131–1137. [Google Scholar] [CrossRef]
- Mosselman, S.; Höppener, J.; De Wit, L.; Soeller, W.; Lips, C.; Jansz, H. IAPP/amylin gene transcriptional control region: Evidence for negative regulation. FEBS Lett. 1990, 271, 33–36. [Google Scholar] [CrossRef]
- German, M.S.; Gene Moss, L.; Wang, J.; Rutter, W.J. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical β-cell nuclear complexes. Mol. Cell. Biol. 1992, 12, 1777–1788. [Google Scholar] [CrossRef]
- De Koning, E.; Morris, E.R.; Hofhuis, F.; Posthuma, G.; Höppener, J.; Morris, J.F.; Capel, P.; Clark, A.; Verbeek, J.S. Intra-and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc. Natl. Acad. Sci. USA 1994, 91, 8467–8471. [Google Scholar] [CrossRef] [PubMed]
- Höppener, J.; Oosterwijk, C.; Nieuwenhuis, M.; Posthuma, G.; Thijssen, J.; Vroom, T.M.; Ahren, B.; Lips, C. Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: Pathogenesis of diabetes in a mouse model. Diabetologia 1999, 42, 427–434. [Google Scholar] [CrossRef][Green Version]
- Kapurniotu, A.; Bernhagen, J.; Greenfield, N.; Al-Abed, Y.; Teichberg, S.; Frank, R.W.; Voelter, W.; Bucala, R. Contribution of advanced glycosylation to the amyloidogenicity of islet amyloid polypeptide. Eur. J. Biochem. 1998, 251, 208–216. [Google Scholar] [CrossRef]
- Höppener, J.W.M.; Ahrén, B.; Lips, C.J.M. Islet Amyloid and Type 2 Diabetes Mellitus. N. Engl. J. Med. 2000, 343, 411–419. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Permert, J.; Larsson, J.; Westermark, G.T.; Herrington, M.K.; Christmanson, L.; Pour, P.M.; Westermark, P.; Adrian, T.E. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N. Engl. J. Med. 1994, 330, 313–318. [Google Scholar] [CrossRef]
- Kanji, S.; Das, H. Advances of Stem Cell Therapeutics in Cutaneous Wound Healing and Regeneration. Mediat. Inflamm. 2017, 2017, 5217967. [Google Scholar] [CrossRef]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bachem, M.G.; Schünemann, M.; Ramadani, M.; Siech, M.; Beger, H.; Buck, A.; Zhou, S.; Schmid-Kotsas, A.; Adler, G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005, 128, 907–921. [Google Scholar] [CrossRef]
- Longnecker, D.; Gorelick, F.; Thompson, E. Anatomy, Histology, and Fine Structure of the Pancreas. In The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery, 3rd ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 10–23. [Google Scholar] [CrossRef]
- Pannala, R.; Basu, A.; Petersen, G.M.; Chari, S.T. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009, 10, 88–95. [Google Scholar] [CrossRef]
- Sah, R.P.; Nagpal, S.J.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef]
- Perera, C.J.; Falasca, M.; Chari, S.T.; Greenfield, J.R.; Xu, Z.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers 2021, 13, 5224. [Google Scholar] [CrossRef]
- Nagpal, S.; Kandlakunta, H.; Sharma, A.; Sannapaneni, S.; Velamala, P.R.; Majumder, S.; Matveyenko, A.; Chari, S. Endocrinopathy in Pancreatic Cancer Is Characterized by Reduced Islet Size and Density with Preserved Endocrine Composition as Compared to Type 2 Diabetes: Presidential Poster Award: 45. Am. J. Gastroenterol. 2018, 113, S26–S28. [Google Scholar] [CrossRef]
- Rozengurt, E.; Sinnett-Smith, J.; Kisfalvi, K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: A novel target for the antidiabetic drug metformin in pancreatic cancer. Clin. Cancer Res. 2010, 16, 2505–2511. [Google Scholar] [CrossRef]
- Haass, C.; Koo, E.H.; Mellon, A.; Hung, A.Y.; Selkoe, D.J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: Alternative processing into amyloid-bearing fragments. Nature 1992, 357, 500–503. [Google Scholar] [CrossRef]
- Cooper, G.J.; Willis, A.C.; Clark, A.; Turner, R.C.; Sim, R.B.; Reid, K.B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA 1987, 84, 8628–8632. [Google Scholar] [CrossRef]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Ben Hmidene, A.; Hanaki, M.; Murakami, K.; Irie, K.; Isoda, H.; Shigemori, H. Inhibitory Activities of Antioxidant Flavonoids from Tamarix gallica on Amyloid Aggregation Related to Alzheimer’s and Type 2 Diabetes Diseases. Biol. Pharm. Bull. 2017, 40, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Murakami, K.; Uno, M.; Ikubo, H.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Irie, K. Structure-activity relationship for (+)-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci. Biotechnol. Biochem. 2013, 77, 1100–1103. [Google Scholar] [CrossRef]
- Kurisu, M.; Miyamae, Y.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Inhibition of amyloid β aggregation by acteoside, a phenylethanoid glycoside. Biosci. Biotechnol. Biochem. 2013, 77, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Chen, X.; Peng, C.; Lin, L. The role of irisin in metabolic flexibility: Beyond adipose tissue browning. Drug Discov. Today 2022, 27, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and quercetin exhibit in vitro antioxidant effect, improve biochemical parameters related to metabolic syndrome, and decrease cellular genotoxicity in humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef] [PubMed]
- Derochette, S.; Franck, T.; Mouithys-Mickalad, A.; Ceusters, J.; Deby-Dupont, G.; Lejeune, J.P.; Neven, P.; Serteyn, D. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem. Biol. Interact. 2013, 206, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200–213. [Google Scholar] [CrossRef]
- Jaishree, V.; Narsimha, S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed. Pharmacother. 2020, 130, 110561. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Li, D.; Yeung, S.C.; Hassan, M.M.; Konopleva, M.; Abbruzzese, J.L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009, 137, 482–488. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chari, S.T. Pancreatic Cancer and Diabetes Mellitus. Curr. Treat. Options Gastroenterol. 2018, 16, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.A.; Law, R.J.; Frank, R.D.; Bamlet, W.R.; Burch, P.A.; Petersen, G.M.; Rabe, K.G.; Chari, S.T. Impact of diabetes mellitus on clinical outcomes in patients undergoing surgical resection for pancreatic cancer: A retrospective, cohort study. Am. J. Gastroenterol. 2014, 109, 1484–1492. [Google Scholar] [CrossRef][Green Version]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef]
- Bhat, K.; Wang, F.; Ma, Q.; Li, Q.; Mallik, S.; Hsieh, T.C.; Wu, E. Advances in biomarker research for pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2439–2451. [Google Scholar] [CrossRef]
- Lumlerdkij, N.; Tantiwongse, J.; Booranasubkajorn, S.; Boonrak, R.; Akarasereenont, P.; Laohapand, T.; Heinrich, M. Understanding cancer and its treatment in Thai traditional medicine: An ethnopharmacological-anthropological investigation. J. Ethnopharmacol. 2018, 216, 259–273. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M. Influence of hydroxyl substitution on flavanone antioxidants properties. Food Chem. 2017, 215, 501–507. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Klein, E.; Dimitrić Marković, J.M.; Milenković, D. Theoretical study of the thermodynamics of the mechanisms underlying antiradical activity of cinnamic acid derivatives. Food Chem. 2018, 246, 481–489. [Google Scholar] [CrossRef]
- Park, M.N.; Rahman, M.A.; Rahman, M.H.; Kim, J.W.; Choi, M.; Kim, J.W.; Choi, J.; Moon, M.; Ahmed, K.R.; Kim, B. Potential Therapeutic Implication of Herbal Medicine in Mitochondria-Mediated Oxidative Stress-Related Liver Diseases. Antioxidants 2022, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. Vitr. 2001, 15, 3–6. [Google Scholar] [CrossRef] [PubMed]
- van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure-Activity Relationship-A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; Vekemans, J.A.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Mainali, K.; Shrestha, B.B.; Sharma, R.K.; Adhikari, A.; Gurarie, E.; Singer, M.; Parmesan, C. Contrasting responses to climate change at Himalayan treelines revealed by population demographics of two dominant species. Ecol. Evol. 2020, 10, 1209–1222. [Google Scholar] [CrossRef]
- Phan, N.D.; Omar, A.M.; Sun, S.; Maneenet, J.; Dibwe, D.F.; Sato, M.; Kalauni, S.K.; Toyooka, N.; Fujii, T.; Awale, S. Abietane diterpenes from Abies spectabilis and their anti-pancreatic cancer activity against the MIA PaCa-2 cell line. Bioorg. Med. Chem. Lett. 2022, 66, 128723. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as Tumor Suppressor in Cancers: Biotherapeutic Activity, Nanodelivery, and Mechanisms With Emphasis on Pancreatic Cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef]
- Johnson, J.L.; de Mejia, E.G. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol. Nutr. Food Res. 2013, 57, 2112–2127. [Google Scholar] [CrossRef]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; O’Neill, E.J.; Gagacev, F.; Tsiani, E. Effects of Berberine against Pancreatitis and Pancreatic Cancer. Molecules 2022, 27, 8630. [Google Scholar] [CrossRef] [PubMed]
- Yassine, F.; Salibi, E.; Gali-Muhtasib, H. Overview of the Formulations and Analogs in the Taxanes’ Story. Curr. Med. Chem. 2016, 23, 4540–4558. [Google Scholar] [CrossRef]
- Qian, Y.; Xiong, Y.; Feng, D.; Wu, Y.; Zhang, X.; Chen, L.; Gu, M. Coix Seed Extract Enhances the Anti-Pancreatic Cancer Efficacy of Gemcitabine through Regulating ABCB1- and ABCG2-Mediated Drug Efflux: A Bioluminescent Pharmacokinetic and Pharmacodynamic Study. Int. J. Mol. Sci. 2019, 20, 5250. [Google Scholar] [CrossRef]
- Liu, P.; Ying, Q.; Liu, H.; Yu, S.Q.; Bu, L.P.; Shao, L.; Li, X.Y. Curcumin enhances anti-cancer efficacy of either gemcitabine or docetaxel on pancreatic cancer cells. Oncol. Rep. 2020, 44, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Dabiri, M.; Alamshahi, A. Chemical composition of the essential oil of Eryngium billardieri F. Delaroche from Iran. J. Essent. Oil Res. 2004, 16, 42–43. [Google Scholar] [CrossRef]
- Roshanravan, N.; Asgharian, P.; Dariushnejad, H.; Mesri Alamdari, N.; Mansoori, B.; Mohammadi, A.; Alipour, S.; Barati, M.; Ghavami, A.; Ghorbanzadeh, V.; et al. Eryngium Billardieri Induces Apoptosis via Bax Gene Expression in Pancreatic Cancer Cells. Adv. Pharm. Bull. 2018, 8, 667–674. [Google Scholar] [CrossRef]
- Song, C.F.; Hu, Y.H.; Mang, Z.G.; Ye, Z.; Chen, H.D.; Jing, D.S.; Fan, G.X.; Ji, S.R.; Yu, X.J.; Xu, X.W.; et al. Hernandezine induces autophagic cell death in human pancreatic cancer cells via activation of the ROS/AMPK signaling pathway. Acta Pharmacol. Sin. 2023, 44, 865–876. [Google Scholar] [CrossRef]
- Ben Marzoug, H.N.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef]
- Benyahia, S.; Benayache, S.; Benayache, F.; León, F.; Quintana, J.; López, M.; Hernández, J.C.; Estévez, F.; Bermejo, J. Cladocalol, a pentacyclic 28-nor-triterpene from Eucalyptus cladocalyx with cytotoxic activity. Phytochemistry 2005, 66, 627–632. [Google Scholar] [CrossRef]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.H.; Amine, G.; Shirazi, M.H.; Hafezi, R.; Mohammadypour, M. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin. Microbiol. Infect. 2006, 12, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; DaCunha, D.C.; Barros, L.; Caires, H.R.; Xavier, C.P.R.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Eucalyptus globulus Labill. decoction extract inhibits the growth of NCI-H460 cells by increasing the p53 levels and altering the cell cycle profile. Food Funct. 2019, 10, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122. [Google Scholar] [CrossRef]
- Sun, S.; Kim, M.J.; Dibwe, D.F.; Omar, A.M.; Athikomkulchai, S.; Phrutivorapongkul, A.; Okada, T.; Tsuge, K.; Toyooka, N.; Awale, S. Anti-Austerity Activity of Thai Medicinal Plants: Chemical Constituents and Anti-Pancreatic Cancer Activities of Kaempferia parviflora. Plants 2021, 10, 229. [Google Scholar] [CrossRef]
- Salman, H.; Bergman, M.; Djaldetti, M.; Bessler, H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed. Pharmacother. 2007, 61, 366–369. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; Maia Gde, A.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef]
- Venier, N.A.; Colquhoun, A.J.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Lycopene enhances the anti-proliferative and pro-apoptotic effects of capsaicin in prostate cancer in vitro. J. Cancer Ther. Res. 2012, 1, 30. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Wang, P.; Gao, S.; Qu, C.; Liu, L. Effects of baicalein on pancreatic cancer stem cells via modulation of sonic Hedgehog pathway. Acta Biochim. Biophys. Sin. 2018, 50, 586–596. [Google Scholar] [CrossRef]
- Chen, P.; Wang, M.; Wang, C. Qingyihuaji formula reverses gemcitabine resistant human pancreatic cancer through regulate lncRNA AB209630/miR-373/EphB2-NANOG signals. Biosci. Rep. 2019, 39, BSR20190610. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Chen, Z.; Chen, S.S.; Liu, L.M.; Zhang, A.Q. Integrated Analyses Identify Immune-Related Signature Associated with Qingyihuaji Formula for Treatment of Pancreatic Ductal Adenocarcinoma Using Network Pharmacology and Weighted Gene Co-Expression Network. J. Immunol. Res. 2020, 2020, 7503605. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.W.; Xu, P.L.; Cheng, C.S.; Jiao, J.Y.; Wu, Y.; Dong, S.; Xie, J.; Zhu, X.Y. Integrating network pharmacology and experimental models to investigate the efficacy of QYHJ on pancreatic cancer. J. Ethnopharmacol. 2022, 297, 115516. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Cheng, C.S.; Xu, P.; Yang, P.; Zhang, K.; Jing, Y.; Chen, Z. Mechanisms of pancreatic tumor suppression mediated by Xiang-lian pill: An integrated in silico exploration and experimental validation. J. Ethnopharmacol. 2022, 298, 115586. [Google Scholar] [CrossRef]
- Karunanithi, S.; Rajkishore, V.B.; Pol, V.G.; Mumthana. M, S.; D, A.; Jayshree. Pharmacognostical and phytochemical studies on leaves of Oxalis corniculata Linn. J. Phytopharm. 2016, 5, 225–229. [Google Scholar] [CrossRef]
- An, E.J.; Kim, Y.; Lee, S.H.; Ko, H.M.; Chung, W.S.; Jang, H.J. Anti-Cancer Potential of Oxialis obtriangulata in Pancreatic Cancer Cell through Regulation of the ERK/Src/STAT3-Mediated Pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef]
- Al-Suede, F.S.R.; Farsi, E.; Ahamed, M.K.B.; Ismail, Z.; Majid, A.S.A.; Majid, A. Marked antitumor activity of cat’s whiskers tea (Orthosiphon stamineus) extract in orthotopic model of human colon tumor in nude mice. J. Biochem. Technol. 2014, 3, S170–S176. [Google Scholar]
- Yehya, A.H.; Asif, M.; Tan, Y.J.; Sasidharan, S.; Majid, A.M.A.; Oon, C.E. Broad spectrum targeting of tumor vasculature by medicinal plants: An updated review. J. Herb. Med. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Yehya, A.H.S.; Asif, M.; Abdul Majid, A.M.S.; Oon, C.E. Complementary effects of Orthosiphon stamineus standardized ethanolic extract and rosmarinic acid in combination with gemcitabine on pancreatic cancer. Biomed. J. 2021, 44, 694–708. [Google Scholar] [CrossRef]
- Yao, L.C.; Wu, L.; Wang, W.; Zhai, L.L.; Ye, L.; Xiang, F.; Tang, Z.G. Panax notoginseng Saponins Promote Cell Death and Chemosensitivity in Pancreatic Cancer through the Apoptosis and Autophagy Pathways. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2021, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Irani, M.; Moazzeni Zehan, H.; Keramatian, B.; Tavakoli Harandi, Z.; Hamzeloo-Moghadam, M. Cytotoxic activity of some ethnic medicinal plants from southwest of Iran. J. Pharmacognosy Res. 2016, 3, 43–47. [Google Scholar]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-inflammatory and anti-oxidant activity of Portulaca oleracea extract on LPS-induced rat lung injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Hashemzehi, M.; Khazdair, M.R.; Askari, V.R. Hydro-ethanolic extract of Portulaca oleracea affects beta-adrenoceptors of guinea pig tracheal smooth muscle. Iran. J. Pharm. Res. IJPR 2016, 15, 867. [Google Scholar]
- Alipour, S.; Pishkar, L.; Chaleshi, V. Cytotoxic Effect of Portulaca Oleracea Extract on the Regulation of CDK1 and P53 Gene Expression in Pancreatic Cancer Cell Line. Nutr. Cancer 2022, 74, 1792–1801. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Shabani Varkani, M.; Clark, C.C.T.; Jafarnejad, S. Quercetin as an anticancer agent: Focus on esophageal cancer. J. Food Biochem. 2020, 44, e13374. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazehkand, A.P.; Soofiyani, S.R.; Hosseini, K.; Martorell, M.; Tarhriz, V.; Ahangari, H.; Cruz-Martins, N.; Sharifi-Rad, J.; Almarhoon, Z.M.; et al. Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects. Oxid. Med. Cell Longev. 2021, 2021, 4393266. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, Y.C.; Ko, S.G. Integrating traditional medicine into modern inflammatory diseases care: Multitargeting by Rhus verniciflua Stokes. Mediators Inflamm. 2014, 2014, 154561. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.-S.; Jang, B.-H.; Shin, Y.-C.; Ko, S.-G. The effect of Rhus verniciflua Stokes (RVS) for anti-aging and whitening of skin. Orient. Pharm. Exp. Med. 2014, 14, 213–222. [Google Scholar] [CrossRef]
- Murtaza, I.; Adhami, V.M.; Hafeez, B.B.; Saleem, M.; Mukhtar, H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int. J. Cancer 2009, 125, 2465–2473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, J.; Chang, Q.; Hu, Z.; Shen, X.; Feng, J.; Zhang, Z.; Wu, X. Total flavonoid aglycones extract in Radix Scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J. Ethnopharmacol. 2019, 235, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Che, Q.; Huang, X.; Li, Y.; Kun, Z.; Teruaki, A.; Masao, H. Studies on metabolites of baicalin in human urine. Zhongguo Zhongyao Zazhi 2001, 26, 768–769. [Google Scholar] [PubMed]
- Wang, Y.; Cao, H.-j.; Sun, S.-j.; Dai, J.-y.; Fang, J.-w.; Li, Q.-h.; Yan, C.; Mao, W.-w.; Zhang, Y.-y. Total flavonoid aglycones extract in Radix scutellariae inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J. Ethnopharmacol. 2016, 194, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef]
- Ji, W.; Sun, X.; Gao, Y.; Lu, M.; Zhu, L.; Wang, D.; Hu, C.; Chen, J.; Cao, P. Natural Compound Shikonin Is a Novel PAK1 Inhibitor and Enhances Efficacy of Chemotherapy against Pancreatic Cancer Cells. Molecules 2022, 27, 2747. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, S.; Gao, W.; Wang, Y.; Liu, P.; Hu, Y.; Dong, H. Extracts of Tripterygium wilfordii Hook F in the Treatment of Rheumatoid Arthritis: A Systemic Review and Meta-Analysis of Randomised Controlled Trials. Evid.-Based Complement. Altern. Med. 2013, 2013, 410793. [Google Scholar] [CrossRef] [PubMed]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Mujumdar, N.; Mackenzie, T.N.; Dudeja, V.; Chugh, R.; Antonoff, M.B.; Borja-Cacho, D.; Sangwan, V.; Dawra, R.; Vickers, S.M.; Saluja, A.K. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology 2010, 139, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Banerjee, S.; Ahmad, A.; Mohammad, R.; Sarkar, F.H. From here to eternity—The secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008, 6, 495–510. [Google Scholar]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone Promotes Pancreatic Cancer Cell Death and Reduction of Tumor Size through Combined Inhibition of Histone Deacetylation and Induction of Histone Acetylation. Adv. Prev. Med. 2016, 2016, 1407840. [Google Scholar] [CrossRef]
- Mu, H.Q.; Yang, S.; Wang, Y.J.; Chen, Y.H. [Role of NF-κB in the anti-tumor effect of thymoquinone on bladder cancer]. Zhonghua Yi Xue Za Zhi 2012, 92, 392–396. [Google Scholar]
- Muralidharan-Chari, V.; Kim, J.; Abuawad, A.; Naeem, M.; Cui, H.; Mousa, S.A. Thymoquinone Modulates Blood Coagulation in Vitro via Its Effects on Inflammatory and Coagulation Pathways. Int. J. Mol. Sci. 2016, 17, 474. [Google Scholar] [CrossRef] [PubMed]
- Yusufi, M.; Banerjee, S.; Mohammad, M.; Khatal, S.; Venkateswara Swamy, K.; Khan, E.M.; Aboukameel, A.; Sarkar, F.H.; Padhye, S. Synthesis, characterization and anti-tumor activity of novel thymoquinone analogs against pancreatic cancer. Bioorg. Med. Chem. Lett. 2013, 23, 3101–3104. [Google Scholar] [CrossRef]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. 2022, 36, 1854–1883. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, Y.J.; Lee, J.H.; Nam, M.J. Naringenin causes ASK1-induced apoptosis via reactive oxygen species in human pancreatic cancer cells. Food Chem. Toxicol. 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Lou, C.; Zhang, F.; Yang, M.; Zhao, J.; Zeng, W.; Fang, X.; Zhang, Y.; Zhang, C.; Liang, W. Naringenin decreases invasiveness and metastasis by inhibiting TGF-β-induced epithelial to mesenchymal transition in pancreatic cancer cells. PLoS ONE 2012, 7, e50956. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H.; Kim, J.H. Combined administration of naringenin and hesperetin with optimal ratio maximizes the anti-cancer effect in human pancreatic cancer via down regulation of FAK and p38 signaling pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Polier, G.; Köhler, R.; Giaisi, M.; Krammer, P.H.; Li-Weber, M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 2012, 287, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Polier, G.; Ding, J.; Konkimalla, B.V.; Eick, D.; Ribeiro, N.; Köhler, R.; Giaisi, M.; Efferth, T.; Desaubry, L.; Krammer, P.H.; et al. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Garg, V.K.; Kumar, N.; Sharma, P.K.; Chaudhary, S.; Upadhyay, A. Pharmacognostical studies on the leaves of Ziziphus nummularia (Burm. F.). Eur. J. Exper. Biol. 2011, 1, 77–83. [Google Scholar]
- Kapoor, B.; Arora, V. Ethnomedicinal plants of jaisalmer district of rajasthan used in herbal and folk remedies. Int. J. Ethnobiol. Ethnomed. 2014, 1, 1–6. [Google Scholar]
- Mesmar, J.; Fardoun, M.M.; Abdallah, R.; Al Dhaheri, Y.; Yassine, H.M.; Iratni, R.; Badran, A.; Eid, A.H.; Baydoun, E. Ziziphus nummularia Attenuates the Malignant Phenotype of Human Pancreatic Cancer Cells: Role of ROS. Molecules 2021, 26, 4295. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.H.; Ko, S.G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Cheng, C.S.; Tan, H.Y.; Wang, N.; Chen, L.; Meng, Z.; Chen, Z.; Feng, Y. Functional inhibition of lactate dehydrogenase suppresses pancreatic adenocarcinoma progression. Clin. Transl. Med. 2021, 11, e467. [Google Scholar] [CrossRef]
- Rotow, J.; Bivona, T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Han, J.W. Targeting epigenetics for cancer therapy. Arch. Pharm. Res. 2019, 42, 159–170. [Google Scholar] [CrossRef]
- Lee, S.K.; Jung, H.S.; Eo, W.K.; Lee, S.Y.; Kim, S.H.; Shim, B.S. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: Report of two cases. Ann. Oncol. 2010, 21, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, W.C.; Kim, K.S.; Park, J.W.; Lee, S.H.; Yoon, S.W. Shrinkage of gastric cancer in an elderly patient who received Rhus verniciflua Stokes extract. J. Altern. Complement. Med. 2010, 16, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.S.; Choi, W.C.; Yoon, S.W. Successful outcome of advanced pulmonary adenocarcinoma with malignant pleural effusion by the standardized rhus verniciflua stokes extract: A case study. Explore 2009, 5, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.; Jung, H.; Lee, S.; Cheon, S.; Kim, S.; Eo, W.; Choi, W. Efficacy and safety of standardized allergen-removed Rhus verniciflua Stokes extract in patients with advanced or metastatic pancreatic cancer: A Korean single-center experience. Oncology 2011, 81, 312–318. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Arena, F.P.; Bienvenu, B.J.; Kaplan, E.H.; Camacho, L.H.; Campos, L.T.; Waymack, J.P.; Tagliaferri, M.A.; Chen, M.M.; Li, D. A Randomized, Open-Label, Safety and Exploratory Efficacy Study of Kanglaite Injection (KLTi) plus Gemcitabine versus Gemcitabine in Patients with Advanced Pancreatic Cancer. J. Cancer 2017, 8, 1872–1883. [Google Scholar] [CrossRef]
- Staupe, H.; Buentzel, J.; Keinki, C.; Buentzel, J.; Huebner, J. Systematic analysis of mistletoe prescriptions in clinical studies. J. Cancer Res. Clin. Oncol. 2023, 149, 5559–5571. [Google Scholar] [CrossRef]
- de Giorgio, A.; Stebbing, J. Mistletoe: For cancer or just for Christmas? Lancet Oncol. 2013, 14, 1264–1265. [Google Scholar] [CrossRef]
- Lordick, F. Mistletoe treatment for cancer--promising or passé? Dtsch. Arztebl. Int. 2014, 111, 491–492. [Google Scholar] [CrossRef]
- Matthes, H.; Friedel, W.E.; Bock, P.R.; Zänker, K.S. Molecular mistletoe therapy: Friend or foe in established anti-tumor protocols? A multicenter, controlled, retrospective pharmaco-epidemiological study in pancreas cancer. Curr. Mol. Med. 2010, 10, 430–439. [Google Scholar] [CrossRef]
- Friedel, W.E.; Matthes, H.; Bock, P.R.; Zänker, K.S. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo- and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: Multicenter, controlled, observational cohort study. J. Soc. Integr. Oncol. 2009, 7, 137–145. [Google Scholar]
- Schad, F.; Atxner, J.; Buchwald, D.; Happe, A.; Popp, S.; Kröz, M.; Matthes, H. Intratumoral Mistletoe (Viscum album L.) Therapy in Patients With Unresectable Pancreas Carcinoma: A Retrospective Analysis. Integr. Cancer Ther. 2014, 13, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, W.; Cheung, F.; Tan, H.Y.; Wang, N.; Feng, Y. Integrating Network Pharmacology and Experimental Models to Investigate the Efficacy of Coptidis and Scutellaria Containing Huanglian Jiedu Decoction on Hepatocellular Carcinoma. Am. J. Chin. Med. 2020, 48, 161–182. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Nouzari, S.; Jalalian, H.; Sahebkar, A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic efficacy: A systematic review of the evidence from randomized controlled trials. Cancer Treat. Rev. 2007, 33, 407–418. [Google Scholar] [CrossRef]
- Ji, B.; Liu, J.; Ma, Y.; Yin, Y.; Xu, H.; Shen, Q.; Yu, J. Minnelide Markedly Reduces Proteinuria in Mice with Adriamycin Nephropathy by Protecting Against Podocyte Injury. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Modi, S.; Giri, B.; Gupta, V.K.; Lavania, S.; Sethi, V.; Sharma, N.S.; Pandey, S.; Vickers, S.; Dudeja, V.; Saluja, A.K. Minnelide synergizes with conventional chemotherapy by targeting both cancer and associated stroma components in pancreatic cancer. Cancer Lett. 2022, 537, 215591. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Qin, S.; Ma, D.; Jiao, S.; Yu, S.; Li, J.; Liu, D.; Song, D.; Li, D. A multicenter randomized phase II trial on Kanglaite Injection (KLT) plus gemcitabine hydrochloride (GEM) versus GEM in patients with local advanced and metastatic pancreatic cancer. J. Clin. Oncol. 2011, 29 (Suppl. 15), e14510. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Zhou, X.; Liu, Y.; Das, A.; Vincent, B.G.; Li, J.; Liu, R.; Huang, L. Tumor neoantigen heterogeneity impacts bystander immune inhibition of pancreatic cancer growth. Transl. Oncol. 2020, 13, 100856. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Tao, Z.; Gu, W.; Sun, L.H. Variation of blood T lymphocyte subgroups in patients with non-small cell lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 4671–4673. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Min, H. Ginseng, the ‘Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- Hwang, I.; Ahn, G.; Park, E.; Ha, D.; Song, J.Y.; Jee, Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol. Lett. 2011, 138, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Lee, K.Y.; Kang, J.; Park, J.S.; Jeong, J. Immune-modulating Effect of Korean Red Ginseng by Balancing the Ratio of Peripheral T Lymphocytes in Bile Duct or Pancreatic Cancer Patients with Adjuvant Chemotherapy. In Vivo 2021, 35, 1895–1900. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef]
- Sohal, D.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef]
- Shojania, K.G.; Sampson, M.; Ansari, M.T.; Ji, J.; Doucette, S.; Moher, D. How quickly do systematic reviews go out of date? A survival analysis. Ann. Intern. Med. 2007, 147, 224–233. [Google Scholar] [CrossRef]
- Ko, A.H.; Tempero, M.A.; Shan, Y.S.; Su, W.C.; Lin, Y.L.; Dito, E.; Ong, A.; Wang, Y.W.; Yeh, C.G.; Chen, L.T. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br. J. Cancer 2013, 109, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Pebole, M.M.; Hall, K.S. Insights Following Implementation of an Exercise Intervention in Older Veterans with PTSD. Int. J. Environ. Res. Public. Health 2019, 16, 2630. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Shen, M.; Xu, M.D.; Yu, Z.Y.; Tao, M. FOLFIRINOX regulated tumor immune microenvironment to extend the survival of patients with resectable pancreatic ductal adenocarcinoma. Gland. Surg. 2020, 9, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Wu, W.; Wang, B.; Cui, C.; Niu, J.; Chen, J.; Chen, Z.; Liu, Y. CXCL5 Plays a Promoting Role in Osteosarcoma Cell Migration and Invasion in Autocrine- and Paracrine-Dependent Manners. Oncol. Res. 2017, 25, 177–186. [Google Scholar] [CrossRef]
- Bonavita, O.; Massara, M.; Bonecchi, R. Chemokine regulation of neutrophil function in tumors. Cytokine Growth Factor. Rev. 2016, 30, 81–86. [Google Scholar] [CrossRef]
- Klaiber, U.; Hackert, T.; Neoptolemos, J.P. Adjuvant treatment for pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019, 4, 27. [Google Scholar] [CrossRef]
- Dahan, L.; Phelip, J.M.; Le Malicot, K.; Williet, N.; Desrame, J.; Volet, J.; Petorin, C.; Malka, D.; Rebischung, C.; Aparicio, T.; et al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRI.3 for first-line treatment of metastatic pancreatic cancer: A randomized phase II trial (PRODIGE 35-PANOPTIMOX). J. Clin. Oncol. 2018, 36 (Suppl. 15), 4000. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.M.; Summers, S.A.; Gunter, M.; et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity 2022, 30, 1323–1334. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018, 155, 730–739. [Google Scholar] [CrossRef]
| Compound | Mechanism | Experimental Model | Reference |
|---|---|---|---|
| Epicatechin and quercetin (1:1 combination) | reductions in total cholesterol, LDL-cholesterol, total triglycerides, and fasting plasma glucose | randomized placebo-controlled trial | [127] |
| Curcumin | nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, which leads to an increase in the activity of antioxidant enzymes | healthy horses (mixed breeds, mean age 6.2 ± 2.3) | [128,129] |
| Combination of swertiamarin and quercetin (CSQ) | activation of viable β cells, reducing levels of total cholesterol, triglycerides, and LDL, while simultaneously increasing HDL | diabetic rats | [140] |
| Metformin | insulin or sulfonylureas | patients | [131,132] |
| Naringenin | upregulation of adenosine-monophosphate-activated protein kinase (AMPK), a key enzyme involved in cellular energy regulation. | [141] |
| Compound | Mechanism | Experimental Model | Reference |
|---|---|---|---|
| Abies spectabilis | 100% cell death at 50 μg/mL | MIA PaCa-2 cells | [150] |
| Apigenin | Increased the expression of the cytokine genes IL17F, LTA, IL17C, IL17A, and IFNB1 | BxPC-3, PANC-1, BxPC-3 cells | [152] |
| Berberine | Release of cytochrome c and caspase 7 | BxPC-3, HPDE-E6E7c7 cells | [154] |
| Coix seed extract | Inhibion of ABCB1 and ABCG2, combined with gemcitabine (combination index = 0.54) IC50 and tumor growth inhibition rate (TGI), bioluminescent pharmacokinetics | PANC-1, BxPC3luc cells, BxPC3luc xenografts nude mice | [156] |
| Combination of docetaxel or gemcitabine with curcumin | Metastasis suppression using a matrigel-transwell test and wound healing Downregulation of MMP2/MMP9 and N-cadherin proteins and the overexpression of TIMP1/TIMP2 | PANC-1, HPAF-II, MIAPaCa-2 cells | [157] |
| Combination of gemcitabine with ethanolic extract of Orthosiphon stamineus | Inhibiting migration triggered caspase-3, causing early apoptosis by cleaved PARP | PANC-1 cells | [182] |
| Eucalyptus globulus Labill | Reduced cell viability | PANC-1 cells | [165] |
| Eryngium Billardieri | Reduction of cyclin D1 expression but increase in Bax leads to apoptosis no effect on normal cells | PANC-1, KDR/293 cells | [159] |
| Hernandezine | Increasing AMPK phosphorylation or ROS and lowering mTOR/p70S6K phosphorylation | Capan-1, SW1990 cells | [160] |
| Kaempferia parviflora | Anti-austerity efficacy Inhibiting colony formation | PANC-1 cells | [168] |
| Lycopene | Inhibiting NF-kB activity and reducing reactive oxygen species The ratio of Bax to Bcl-2 is increased, but active caspase-3 and cIAP1 and cIAP2 are downregulated | PANC-1 cells | [172] |
| Naringenin | Increased activity of ASK1, P38, P53, JNK, and reactive oxygen species Gemcitabine’s ability to prevent cancer drug resistance also limits tumor cell invasion | SNU-213, AsPC-1, PANC-1 cells | [211] |
| Combination with naringenin | Effects that suppress cell proliferation, invasion, and p38 signaling | MIA PaCa-2, PANC-1, SNU-213, BALB/c nude mice | [213] |
| Obtriangulata methanol extract | Accumulation of apoptotic effects in the G2/M phase due to ERK, Src, and STAT3 downregulation | BxPC3 cells | [179] |
| Panax notoginseng Saponins | Apoptosis by flow cytometry, colony formation, and EdU for cell proliferation; wound healing and transwell for cell migration and invasiveness; CCK-8 for cell viability | MIA PaCa-2, PANC-1 cells | [183] |
| Portulaca oleracea | Induction of apoptosis as decreasing CDK1 and increasing P53 gene expression | PANC-1, HUVEC cells | [187] |
| Pomegranate extract | Using a chick chorioallantoic membrane (CAM) model to examine the antiangiogenic effect | Suit-2 cells | [188] |
| QYHJ | Keap1/Nrf2/HO-1/NQO1 axis, p-PI3K/p-AKT/p-mTOR, and p-AKT/mTOR inhibition | PANC-1, MIA PaCa-2 cells | [176] |
| Quercetin | Reductions in EMT, invasion, and metastasis with downregulation of N-cadherin, MMP-9, and STAT-3 | PANC-1 cells | [191] |
| Rhus verniciflua Stokes | Receptor DR3 mediates NF-kappaB pIkBalpha/beta kinases (pIKKs), MMP9, and XIAP downregulation | PaC cells | [194] |
| Shikonin | PAK1 inhibitors | BxPC−3, PANC−1 cells | [200] |
| Total flavonoid aglycone extracted | Inhibition of the Akt/mTOR/PI3K signaling cascade Cleavage of caspase proteins as part of the caspase signaling cascade in annexin V/PI-positive cells but showed low cytotoxicity toward normal cells | BxPC3, HPDE6-C7 cells | [195] |
| Triptolide | Inducing caspase-independent apoptosis Induces autophagic death | MiaPaCa-2, Capan-1, BxPC-3, S2-013, S2-VP10, Hs766T cells | [202] |
| Thymoquinone | Increased p21 and p53; downregulation of Bcl-xL, Bcl-2, XIAP, Notch1, NICD, PTEN, Akt/mTOR/S6 signaling Upregulation of caspases-3, -9, Bax, and cytochrome c; G2 cycle arrest and Sub G0/G1 arrest | MiaPaCa-2, AsPC-1 cells | [206,207,208,209] |
| Wogonin | p53 expression was stimulated, and the Beclin-1/PI3K, Akt/ULK1/4E-BP1/CYLD, and mTOR pathways were all inhibited | PANC-1, Colo-357, HPCCs4, Capan-1, Colo-357 cells | [144] |
| Wogonin | Suppressing Mcl-1, CDK-9, c-FLIP, and MDM2 | Capan-1, Colo-357 cells | [215,216] |
| XLP | A reduction in the activity of MMP2, PTGS2, CASP9, IL4, and CTSD | Mouse PC Panc-02 cell transfection pCDNA3.1(+)-PTGS2 and pCDNA3.1(+)-NC, male C57BL/6 mice, 4–6 weeks old | [177,222] |
| Ziziphus nummularia ethanolic extract | Suppresses of MMP-9, proliferation, migration, invasive potential, ERK1/2(MAPK), NFκB pathways, collagen adhesion, integrin α2 expression, and VEGF production Induction of caspase-3-dependent | Capan-2 cells | [219] |
| Compound | Efficacy | Reference |
|---|---|---|
| Curcuminoids | Health benefits, including fewer unwanted consequences, are increasing | [238,239,240] |
| Mistletoe (Viscum album) | Improve survival rates from the current 2.7 months to 4.8 | [233,235] |
| QYHJ | Improved 1-, 3-, and 5-year survival with no obvious side effects | [177] |
| aRVS | Improve survival rates with single or combined treatment | [228] |
| Combination of curcuminoids and gemcitabine | Curcumin increases chemotherapy’s efficacy | [237] |
| Combination of kanglaite injection (KLTi) and gemcitabine | Improve survival rates with quality of life Or no obvious side effects | [229] |
| Korean red ginseng | Elevated CD4+ lymphocytes and a higher CD4+/CD8+ T lymphocyte counts | [249,250] |
| Drug | Target | Characterization | Reference |
|---|---|---|---|
| Olaparib | PDAC drivers with KRAS mutations, NTRK1–3 fusions, and BRCA1/2 mutations | BRCA1/2 mutations | [266] |
| Afatinib and zenocutuzumab | PDAC drivers, which are characterized by the presence of wild-type KRAS, include ERBB inhibitors | ERBB mutations | [12] |
| Larotrectinib and entrectinib | PDAC drivers include TRK inhibitors, which are activated by the presence of wild-type KRAS | TRK mutations | [12] |
| Crizotinib | PDAC drivers with wild-type KRAS include sensitivity to ALK/ROS inhibitors | ALK/ROS mutations | [12] |
| Pembrolizumab | Programmed cell death protein (PD-1) inhibitor | Microsatellite instability high (MSI-H) | [253,267] |
| FOLFIRINOX; leucovorin (LV), 5-fluorouracil (5-FU), irinotecan, and oxaliplatin | C-X-C motif chemokine 5 (CXCL5) | Influence on tumor immunity regulation | [259] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.N. Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products. Int. J. Mol. Sci. 2023, 24, 15906. https://doi.org/10.3390/ijms242115906
Park MN. Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products. International Journal of Molecular Sciences. 2023; 24(21):15906. https://doi.org/10.3390/ijms242115906
Chicago/Turabian StylePark, Moon Nyeo. 2023. "Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products" International Journal of Molecular Sciences 24, no. 21: 15906. https://doi.org/10.3390/ijms242115906
APA StylePark, M. N. (2023). Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products. International Journal of Molecular Sciences, 24(21), 15906. https://doi.org/10.3390/ijms242115906






