Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Purification of PFSP-2-1
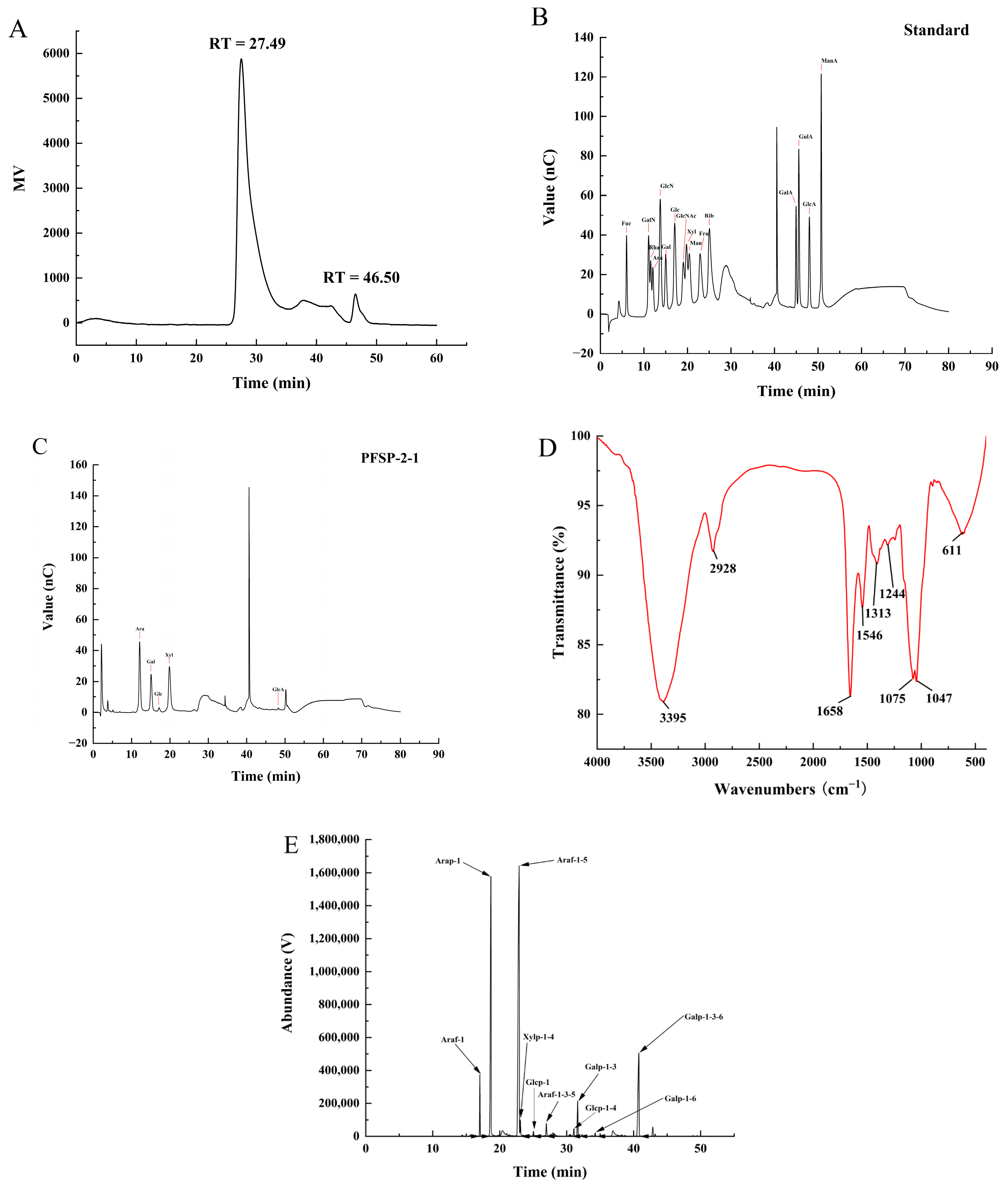
2.2. Characterization of PFSP-2-1
2.3. Antitumor Activity In Vitro
2.4. Discussion
3. Materials and Methods
3.1. Materials
3.2. Extraction and Purification of PFSP-2-1
3.3. Structural Characterization of PFSP-2-1
3.3.1. Physicochemical Characterization of PFSP-2-1
3.3.2. Relative Molecular Weight (Mw) of PFSP-2-1
3.3.3. Monosaccharide Composition Analysis
3.3.4. Methylation Analysis
3.3.5. FT-IR and NMR Spectroscopy
3.3.6. Microscopic Analysis
3.3.7. Triple-Helix Structure Determination
3.4. Evaluation of Antitumor Activity
3.4.1. Cell Culture
3.4.2. Cytotoxicity Experiment
3.5. Statistical Analysis
4. Conclusions
5. Limitation
- (1)
- We did not compare the structure of polysaccharides via different extraction methods and, thus, could not possibly understand the entire structure of polysaccharides;
- (2)
- This study only investigated the antitumor activity of polysaccharides from Perilla frutescens, but the mechanism of antitumor activity will need to be explored in the further.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbieri, C.; Ferrazzi, P. Perilla frutescens: Interesting New Medicinal and Melliferous Plant in Italy. Nat. Prod. Commun. 2011, 6, 1934578X1100601. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound Pre-Treatment Combined with Microwave-Assisted Hydrodistillation of Essential Oils from Perilla frutescens (L.) Britt. Leaves and Its Chemical Composition and Biological Activity. Ind. Crops Prod. 2020, 143, 111908. [Google Scholar] [CrossRef]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-J.; Yu, C.-H.; Ying, K.-J.; Hua, J.; Dai, X.-Y. Hypolipidemic and Antioxidant Effects of Total Flavonoids of Perilla frutescens Leaves in Hyperlipidemia Rats Induced by High-Fat Diet. Food Res. Int. 2011, 44, 404–409. [Google Scholar] [CrossRef]
- He, Y.-K.; Yao, Y.-Y.; Chang, Y.-N. Characterization of Anthocyanins in Perilla frutescens Var. Acuta Extract by Advanced UPLC-ESI-IT-TOF-MSn Method and Their Anticancer Bioactivity. Molecules 2015, 20, 9155–9169. [Google Scholar] [CrossRef]
- Osakabe, N. Rosmarinic Acid Inhibits Epidermal Inflammatory Responses: Anticarcinogenic Effect of Perilla frutescens Extract in the Murine Two-Stage Skin Model. Carcinogenesis 2003, 25, 549–557. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, Structural Characterization, Biological Activity, and Application of Glycyrrhiza Polysaccharides: Systematic Review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, L.-L.; Liu, H.-P.; Wu, Y.-R.; Li, M.-Y.; Jia, Q. Structural Characterization of a Novel Polysaccharide from Pleurotus citrinopileatus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Int. J. Biol. Macromol. 2021, 168, 251–260. [Google Scholar] [CrossRef]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective Effect of Polysaccharides from Sargassum fusiforme against UVB-Induced Oxidative Stress in HaCaT Human Keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Yang, X.; Lin, P.; Wang, J.; Liu, N.; Yin, F.; Shen, N.; Guo, S. Purification, Characterization and Anti-Atherosclerotic Effects of the Polysaccharides from the Fruiting Body of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 181, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, Y.; Wei, X.; Yang, Z.; Xiao, J.; Jin, Z. Sulfation of Tea Polysaccharides: Synthesis, Characterization and Hypoglycemic Activity. Int. J. Biol. Macromol. 2010, 46, 270–274. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhao, L.; Li, S.; Zhao, X.; Zhang, Q.; Xiong, Q. Preliminary Characterization and Immunostimulatory Activity of Polysaccharides from Glossaulax didyma. Food Chem. Toxicol. 2013, 62, 226–230. [Google Scholar] [CrossRef]
- Li, S.; Dai, S.; Shah, N.P. Sulfonation and Antioxidative Evaluation of Polysaccharides from Pleurotus Mushroom and Streptococcus thermophilus Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 282–294. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Liao, D.; Cheng, C.; Liu, J.; Zhao, L.; Huang, D.-C.; Chen, G. Characterization and Antitumor Activities of Polysaccharides Obtained from Ginger (Zingiber Officinale) by Different Extraction Methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, G.; Wang, M.; Lv, Z.; Du, P. A Selenium Polysaccharide from Platycodon Grandiflorum rescues PC12 Cell Death Caused by H2O2 via Inhibiting Oxidative Stress. Int. J. Biol. Macromol. 2017, 104, 393–399. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between Antitumor Activity, Molecular Weight, and Conformation of Lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Q.; Lin, R.; He, P.; Lai, F.; Zhang, M.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Acid Polysaccharide Isolated from the Pulp of Rosa laevigata Michx Fruit. Int. J. Biol. Macromol. 2020, 145, 1080–1090. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A Novel Low-Molecular-Mass Pumpkin Polysaccharide: Structural Characterization, Antioxidant Activity, and Hypoglycemic Potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from Fruit Bodies of Cultivated Mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and Potential Prebiotic Activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, R.; Li, H.; Wang, Y.; Luo, Y.; Zou, J.; Zhao, B.; Chen, S. New Insight into the Joint Significance of Dietary Jujube Polysaccharides and 6-Gingerol in Antioxidant and Antitumor Activities. RSC Adv. 2021, 11, 33219–33234. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Feng, W.; Zhang, Z.; Li, H. Structural Characterization and Immunomodulatory Activities of Two Polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef]
- Xiong, Q.; Luo, G.; Zheng, F.; Wu, K.; Yang, H.; Chen, L.; Tian, W. Structural Characterization and Evaluation the Elicitors Activity of Polysaccharides from Chrysanthemum indicum. Carbohydr. Polym. 2021, 263, 117994. [Google Scholar] [CrossRef]
- Dourado, F.; Cardoso, S.; Silva, A.; Gama, F.; Coimbra, M. NMR Structural Elucidation of the Arabinan from Prunus dulcis Immunobiological Active Pectic Polysaccharides. Carbohydr. Polym. 2006, 66, 27–33. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Yu, J.; Liu, Y.; Lu, W.; Chai, Y.; Liu, C.; Pan, C.; Yao, W.; Gao, X. Structure, Chain Conformation, and Immunomodulatory Activity of the Polysaccharide Purified from Bacillus Calmette Guerin Formulation. Carbohydr. Polym. 2016, 150, 149–158. [Google Scholar] [CrossRef]
- Fu, Z.; Xia, L.; De, J.; Zhu, M.; Li, H.; Lu, Y.; Chen, D. Beneficial Effects on H1N1-Induced Acute Lung Injury and Structure Characterization of Anti-Complementary Acidic Polysaccharides from Juniperus pingii Var. Wilsonii. Int. J. Biol. Macromol. 2019, 129, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Gao, X.; Cheng, C.; Zhao, H.; Wang, Z.; Yi, J. Hepatoprotection Mechanism against Alcohol-Induced Liver Injury in Vivo and Structural Characterization of Pinus koraiensis Pine Nut Polysaccharide. Int. J. Biol. Macromol. 2020, 154, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Q.; Pang, Z.; Xie, J.; Lin, L.; Yao, Q. Optimization Extraction, Characterization and Anticancer Activities of Polysaccharides from Mango Pomace. Int. J. Biol. Macromol. 2018, 117, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, Structure, Pharmacological Activities and Drug Carrier Applications of Angelica sinensis Polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, X.; Ma, J.; Zhang, M.; Wu, H. Structural Elucidation of a Novel Pectin-Polysaccharide from the Petal of Saussurea laniceps and the Mechanism of Its Anti-HBV Activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural Characterization and Anti-Inflammatory Activity of a Polysaccharide from the Lignified okra. Carbohydr. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, Purification, Structural Characterization, and Antioxidant Activity of Polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Qian, T.; Sun, G.; Guo, Y.; Chang, F.; Zhou, S.; Sun, X. Antitumor and Immunomodulatory Activity of Arabinoxylans: A Major Constituent of Wheat Bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Wang, D.; Li, X.; Wu, X.; Liu, X.; Du, G.; Li, X.; Qin, X.; Du, Y. Extraction, Characterization, Antitumor and Immunological Activities of Hemicellulose Polysaccharide from Astragalus Radix Herb Residue. Molecules 2019, 24, 3644. [Google Scholar] [CrossRef]
- Hu, J.; Yao, W.; Chang, S.; You, L.; Zhao, M.; Chi-Keung Cheung, P.; Hileuskaya, K. Structural Characterization and Anti-Photoaging Activity of a Polysaccharide from Sargassum fusiforme. Food Res. Int. 2022, 157, 111267. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Siu, K.-C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Li, X.-Q.; Liu, A.-J. Relationship between Heat Treatment on Structural Properties and Antitumor Activity of the Cold-Water Soluble Polysaccharides from Grifola frondosa. Glycoconj J 2020, 37, 107–117. [Google Scholar] [CrossRef]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of Molecular Structure on Antitumor Activities of (1→3)-β-d-Glucans from Different Lentinus Edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Gong, P.; Yang, W.; Wang, L.; Liu, N.; Su, Y.; Zhao, Y. Anti-oxidant and anti-fatigue properties of apple pomace polysaccharides by acid or alkali extraction. Int. J. Food Sci. Technol. 2021, 57, 78–91. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.; Lin, Y.M. Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Xie, F.; Yaseen, A.; Chen, B.; Zhang, G.-L.; Wang, M.-K.; Shen, X.-F.; Li, F. A polysaccharide TKP-2-1 from Tamarindus indica L: Purification, structural characterization and immunomodulating activity. J. Funct. Foods 2021, 78, 104384. [Google Scholar] [CrossRef]
- Niu, L.; Wu, Y.; Liu, H.; Wang, Q.; Li, M.; Jia, Q. The Structural Characterization of a Novel Water-Soluble Polysaccharide from Edible Mushroom Leucopaxillus giganteus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Chem. Biodivers. 2021, 18, e2001010. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shen, W.; Zhou, Y.; Ma, L.; Xu, D.; Ding, J.; He, L.; Shen, B.; Zhou, C. Characterization of a polysaccharide from Eupolyphaga sinensis walker and its effective antitumor activity via lymphocyte activation. Int. J. Biol. Macromol. 2020, 162, 31–42. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Mac Regenstein, J.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2020, 251, 117078. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Onyango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochemistry 2020, 70, 105355. [Google Scholar] [CrossRef]
- Xiang, B.; Yu, X.; Li, B.; Xiong, Y.; Long, M.; He, Q. Characterization, antioxidant, and anticancer activities of a neutral polysaccharide from Duchesnea indica (Andr.) Focke. J. Food Biochem. 2019, 43, e12899. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, X.; Lu, X.-T.; Cui, C.-B.; Gao, X.-F. Study on hypoglycemic effects of irradiated ginseng adventitious roots. Food Chem. X 2022, 13, 100234. [Google Scholar] [CrossRef]






| Retention Time (min) | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Linage Pattern |
|---|---|---|---|---|
| 17.040 | 2,3,5-Me3-Araf | 43, 71, 87, 101, 117, 129, 145, 161 | 0.054 | Araf-(1→ |
| 18.679 | 2,3,5-Me3-Arap | 43, 71, 87, 101, 117, 129, 145, 161 | 0.238 | Arap-(1→ |
| 22.895 | 2,3,5-Me3-Arap | 43, 71, 87, 99, 101, 117, 129, 161, 189 | 0.503 | →5)-Araf-(1→ |
| 23.081 | 2,3-Me2-Xylp | 43, 71, 87, 99, 101, 117, 129, 161, 189 | 0.012 | →4)-Xylp-(1→ |
| 25.036 | 2,3,4,6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 0.007 | Glcp-(1→ |
| 26.967 | 2-Me1-Araf | 43, 58, 85, 99, 117, 127, 159, 201 | 0.008 | →3,5)-Araf-(1→ |
| 31.079 | 2,3,6-Me3-Glcp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 0.005 | →4)-Glcp-(1→ |
| 31.639 | 2,4,6-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | 0.037 | →3)-Galp-(1→ |
| 34.249 | 2,3,4-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 189, 233 | 0.003 | →6)-Galp-(1→ |
| 40.762 | 2,4-Me2-Galp | 43, 87, 117, 129, 159, 189, 233 | 0.132 | →3,6)-Galp-(1→ |
| Sugar Residues | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
|---|---|---|---|---|---|---|---|
| A | Araf-(1→ | 5.09/108.73 | 4.45/103.22 | 3.91/66.87 | 3.98/83.77 | 3.62/61.02 | 3.54 |
| B | Arap-(1→ | 4.45/105.42 | — | — | — | — | — |
| C | →5)-Araf-(1→ | 5.09/106.95 | 4.04/81.17 | 3.94/76.62 | 4.07/86.59 | 3.83/67.09 | — |
| D | →4)-Xylp-(1→ | 5.09/106.95 | 3.36/73.15 | 3.54/75.54 | 3.83/67.09 | 4.13/54.31 | — |
| E | Glcp-(1→ | 4.35/103.22 | 3.98/83.77 | 4.09/82.47 | 3.54/65.36 | 3.80/68.60 | 4.08 |
| F | →3,5)-Araf-(1→ | 5.10/106.78 | 4.07/86.59 | 4.04/81.17 | 3.80/68.60 | 3.54/65.36 | — |
| G | →4)-Glcp-(1→ | 4.44/104.94 | 3.23/65.14 | 3.62/61.02 | 3.80/68.60 | 3.91/66.87 | — |
| H | →3)-Galp-(1→ | 4.46/104.99 | 3.62/61.02 | 3.98/83.77 | 4.05/87.24 | 4.07/86.59 | 3.83 |
| I | →6)-Galp-(1→ | 4.46/104.63 | 3.62/61.02 | 3.80/68.60 | 3.94/76.62 | 3.89/65.14 | 4.09 |
| J | →3,6)-Galp-(1 | 4.45/103.22 | 3.92/68.60 | 3.63/61.03 | 4.01/81.17 | 3.85/67.09 | 3.85 |
| Cell | Concentration (μg/mL) | F | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polysaccharide | Control | 100 | 200 | 400 | 800 | 1600 | |||
| HepG2 | PFSP-2-1 | 104.99 ± 5.84 a | 96.12 ± 2.05 ab | 94.73 ± 1.36 b | 87.98 ± 2.54 c | 71.86 ± 3.12 d | 53.34 ± 6.62 e | 64.78808 | <0.001 |
| PFSP-2-2 | 104.99 ± 5.84 a | 100.37 ± 6.95 ab | 94.73 ± 3.21 bc | 90.34 ± 4.50 c | 80.62 ± 4.08 d | 61.07 ± 3.89 e | 31.55905 | <0.001 | |
| Hep3b | PFSP-2-1 | 100.00 ± 5.29 a | 98.41 ± 3.59 a | 95.04 ± 4.00 ab | 89.74 ± 4.26 b | 78.16 ± 6.40 c | 70.33 ± 1.76 c | 21.52656 | <0.001 |
| PFSP-2-2 | 100.00 ± 5.29 a | 97.32 ± 2.03 ab | 94.74 ± 3.82 ab | 90.52 ± 4.46 bc | 85.01 ± 5.85 c | 75.58 ± 4.92 d | 11.73893 | <0.001 | |
| SK-Hep-1 | PFSP-2-1 | 100.00 ± 2.00 a | 97.77 ± 1.37 ab | 94.01 ± 2.82 b | 88.62 ± 2.51 c | 84.31 ± 3.39 c | 71.06 ± 3.82 d | 44.13431 | <0.001 |
| PFSP-2-2 | 100.00 ± 2.00 a | 97.26 ± 3.75 a | 93.11 ± 4.20 a | 92.73 ± 6.48 a | 73.94 ± 3.96 b | 64.02 ± 4.89 c | 32.00001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, M.; Liu, Z.; Cheng, L.; Li, M.; Li, C. Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds. Int. J. Mol. Sci. 2023, 24, 15904. https://doi.org/10.3390/ijms242115904
Li H, Liu M, Liu Z, Cheng L, Li M, Li C. Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds. International Journal of Molecular Sciences. 2023; 24(21):15904. https://doi.org/10.3390/ijms242115904
Chicago/Turabian StyleLi, Hui, Ming Liu, Zikun Liu, Li Cheng, Mengsha Li, and Chongwei Li. 2023. "Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds" International Journal of Molecular Sciences 24, no. 21: 15904. https://doi.org/10.3390/ijms242115904
APA StyleLi, H., Liu, M., Liu, Z., Cheng, L., Li, M., & Li, C. (2023). Purification, Structural Characterization, and Antitumor Activity of a Polysaccharide from Perilla Seeds. International Journal of Molecular Sciences, 24(21), 15904. https://doi.org/10.3390/ijms242115904







