Bacillus amyloliquefaciens AK-12 Helps Rapeseed Establish a Protection against Brevicoryne brassicae
Abstract
:1. Introduction
2. Results
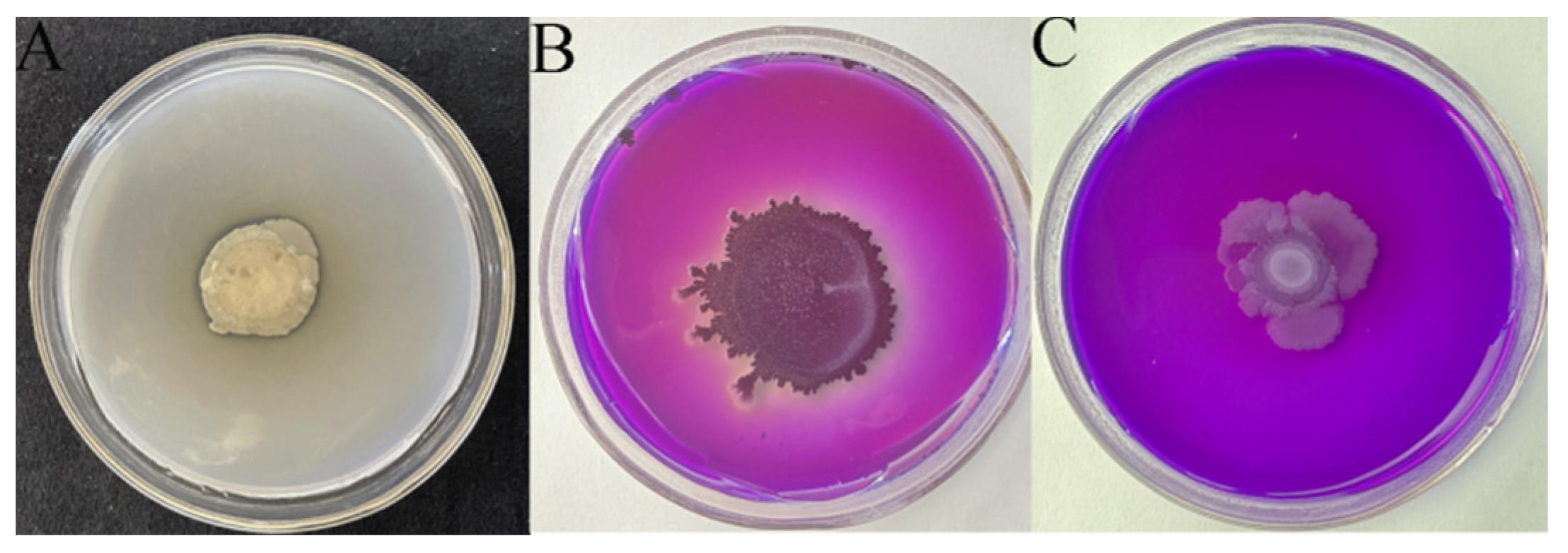
2.1. Comparison of Strain Stability and Growth Curves
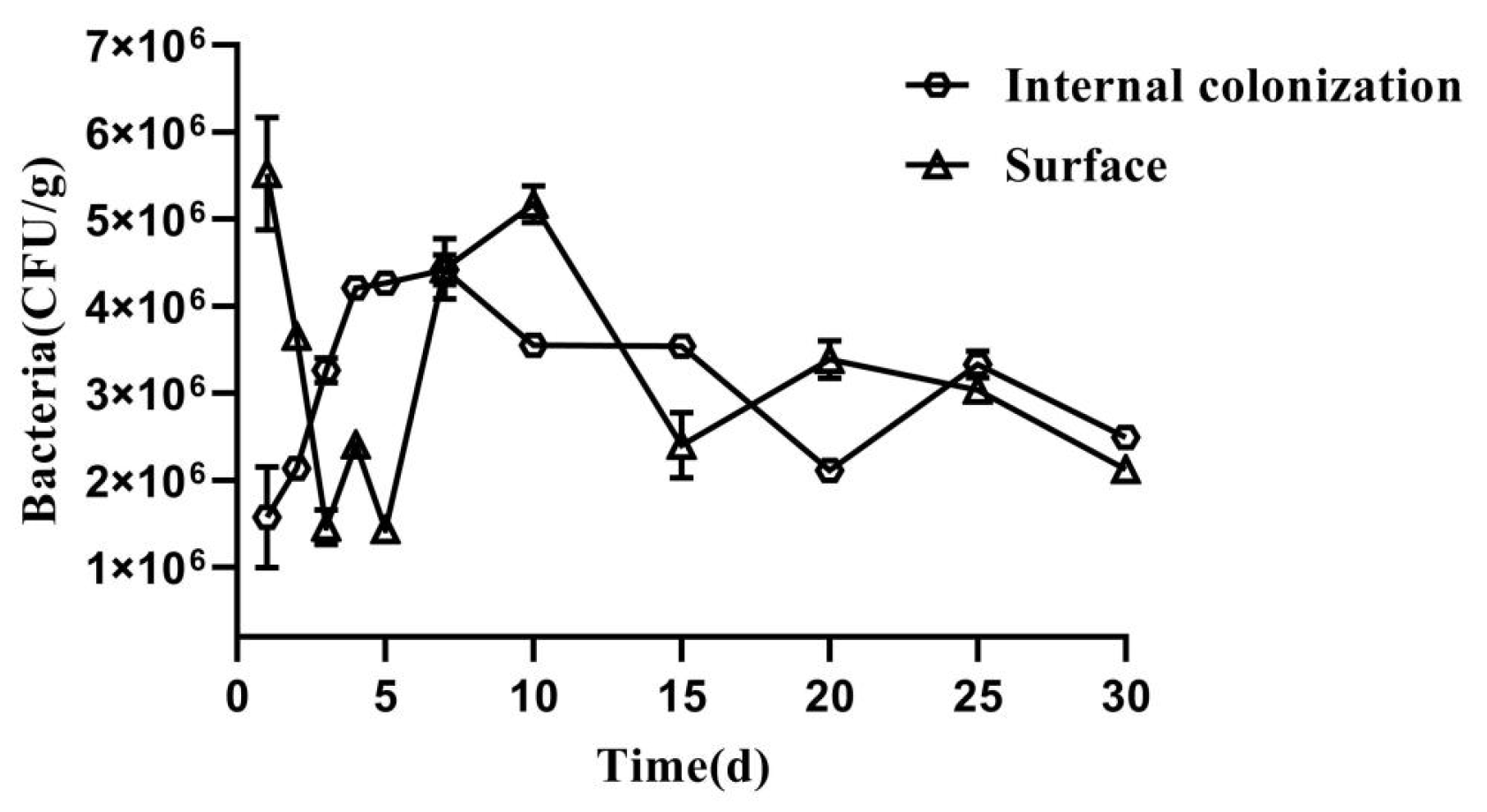
2.2. Colonization Dynamics of AK-12
2.3. Strain Colonization Ability to Establish Green Barrier and Control Cabbage Aphids
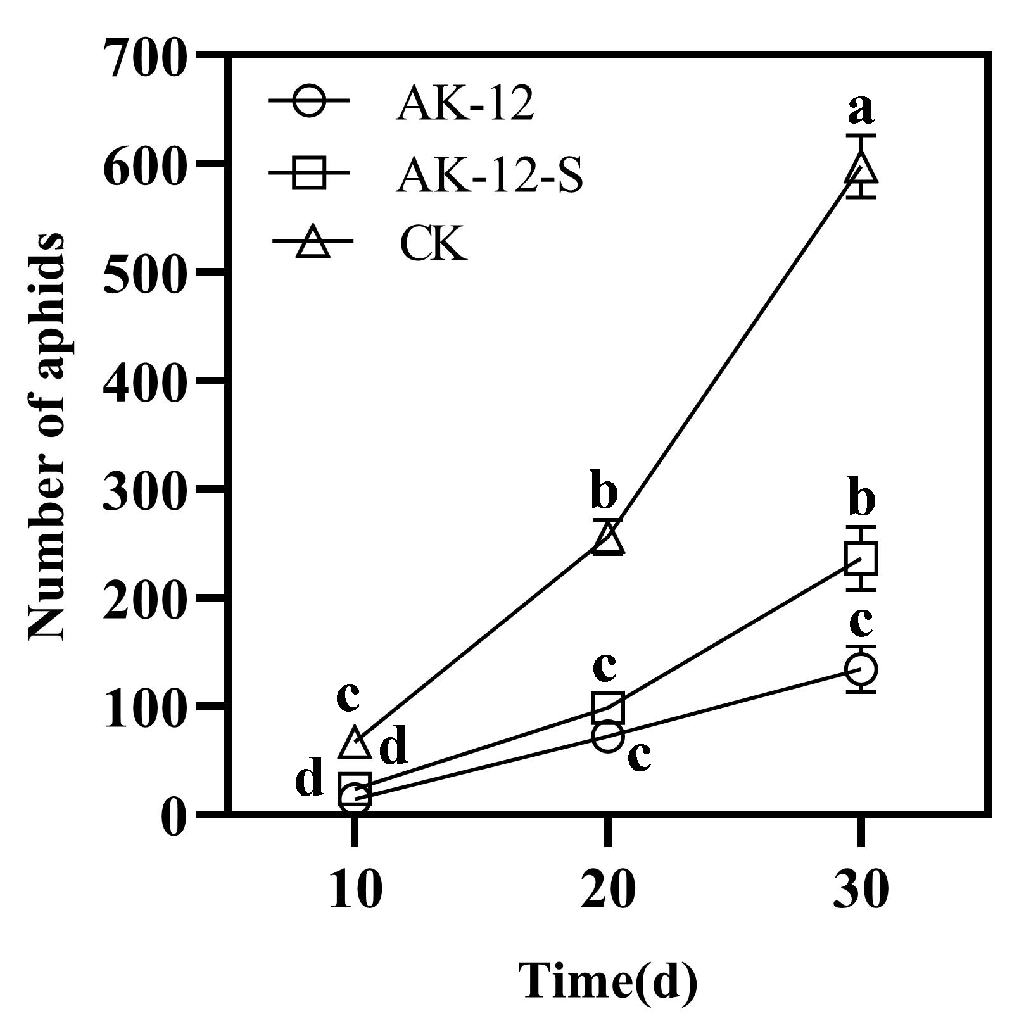
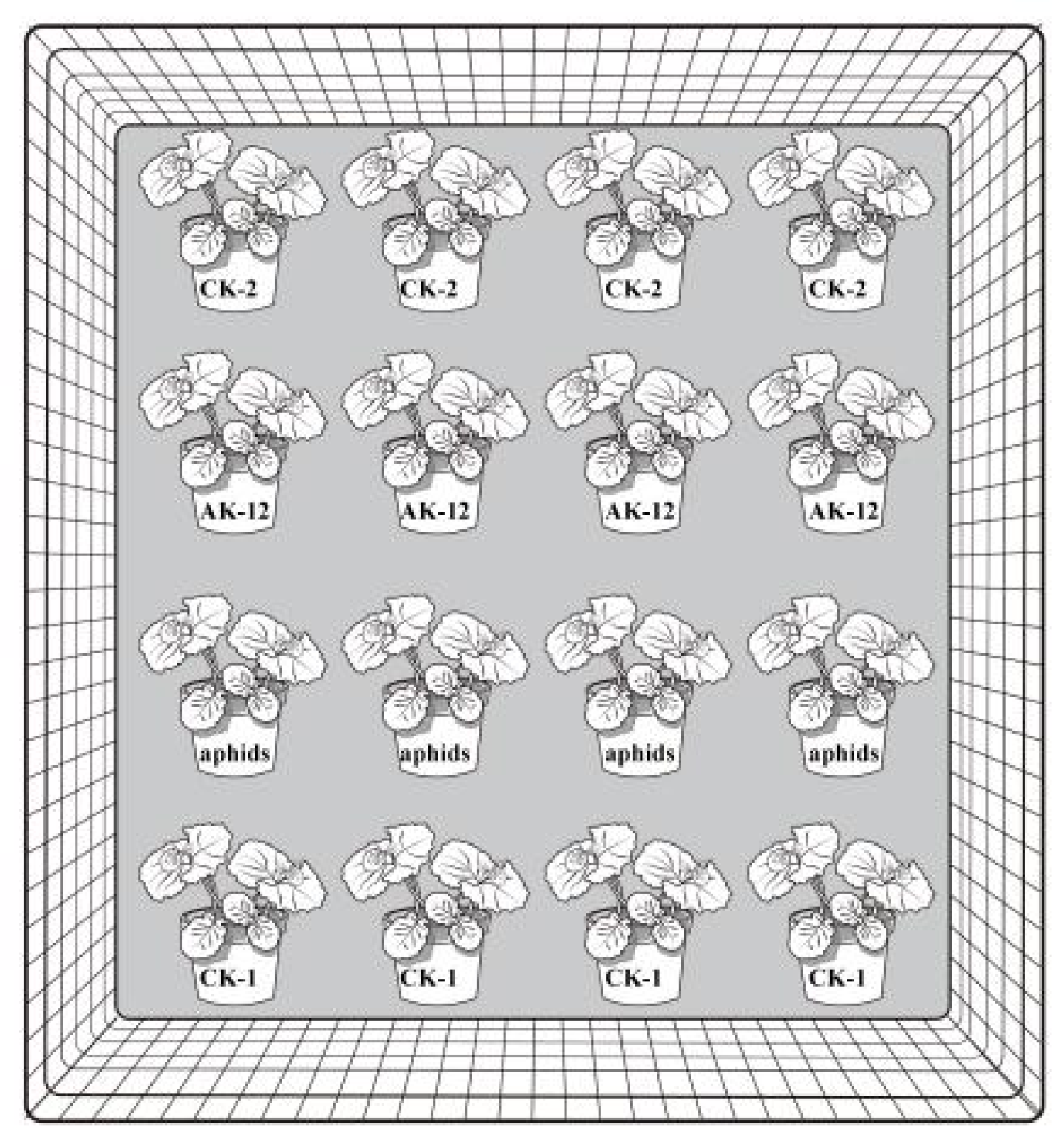
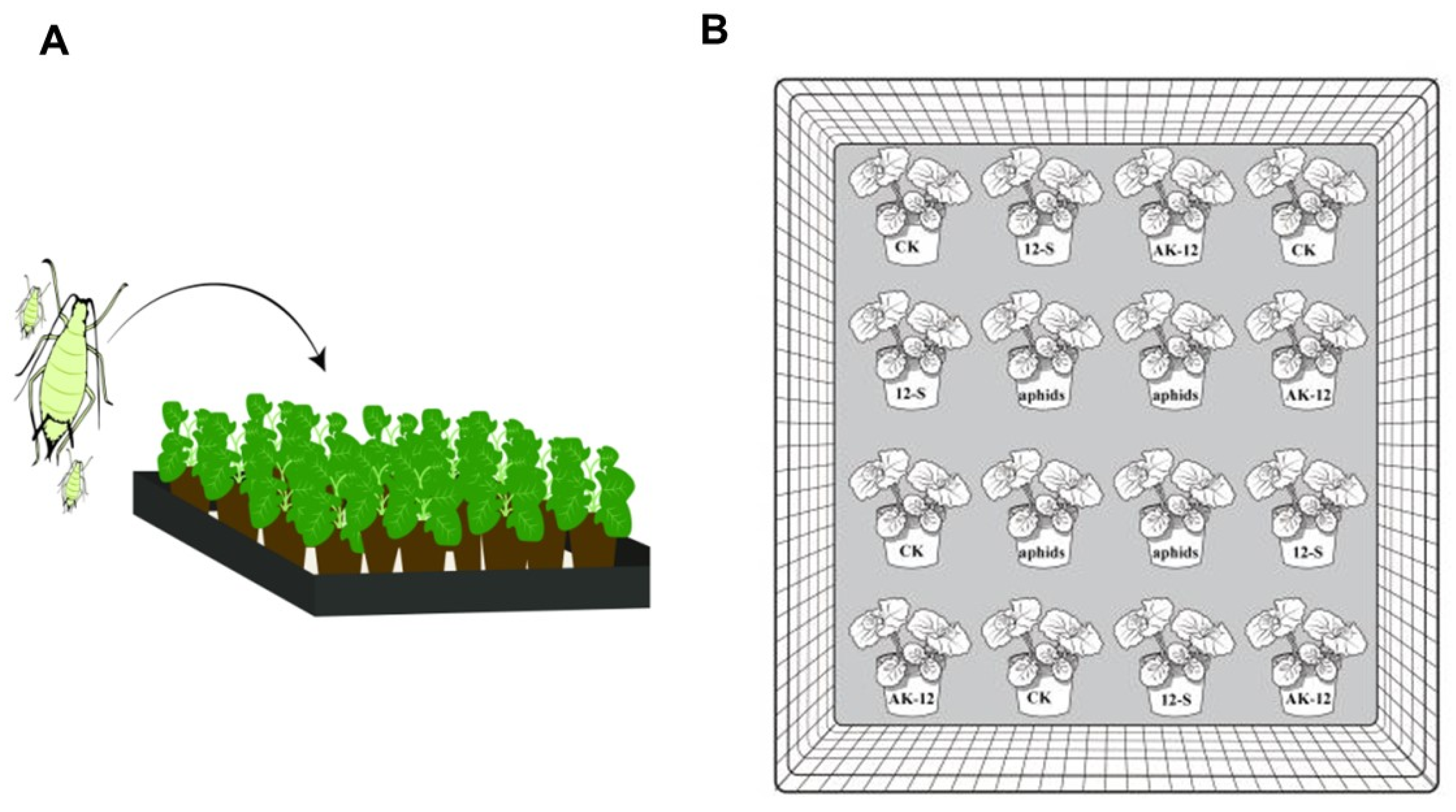
2.3.1. Effect of Strains on Cabbage Aphid Transmission
2.3.2. Effect of AK-12 on Cabbage Aphid Feeding
2.4. Growth Promoting Effect of AK-12 on Rapeseed Plants
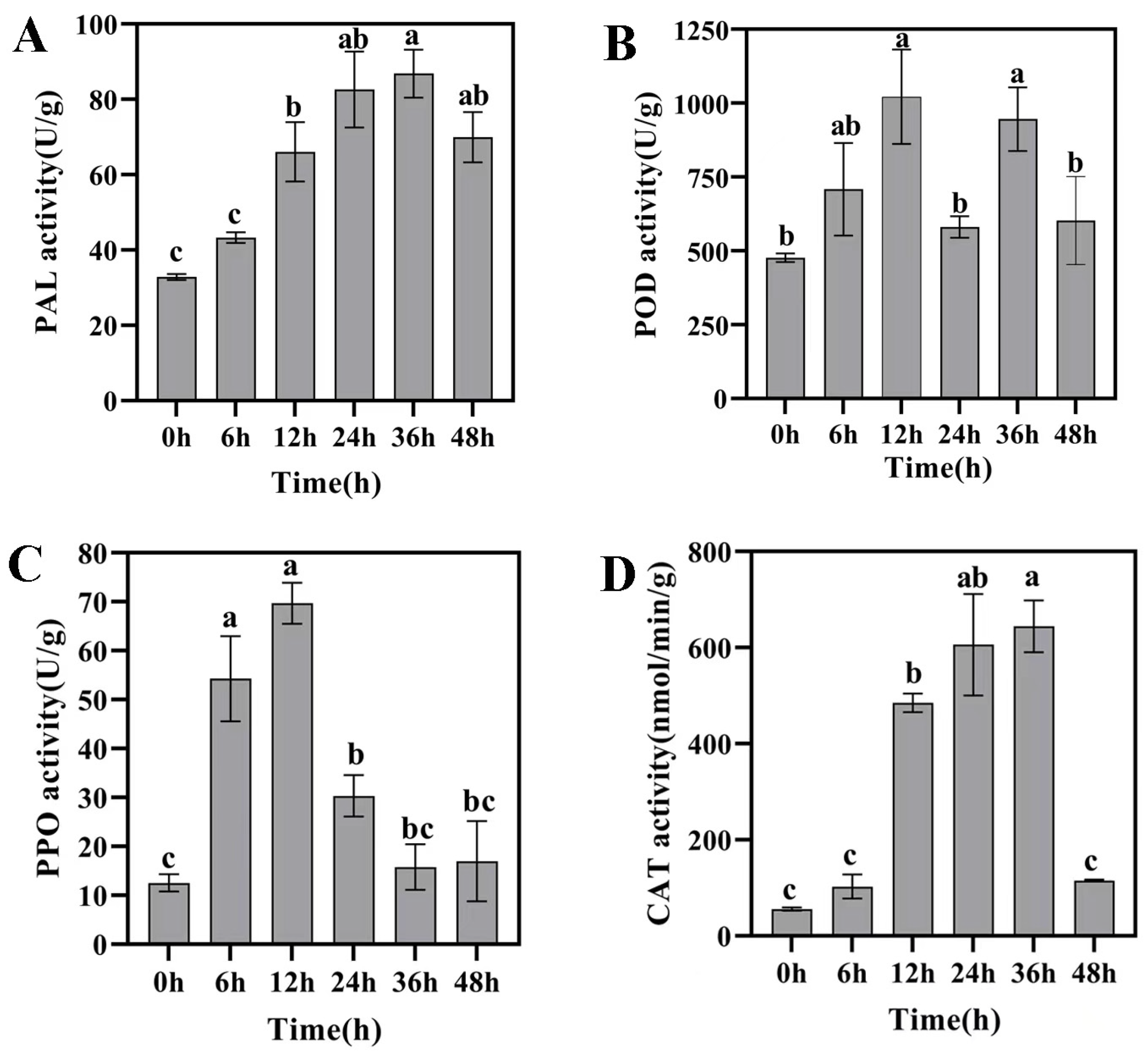
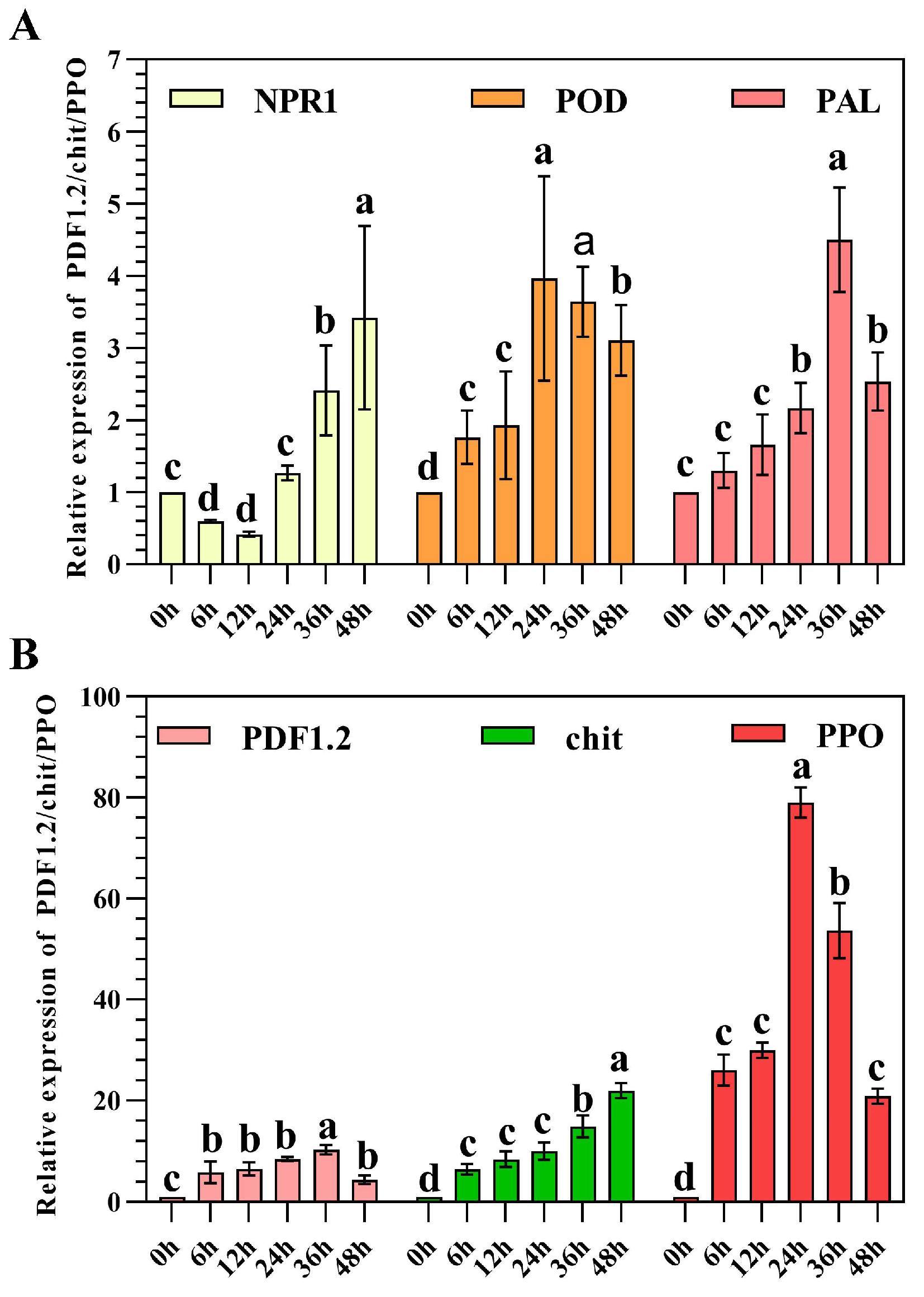
2.5. Induced Resistance Studies of Strain
3. Discussion
4. Materials and Methods
4.1. Materials, Bacterial Strains, and Reagents
4.2. Natural Transformation of GFP-Harboring Vactor in the Bacterial Strains
4.3. Comparison of Strain Stability and Growth Curves
4.4. External and Internal Colonization of AK-12 in Rapeseed Leaves
4.5. AK-12 Colonization Ability to Establish Green Barrier against Aphids
4.5.1. Effect of AK-12 on Aphid Transmission
4.5.2. Effect of Strains on Aphid Feeding
4.6. Growth Promotion in Rapeseed Treated with AK-12
4.7. Study of Induced Resistance in Rapeseed Due to AK-12
4.8. Data Statistics and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, utilization, and genetic improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Zheng, C.; Yang, Y.; Wang, X.; Hui, J.; Zhou, Q. Key odorant differences in fragrant Brassica napus and Brassica juncea oils revealed by gas chromatography–olfactometry, odor activity values, and aroma recombination. J. Agric. Food Chem. 2020, 68, 14950–14960. [Google Scholar] [CrossRef]
- Bian, X.; Yang, X.; Li, Q.; Sun, X. Effects of planting of two common crops, Allium fistulosum and Brassica napus, on soil properties and microbial communities of ginseng cultivation in northeast China. BMC Microbiol. 2022, 22, 182. [Google Scholar] [CrossRef]
- Maroofpour, N.; Mousavi, M.; Hejazi, M.J.; Iranipour, S.; Hamishehkar, H.; Desneux, N.; Biondi, A.; Haddi, K. Comparative selectivity of nano and commercial formulations of pirimicarb on a target pest, Brevicoryne brassicae, and its predator Chrysoperla carnea. Ecotoxicology 2021, 30, 361–372. [Google Scholar] [CrossRef]
- Simon, S.; Morel, K.; Durand, E.; Brevalle, G.; Girard, T.; Lauri, P.E. Aphids at crossroads: When branch architecture alters aphid infestation patterns in the apple tree. Trees 2012, 26, 273–282. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, P.K.; Das, B.C. Effect of planting date on the incidence of eggplant aphid, Aphis gossypii Glover and yield of eggplant. Bangladesh J. Zool. 2011, 39, 187–194. [Google Scholar] [CrossRef]
- Tabasum, S.; Noorka, I.R.; Afzal, M.; Ali, A. Screening best adopted wheat lines against aphid (Schizaphis graminum Rondani) population. Pak. Entomol. 2012, 34, 51–53. [Google Scholar]
- Ohnishi, S.; Miyake, N.; Takeuchi, T.; Kousaka, F.; Hiura, S.; Kanehira, O.; Saito, M.; Sayama, T.; Higashi, A.; Ishimoto, M.; et al. Fine mapping of foxglove aphid (Aulacorthum solani) resistance gene Raso1 in soybean and its effect on tolerance to Soybean dwarf virus transmitted by foxglove aphid. Breed. Sci. 2012, 61, 618–624. [Google Scholar] [CrossRef]
- Ateyyat, M. Selectivity of four insecticides to woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.). World Appl. Sci. J. 2012, 16, 1060–1064. [Google Scholar]
- Pang, Y.-P.; Singh, S.K.; Gao, Y.; Lassiter, T.L.; Mishra, R.K.; Zhu, K.Y.; Brimijoin, S. Selective and irreversible inhibitors of aphid acetylcholinesterases: Steps toward human-safe insecticides. PLoS ONE 2009, 4, e4349. [Google Scholar] [CrossRef]
- Sadeghi, A.; Van Damme, E.J.; Smagghe, G. Evaluation of the susceptibility of the pea aphid, Acyrthosiphon pisum, to a selection of novel biorational insecticides using an artificial diet. J. Insect Sci. 2009, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.A.; Seo, M.J.; Hwang, I.C.; Jang, C.; Park, H.J.; Yu, Y.M.; Youn, Y.N. Insecticidal activity and feeding behavior of the green peach aphid, Myzus persicae, after treatment with nano types of pyrifluquinazon. J. Asia-Pac. Entomol. 2012, 15, 533–541. [Google Scholar] [CrossRef]
- Boiteau, G.; Singh, M.; Lavoie, J. Crop border and mineral oil sprays used in combination as physical control methods of the aphid-transmitted potato virus Y in potato. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 255–259. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gao, H.H.; Zhao, H.Y.; Monika, W.; Hu, Z.A.; Hu, X.S. Effect of static magnetic fields (SMF) on the viability and fecundity of aphid Sitobion avenae (Homoptera: Aphididae) under laboratory conditions. Arch. Biol. Sci. 2012, 64, 693–702. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, Y.; Singh, B.K.; Wu, Y.; Munir, S.; He, P. Biocontrol of plant pathogens in omics era—With special focus on endophytic bacilli. Crit. Rev. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jayasimha, G.T.; Rachana, R.R.; Rajkumar, V.B.; Manjunatha, M. Evaluation of fungal pathogen, Fusarium semitectum Berk and Ravenel against okra aphid, Aphis gossypii Glover under laboratory and green house conditions. Pest Manag. Hortic. Ecosyst. 2012, 18, 139–142. [Google Scholar]
- Diaz, B.M.; Oggerin, M.; Lastra, C.C.L.; Rubio, V.; Fereres, A. Characterization and virulence of Lecanicillium lecanii against different aphid species. BioControl 2009, 54, 825–835. [Google Scholar] [CrossRef]
- Nawaz, A.; Razzaq, F.; Razzaq, A.; Gogi, M.D.; Fernández-Grandon, G.M.; Tayib, M.; Ayub, M.A.; Sufyan, M.; Shahid, M.R.; Qayyum, M.A.; et al. Compatibility and synergistic interactions of fungi, Metarhizium anisopliae, and insecticide combinations against the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2022, 12, 4843. [Google Scholar] [CrossRef]
- Rodríguez, M.; Marín, A.; Torres, M.; Béjar, V.; Campos, M.; Sampedro, I. Aphicidal activity of surfactants produced by Bacillus atrophaeus L193. Front. Microbiol. 2018, 9, 3114. [Google Scholar] [CrossRef]
- López-Isasmendi, G.; Alvarez, A.E.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol. Res. 2019, 226, 41–47. [Google Scholar] [CrossRef]
- Lim, D.J.; Yang, S.Y.; Noh, M.Y.; Lee, C.W.; Kim, J.C.; Kim, I.S. Identification of lipopeptide xantholysins from Pseudomonas sp. DJ15 and their insecticidal activity against Myzus persicae. Entomol. Res. 2017, 47, 337–343. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae). J. Agric. Food Chem. 2011, 59, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, F.X.; Jandricic, S.; Bolckmans, K.; Wäckers, F.L.; Pekas, A. Optimizing aphid biocontrol with the predator Aphidoletes aphidimyza, based on biology and ecology. Pest Manag. Sci. 2019, 75, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, G. Aphids and their biocontrol. In Ecofriendly Pest Management for Food Security; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–108. [Google Scholar]
- Ahmed, A.; Munir, S.; He, P.; Li, Y.; He, P.; Yixin, W.; He, Y. Biocontrol arsenals of bacterial endophyte: An imminent triumph against clubroot disease. Microbiol. Res. 2020, 241, 126565. [Google Scholar] [CrossRef] [PubMed]
- Choub, V.; Ajuna, H.B.; Won, S.J.; Moon, J.H.; Choi, S.I.; Maung, C.E.H.; Kim, C.W.; Ahn, Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-J.; Choub, V.; Kwon, J.-H.; Kim, D.-H.; Ahn, Y.-S. The control of fusarium root rot and development of coastal pine (Pinus thunbergii Parl.) seedlings in a container nursery by use of Bacillus licheniformis MH48. Forests 2018, 10, 6. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chakraborty, B.; Basnet, M. Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J. Basic Microbiol. 2006, 46, 186–195. [Google Scholar] [CrossRef]
- Denoirjean, T.; Ameline, A.; Couty, A.; Dubois, F.; Coutte, F.; Doury, G. Effects of surfactins, Bacillus lipopeptides, on the behavior of an aphid and host selection by its parasitoid. Pest Manag. Sci. 2022, 78, 929–937. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [PubMed]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Li, Y.; He, P.; He, P.; He, P.; Cui, W.; Wu, Y.; Li, X.; He, Y. Bacillus subtilis L1-21 possible assessment of inhibitory mechanism against phytopathogens and colonization in different plant hosts. Pak. J. Agric. Sci. 2018, 55, 996–1002. [Google Scholar]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; pp. 147–203. [Google Scholar]
- Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S. Biological control of fall webworm larva (Hyphantria cunea Drury) and growth promotion of poplar seedlings (Populus × canadensis Moench) with Bacillus licheniformis PR2. For. Ecol. Manag. 2022, 525, 120574. [Google Scholar] [CrossRef]
- Figueredo, E.F.; da Cruz, T.A.; de Almeida, J.R.; Batista, B.D.; Marcon, J.; de Andrade, P.A.M.; de Almeida Hayashibara, C.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the CRISPR-Cas9 system. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef]
- Mehta, P.; Walia, A.; Kulshrestha, S.; Chauhan, A.; Shirkot, C.K. Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microbiol. 2015, 55, 33–44. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Wanner, L.A.; Li, G.; Ware, D.; Somssich, I.E.; Davis, K.R. The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana. Plant Mol. Biol. 1995, 27, 327–338. [Google Scholar] [CrossRef]
- Qin, Q.; Shi, X.; Liang, P.; Gao, X. Induction of Phenylalanine ammonia-lyase and lipoxtgenase in cotton seedlings by mechanical wounding and aphid infestation. Prog. Nat. Sci. 2005, 15, 419–423. [Google Scholar]
- Tang, F.; Zhao, W.L.; Gao, X.W. Communication between plants: Induced resistance in poplar seedlings following herbivore infestation, mechanical wounding, and volatile treatment of the neighbors. Entomol. Exp. Appl. 2013, 149, 110–117. [Google Scholar] [CrossRef]
- Polidoros, A.N.; Mylona, P.V.; Scandalios, J.G. Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant-pathogen interactions and resistance to oxidative stress. Transgenic Res. 2001, 10, 555–569. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Wang, N.; Guo, Z.X.; Ou, Y.; Li, N.; Peng, C. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob. Ecol. Conserv. 2020, 23, e01141. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]





| Stem Length (cm) | Stem Thick (mm) | Fresh Weight (g) | Dry Weight (g) | |
|---|---|---|---|---|
| Foliar spray | 27.92 ± 1.98 ab | 3.62 ± 0.29 ab | 4.06 ± 0.51 ab | 0.46 ± 0.12 ab |
| Irrigation | 30.7 ± 2.68 a | 4.06 ± 0.31 a | 5.17 ± 0.63 a | 0.59 ± 0.06 a |
| Foliar spraying and irrigation | 29.82 ± 1.81 a | 4.08 ± 0.32 a | 5.5 ± 1.49 a | 0.65 ± 0.17 a |
| CK | 24.6 ± 0.76 b | 3.08 ± 0.28 b | 2.69 ± 0.20 b | 0.28 ± 0.05 b |
| Primers | Primer-F Sequence (5′-3′) | Primers-R Sequence (5′-3′) |
|---|---|---|
| Tubulin | GAGCGACCCACATACACCAATC | AACCTCAACGAAGCAGTCAACG |
| NPR1 | ACGCTTCTTTCCACGATGTTCAG | GCTTCTTCAGTTGACGCTCTTCC |
| PDF1.2 | GGGACCATGCTCAAGAGACAG | AACAACGGCGGCGGAATC |
| chit | TCGGCAGTATCATCTCAAGTTCC | TTTACGGGCAGTGGTATCGC |
| POD | ACACACATTTGGAAGAGCAAGATG | CGTCTACGGTTGGATCAGGATTAC |
| PPO | TGGGTTTAGGAGGGCTGTATGG | TGAGATCAGGAGGTGGTATAGGAG |
| PAL | CGGTTTGCCCTCTAATCTCACTG | GACATCTTGGTTGTGTTGTTCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, S.; Ahmed, A.; He, P.; He, P.; Munir, S.; Xia, M.; Tang, C.; Tang, P.; Wang, Z.; Khan, R.; et al. Bacillus amyloliquefaciens AK-12 Helps Rapeseed Establish a Protection against Brevicoryne brassicae. Int. J. Mol. Sci. 2023, 24, 15893. https://doi.org/10.3390/ijms242115893
Qian S, Ahmed A, He P, He P, Munir S, Xia M, Tang C, Tang P, Wang Z, Khan R, et al. Bacillus amyloliquefaciens AK-12 Helps Rapeseed Establish a Protection against Brevicoryne brassicae. International Journal of Molecular Sciences. 2023; 24(21):15893. https://doi.org/10.3390/ijms242115893
Chicago/Turabian StyleQian, Shixiong, Ayesha Ahmed, Pengbo He, Pengfei He, Shahzad Munir, Mengyuan Xia, Chaoyun Tang, Ping Tang, Zaiqiang Wang, Rizwan Khan, and et al. 2023. "Bacillus amyloliquefaciens AK-12 Helps Rapeseed Establish a Protection against Brevicoryne brassicae" International Journal of Molecular Sciences 24, no. 21: 15893. https://doi.org/10.3390/ijms242115893
APA StyleQian, S., Ahmed, A., He, P., He, P., Munir, S., Xia, M., Tang, C., Tang, P., Wang, Z., Khan, R., Li, X., Wu, Y., & He, Y. (2023). Bacillus amyloliquefaciens AK-12 Helps Rapeseed Establish a Protection against Brevicoryne brassicae. International Journal of Molecular Sciences, 24(21), 15893. https://doi.org/10.3390/ijms242115893





