Bleeding and Thrombosis in Multiple Myeloma: Platelets as Key Players during Cell Interactions and Potential Use as Drug Delivery Systems
Abstract
1. Multiple Myeloma: Clinical Manifestations and Treatment
2. Hemostatic Complications Associated with Multiple Myeloma
3. Hemostatic Complications Associated with Multiple Myeloma Treatment
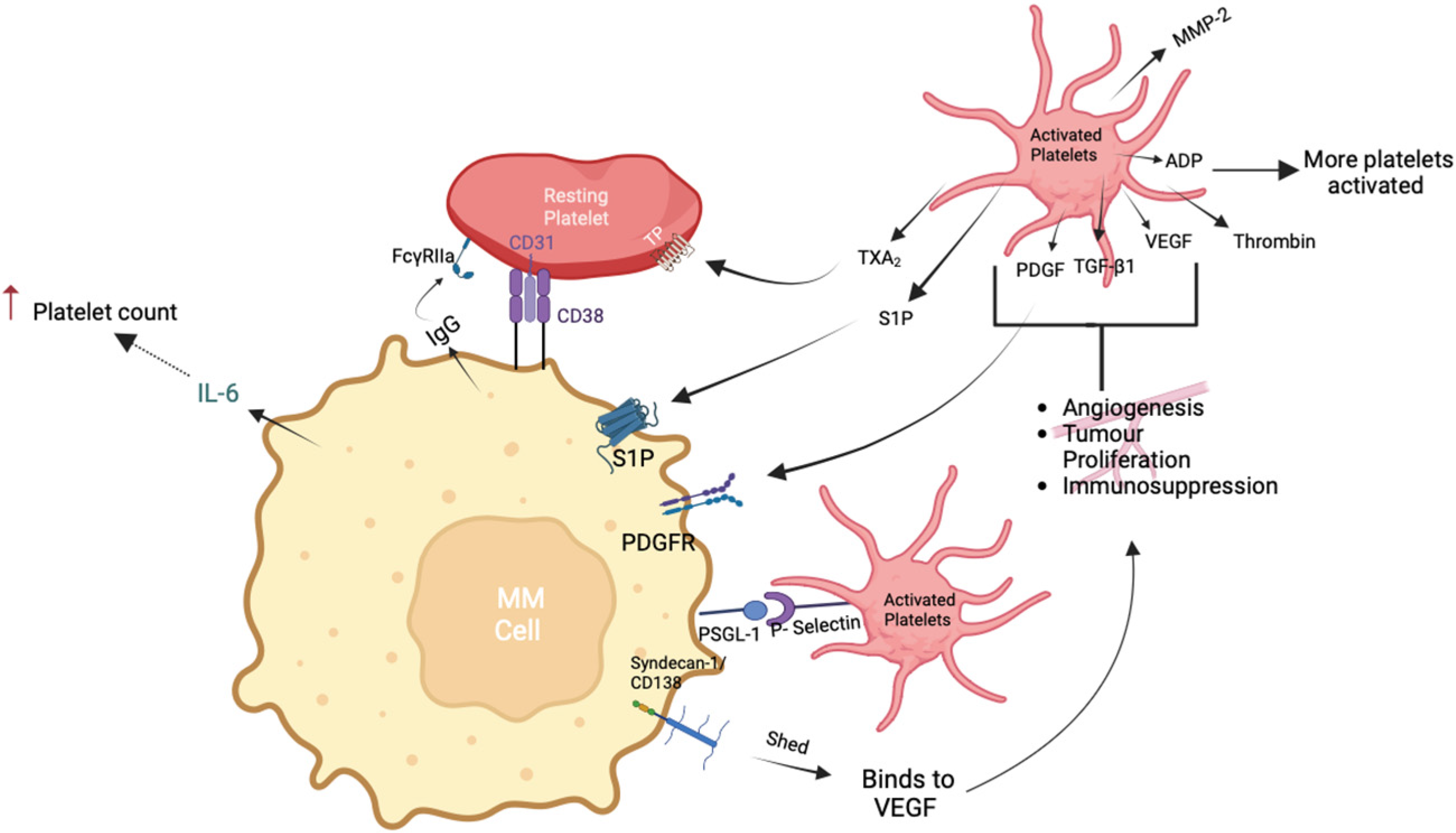
4. Platelet–Myeloma Cell Interactions
5. Platelets as Diagnostic and Therapeutic Tools in Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Multiple Myeloma Source: Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/35-Multiple-myeloma-fact-sheet.pdf (accessed on 5 November 2022).
- Multiple myeloma|Irish Cancer Society. Available online: https://www.cancer.ie/cancer-information-and-support/cancer-types/multiple-myeloma (accessed on 5 November 2022).
- Key Statistics for Multiple Myeloma. Available online: https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html (accessed on 2 April 2023).
- Incidence of Myeloma—Myeloma Patients Europe. Available online: https://www.mpeurope.org/about-myeloma/incidence-of-myeloma/ (accessed on 5 November 2022).
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Waldenstrom, J. Studies on Conditions Associated with Disturbed Gamma Globulin Formation (Gammopathies). Harvey Lect. 1960, 56, 211–231. [Google Scholar] [PubMed]
- Moser-Katz, T.; Joseph, N.S.; Dhodapkar, M.V.; Lee, K.P.; Boise, L.H. Game of Bones: How Myeloma Manipulates Its Microenvironment. Front. Oncol. 2020, 10, 625199. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. MGUS and Smoldering Multiple Myeloma: Update on Pathogenesis, Natural History, and Management. Hematology 2005, 2005, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Jiang, D.; Singh, D.J.; Hasipek, M.; Shah, H.S.; Ullah, F.; Khouri, J.; Maciejewski, J.P.; Jha, B.K. Multiple Myeloma Therapy: Emerging Trends and Challenges. Cancers 2022, 14, 4082. [Google Scholar] [CrossRef] [PubMed]
- International Staging System for Multiple Myeloma | The IMF. Available online: https://www.myeloma.org/international-staging-system-iss-reivised-iss-r-iss (accessed on 14 May 2023).
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic Abnormalities and Survival in Multiple Myeloma: The Experience of the Intergroupe Francophone Du Myélome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef]
- Flynt, E.; Bisht, K.; Sridharan, V.; Ortiz, M.; Towfic, F.; Thakurta, A. Prognosis, Biology, and Targeting of TP53 Dysregulation in Multiple Myeloma. Cells 2020, 9, 287. [Google Scholar] [CrossRef]
- Wallington-Beddoe, C.T.; Mynott, R.L. Prognostic and Predictive Biomarker Developments in Multiple Myeloma. J. Hematol. Oncol. 2021, 14, 151. [Google Scholar] [CrossRef]
- Multiple Myeloma Treatment Overview | Int’l Myeloma Foundation. Available online: https://www.myeloma.org/multiple-myeloma-treatment (accessed on 1 September 2023).
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Goldman-Mazur, S.; Visram, A.; Rajkumar, S.V.; Kapoor, P.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Hayman, S.R.; Dingli, D.; et al. Second Line Treatment Strategies in Multiple Myeloma: A Referral-Center Experience. Blood 2021, 138 (Suppl. S1), 819. [Google Scholar] [CrossRef]
- A Study of Talquetamab in Participants With Relapsed or Refractory Multiple Myeloma—Full Text View—ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04634552 (accessed on 28 August 2023).
- MagnetisMM-3: Study Of Elranatamab (PF-06863135) Monotherapy in Participants With Multiple Myeloma Who Are Refractory to at Least One PI, One IMiD and One Anti-CD38 mAb—Full Text View—ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04649359 (accessed on 30 August 2023).
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Mukai, M.; Oka, T. Mechanism and Management of Cancer-Associated Thrombosis. J. Cardiol. 2018, 72, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Eby, C. Pathogenesis and Management of Bleeding and Thrombosis in Plasma Cell Dyscrasias. Br. J. Haematol. 2009, 145, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Fears, T.R.; Gridley, G.; Turesson, I.; Mellqvist, U.H.; Björkholm, M.; Landgren, O. Deep Vein Thrombosis after Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. Blood 2008, 112, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Luo, S.; Wang, T.-F.; Wildes, T.; Mikhael, J.; Keller, J.W.; Thomas, T.S.; Carson, K.R.; Gage, B.F. Predicting Risk of Venous Thromboembolism in Multiple Myeloma: The Impede VTE Score. Blood 2018, 132 (Suppl. S1), 141. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug–Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2019, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Rybicki, L.; Wei, W.; Valent, J.; Faiman, B.M.; Samaras, C.J.; Anwer, F.; Khorana, A.A. Abnormal Metaphase Cytogenetics Predicts Venous Thromboembolism in Myeloma: Derivation and Validation of the PRISM Score. Blood 2022, 140, 2443–2450. [Google Scholar] [CrossRef]
- Kapur, S.; Feehan, K.; Mosiman, S.; Frankki, S.; Rosenstein, L.J. Real World Validation of VTE Risk Models in Newly Diagnosed Multiple Myeloma in a Community Setting. Blood 2021, 138 (Suppl. S1), 2971. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Mackman, N.; Metz-Schimmerl, S.; Stift, J.; Kaider, A.; Müllauer, L.; Gnant, M.; Scheithauer, W.; Pabinger, I. Microparticle-Associated Tissue Factor Activity in Patients with Pancreatic Cancer: Correlation with Clinicopathological Features. Eur. J. Clin. Invest. 2013, 43, 277–285. [Google Scholar] [CrossRef]
- Lwaleed, B.A.; Lam, L.; Lasebai, M.; Cooper, A.J. Expression of Tissue Factor and Tissue Factor Pathway Inhibitor in Microparticles and Subcellular Fractions of Normal and Malignant Prostate Cell Lines. Blood Coagul. Fibrinolysis 2013, 24, 339–343. [Google Scholar] [CrossRef]
- Ueno, T.; Toi, M.; Koike, M.; Nakamura, S.; Tominaga, T. Tissue Factor Expression in Breast Cancer Tissues: Its Correlation with Prognosis and Plasma Concentration. Br. J. Cancer 2000, 83, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Homma, S.; Satoh, T.; Nakanishi, K.; Abe, D.; Matsumoto, K.; Oki, A.; Tsunoda, H.; Yamaguchi, I.; Nagasawa, T.; et al. Tissue Factor Expression as a Possible Determinant of Thromboembolism in Ovarian Cancer. Br. J. Cancer 2007, 96, 290. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Preusser, M.; Ay, C.; Kaider, A.; Marosi, C.; Zielinski, C.; Pabinger, I.; Hainfellner, J.A. Intratumoral Tissue Factor Expression and Risk of Venous Thromboembolism in Brain Tumor Patients. Thromb. Res. 2013, 131, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, R.S.; Taubman, M.B.; Mackman, N. Role of Tissue Factor in Cancer. J. Clin. Oncol. 2009, 27, 4834. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Whur, P. Factor X-Activating Activity from Lewis Lung Carcinoma. Br. J. Cancer 1980, 41, 642–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falanga, A.; Alessio, M.; Donati, M.; Barbui, T. A New Procoagulant in Acute Leukemia. Blood 1988, 71, 870–875. [Google Scholar] [CrossRef]
- van Marion, A.M.W.; Auwerda, J.J.A.; Lisman, T.; Sonneveld, P.; de Maat, M.P.M.; Lokhorst, H.M.; Leebeek, F.W.G. Prospective Evaluation of Coagulopathy in Multiple Myeloma Patients before, during and after Various Chemotherapeutic Regimens. Leuk. Res. 2008, 32, 1078–1084. [Google Scholar] [CrossRef]
- Fotiou, D.; Gavriatopoulou, M.; Terpos, E. Multiple Myeloma and Thrombosis: Prophylaxis and Risk Prediction Tools. Cancers 2020, 12, 191. [Google Scholar] [CrossRef]
- Ghansah, H.; Debreceni, I.B.; Váróczy, L.; Rejtő, L.; Lóczi, L.; Bagoly, Z.; Kappelmayer, J. Patients with Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance Have Variably Increased Thrombin Generation and Different Sensitivity to the Anticoagulant Effect of Activated Protein C. Thromb. Res. 2023, 223, 44–52. [Google Scholar] [CrossRef]
- Nielsen, T.; Kristensen, S.R.; Gregersen, H.; Teodorescu, E.M.; Christiansen, G.; Pedersen, S. Extracellular Vesicle-Associated Procoagulant Phospholipid and Tissue Factor Activity in Multiple Myeloma. PLoS ONE 2019, 14, e0210835. [Google Scholar] [CrossRef]
- Shaw, J.L.; Nielson, C.M.; Park, J.K.; Marongiu, A.; Soff, G.A. The Incidence of Thrombocytopenia in Adult Patients Receiving Chemotherapy for Solid Tumors or Hematologic Malignancies. Eur. J. Haematol. 2021, 106, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Suppapanya, N.; Grotzinger, K. Thrombocytopenia in Hematologic Malignancy and Solid Tumors in the United States. J. Clin. Oncol. 2012, 30 (Suppl. S15), e12001. [Google Scholar] [CrossRef]
- Rahman, S.; Veeraballi, S.; Chan, K.H.; Shaaban, H.S. Bleeding Diathesis in Multiple Myeloma: A Rare Presentation of a Dreadful Emergency with Management Nightmare. Cureus 2021, 13, e13990. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, N.; Bergstrom, C.; Anderson, L.D.; Nagalla, S. Bleeding Due to Acquired Dysfibrinogenemia as the Initial Presentation of Multiple Myeloma. BMJ Case Rep. CP 2019, 12, e229312. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, A.; Kojima, H.; Berndt, M.C.; Kaneko, S.; Suzukawa, K.; Hasegawa, Y.; Shigeta, O.; Nagasawa, T. Characterization of a Myeloma Patient with a Life-Threatening Hemorrhagic Diathesis: Presence of a Lambda Dimer Protein Inhibiting Shear-Induced Platelet Aggregation by Binding to the A1 Domain of von Willebrand Factor. Thromb. Haemost. 2005, 93, 889–896. [Google Scholar] [CrossRef]
- Saif, M.W.; Allegra, C.J.; Greenberg, B. Bleeding Diathesis in Multiple Myeloma. J. Hematother. Stem. Cell Res. 2001, 10, 657–660. [Google Scholar] [CrossRef]
- Li, L.; Roest, M.; Sang, Y.; Remijn, J.A.; Fijnheer, R.; Smit, K.; Huskens, D.; Wan, J.; de Laat, B.; Konings, J. Patients with Multiple Myeloma Have a Disbalanced Whole Blood Thrombin Generation Profile. Front. Cardiovasc. Med. 2022, 9, 919495. [Google Scholar] [CrossRef]
- Gibbins, J.; Rana, R.; Khan, D.; Shapiro, S.; Grech, H.; Ramasamy, K. Multiple Myeloma Treatment Is Associated with Enhanced Platelet Reactivity. Blood 2018, 132 (Suppl. S1), 3300. [Google Scholar] [CrossRef]
- Knight, R.; DeLap, R.J.; Zeldis, J.B. Lenalidomide and Venous Thrombosis in Multiple Myeloma. N. Engl. J. Med. 2006, 354, 2079–2080. [Google Scholar] [CrossRef]
- Robak, M.; Treliński, J.; Chojnowski, K. Hemostatic Changes after 1 Month of Thalidomide and Dexamethasone Therapy in Patients with Multiple Myeloma. Med. Oncol. 2012, 29, 3574–3580. [Google Scholar] [CrossRef][Green Version]
- Murai, K.; Kowata, S.; Shimoyama, T.; Yashima-Abo, A.; Fujishima, Y.; Ito, S.; Ishida, Y. Bortezomib Induces Thrombocytopenia by the Inhibition of Proplatelet Formation of Megakaryocytes. Eur. J. Haematol. 2014, 93, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Waller, E.K.; Richardson, P.G.; Jagannath, S.; Orlowski, R.Z.; Giver, C.R.; Jaye, D.L.; Francis, D.; Giusti, S.; Torre, C.; et al. Risk Factors and Kinetics of Thrombocytopenia Associated with Bortezomib for Relapsed, Refractory Multiple Myeloma. Blood 2005, 106, 3777. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Prince, M.H.; Honemann, D.; Westerman, D.; Milner, A.D.; Barron, A.; Harrison, S. High-Dose Dexamethasone Reduces Bortezomib-Induced Thrombocytopenia. Blood 2007, 110, 4820. [Google Scholar] [CrossRef]
- Avcu, F.; Ural, A.U.; Cetin, T.; Nevruz, O. Effects of Bortezomib on Platelet Aggregation and ATP Release in Human Platelets, In Vitro. Thromb. Res. 2008, 121, 567–571. [Google Scholar] [CrossRef]
- Ayed, A.O.; Moreb, J.S.; Hsu, J.W.; Hiemenz, J.W.; Wingard, J.R.; Norkin, M. Severe Diffuse Alveolar Hemorrhage Associated with Bortezomib Administration in Patients with Multiple Myeloma. Blood 2014, 124, 5761. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Rajkumar, S.V.; Dimopoulos, M.A.; Richardson, P.G.; San Miguel, J.; Barlogie, B.; Harousseau, J.; Zonder, J.A.; Cavo, M.; Zangari, M.; et al. Prevention of Thalidomide- and Lenalidomide-Associated Thrombosis in Myeloma. Leukemia 2008, 22, 414–423. [Google Scholar] [CrossRef]
- Adrianzen Herrera, D.; Lutsey, P.L.; Giorgio, K.; Zakai, N.A. Bleeding Risk in Patients with Multiple Myeloma Treated for Venous Thromboembolism. Blood 2021, 138 (Suppl. S1), 3023. [Google Scholar] [CrossRef]
- Foss, B.; Bruserud, Ø.; Hervig, T. Platelet-Released Supernatants Enhance Hematopoietic Stem Cell Proliferation in Vitro. Platelets 2008, 19, 155–159. [Google Scholar] [CrossRef]
- Jungi, T.W.; Spycher, M.O.; Nydegger, U.E.; Barandun, S. Platelet-Leukocyte Interaction: Selective Binding of Thrombin-Stimulated Platelets to Human Monocytes, Polymorphonuclear Leukocytes, and Related Cell Lines. Blood 1986, 67, 629–636. [Google Scholar] [CrossRef]
- Moore, K.L.; Stults, N.L.; Diaz, S.; Smith, D.F.; Cummings, R.D.; Varki, A.; McEver, R.P. Identification of a Specific Glycoprotein Ligand for P-Selectin (CD62) on Myeloid Cells. J. Cell Biol. 1992, 118, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Apta, B.H.R.; Bonna, A.M.; Harper, M.T. Platelet P-Selectin Triggers Rapid Surface Exposure of Tissue Factor in Monocytes. Sci. Rep. 2019, 9, 13397. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Tsukamoto, S.; Park, J.; Johnson, K.E.; Kawano, Y.; Moschetta, M.; Liu, C.J.; Mishima, Y.; Kokubun, K.; Manier, S.; et al. Platelets Enhance Multiple Myeloma Progression via Il-1b Upregulation. Clin. Cancer Res. 2018, 24, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Komai, M.; Itoh, T.; Imano, M.; Sakamoto, K.; Shimaoka, H.; Ogawa, N.; Mashimo, K.; Fujiwara, D.; Takeda, T.; et al. Inhibition of the Tumour Necrosis Factor-Alpha Autocrine Loop Enhances the Sensitivity of Multiple Myeloma Cells to Anticancer Drugs. Eur. J. Cancer 2013, 49, 3708–3717. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; De Biase, L.; Lenti, L.; Tocci, G.; Brunelli, A.; Cangemi, R.; Riondino, S.; Grego, S.; Volpe, M.; Violi, F. Tumor Necrosis Factor-α as Trigger of Platelet Activation in Patients with Heart Failure. Blood 2005, 106, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.R.; Meade-Murphy, G.; Gilligan, O.M.; Mykytiv, V.; Young, P.W.; Cahill, M.R. Platelet Hyperactivation in Multiple Myeloma Is Also Evident in Patients with Premalignant Monoclonal Gammopathy of Undetermined Significance. Br. J. Haematol. 2021, 192, 322–332. [Google Scholar] [CrossRef]
- Lemancewicz, D.; Bolkun, L.; Mantur, M.; Semeniuk, J.; Kloczko, J.; Dzieciol, J. Bone Marrow Megakaryocytes, Soluble P-Selectin and Thrombopoietic Cytokines in Multiple Myeloma Patients. Platelets 2014, 25, 181–187. [Google Scholar] [CrossRef]
- Egan, K.; Cooke, N.; Dunne, E.; Murphy, P.; Quinn, J.; Kenny, D. Platelet Hyporeactivity in Active Myeloma. Thromb. Res. 2014, 134, 747–749. [Google Scholar] [CrossRef]
- Kawano, M.; Hirano, T.; Matsuda, T.; Taga, T.; Horii, Y.; Iwato, K.; Asaoku, H.; Tang, B.; Tanabe, O.; Tanaka, H.; et al. Autocrine Generation and Requirement of BSF-2/IL-6 for Human Multiple Myelomas. Nature 1988, 332, 83–85. [Google Scholar] [CrossRef]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of INTERLEUKIN-6 in the Pathogenesis of Multiple Myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-Alpha/IL-1: Interrelations in Bone Resorption Pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 Stimulates Thrombopoiesis through Thrombopoietin: Role in Inflammatory Thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, A.; Pouliou, E.; Roussou, M.; Papanikolaou, A.; Tassidou, A.; Economopoulos, T.; Terpos, E. Increased Expression of Platelet Derived Growth Factor Receptor β on Trephine Biopsies Correlates with Advanced Myeloma. J. BUON 2017, 22, 1032–1037. [Google Scholar] [PubMed]
- Tsirakis, G.; Pappa, C.A.; Kanellou, P.; Stratinaki, M.A.; Xekalou, A.; Psarakis, F.E.; Sakellaris, G.; Alegakis, A.; Stathopoulos, E.N.; Alexandrakis, M.G. Role of Platelet-Derived Growth Factor-AB in Tumour Growth and Angiogenesis in Relation with Other Angiogenic Cytokines in Multiple Myeloma. Hematol. Oncol. 2012, 30, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol. 2017, 38, 323. [Google Scholar] [CrossRef] [PubMed]
- Atalay, F.; Ateşoğlu, E.B.; Yildiz, S.; Firatli-Tuglular, T.; Karakuş, S.; Bayik, M. Relationship of P-Selectin Glycoprotein Ligand-1 to Prognosis in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2015, 15, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; MacLeod, V.; Dai, Y.; Khotskaya-Sample, Y.; Shriver, Z.; Venkataraman, G.; Sasisekharan, R.; Naggi, A.; Torri, G.; Casu, B.; et al. The Syndecan-1 Heparan Sulfate Proteoglycan Is a Viable Target for Myeloma Therapy. Blood 2007, 110, 2041–2048. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Kelly, T.; Theus, A.; Athota, A.B.; Barlogie, B.; Sanderson, R.D. Elevated Levels of Shed Syndecan-1 Correlate with Tumour Mass and Decreased Matrix Metalloproteinase-9 Activity in the Serum of Patients with Multiple Myeloma. Br. J. Haematol. 1997, 99, 368–371. [Google Scholar] [CrossRef]
- Dowling, P.; Hayes, C.; Ting, K.R.; Hameed, A.; Meiller, J.; Mitsiades, C.; Anderson, K.C.; Clynes, M.; Clarke, C.; Richardson, P.; et al. Identification of Proteins Found to Be Significantly Altered When Comparing the Serum Proteome from Multiple Myeloma Patients with Varying Degrees of Bone Disease. BMC Genom. 2014, 15, 904. [Google Scholar] [CrossRef]
- Liang, P.; Cheng, S.H.; Cheng, C.K.; Lau, K.M.; Lin, S.Y.; Chow, E.Y.D.; Chan, N.P.H.; Ip, R.K.L.; Wong, R.S.M.; Ng, M.H.L. Platelet Factor 4 Induces Cell Apoptosis by Inhibition of STAT3 via Up-Regulation of SOCS3 Expression in Multiple Myeloma. Haematologica 2013, 98, 288. [Google Scholar] [CrossRef]
- Tukijan, F.; Chandrakanthan, M.; Nguyen, L.N. The Signalling Roles of Sphingosine-1-phosphate Derived from Red Blood Cells and Platelets. Br. J. Pharmacol. 2018, 175, 3741. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wu, C.T.; Guo, Q.; Wang, H.; Wang, L.S. Sphingosine 1-Phosphate Induces Mcl-1 Upregulation and Protects Multiple Myeloma Cells against Apoptosis. Biochem. Biophys. Res. Commun. 2008, 371, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Wang, T.; Jiang, Q.; Xu, F.; Liu, Z. Living Cell for Drug Delivery. Eng. Regen. 2022, 3, 131–148. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet Secretion: From Haemostasis to Wound Healing and Beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L.E. Platelets and Extracellular Vesicles and Their Cross Talk with Cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef]
- Cohen, S.A.; Trikha, M.; Mascelli, M.A. Potential Future Clinical Applications for the GPIIb/IIIa Antagonist, Abciximab in Thrombosis, Vascular and Oncological Indications. Pathol. Oncol. Res. 2000, 6, 163–174. [Google Scholar] [CrossRef]
- Boilard, E. Extracellular Vesicles and Their Content in Bioactive Lipid Mediators: More than a Sack of MicroRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef]
- Dai, Z.; Zhao, T.; Song, N.; Pan, K.; Yang, Y.; Zhu, X.; Chen, P.; Zhang, J.; Xia, C. Platelets and Platelet Extracellular Vesicles in Drug Delivery Therapy: A Review of the Current Status and Future Prospects. Front. Pharmacol. 2022, 13, 1026386. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular Vesicle-Based Drug Delivery Systems for Cancer Treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- McNamee, N.; de la Fuente, L.R.; Santos-Martinez, M.J.; O’Driscoll, L. Proteomics Profiling Identifies Extracellular Vesicles’ Cargo Associated with Tumour Cell Induced Platelet Aggregation. BMC Cancer 2022, 22, 1023. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Z.; Chen, Q.; Wu, T.; Shi, W.; Gan, L.; Liu, X.; Huang, Y.; Lv, M.; Zhao, Y.; et al. Platelets for Cancer Treatment and Drug Delivery. Clin. Transl. Oncol. 2022, 24, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-Loaded Platelets as a Smart Drug Delivery System: An Improved Therapy for Lymphoma OPEN. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.; Ye, Y.; Hu, Q.; Bomba, H.N.; Gu, Z. In Situ Activation of Platelets with Checkpoint Inhibitors for Post-Surgical Cancer Immunotherapy. Nat. Biomed. Eng. 2017, 1, 0011. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, J.; Ruan, H.; Zhang, X.; Ye, Y.; Shen, S.; Wang, C.; Lu, W.; Cheng, K.; et al. Conjugation of Haematopoietic Stem Cells and Platelets Decorated with Anti-PD-1 Antibodies Augments Anti-Leukaemia Efficacy. Nat. Biomed. Eng. 2018, 2, 831. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell Membrane-Based Nanoparticles: A New Biomimetic Platform for Tumor Diagnosis and Treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef]
- Hu, Q.; Qian, C.; Sun, W.; Wang, J.; Chen, Z.; Bomba, H.N.; Xin, H.; Shen, Q.; Gu, Z. Engineered Nano-Platelets for Enhanced Treatment of Multiple Myeloma and Thrombus. Adv. Mater. 2016, 28, 9573. [Google Scholar] [CrossRef]
- Dai, L.; Liu, Y.; Ding, S.; Wei, X.; Chen, B. Human Nanoplatelets as Living Vehicles for Tumor-Targeted Endocytosis In Vitro and Imaging In Vivo. J. Clin. Med. 2023, 12, 1592. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Lam, M.; Menter, D.G.; Kopetz, S. Platelets, Circulating Tumor Cells, and the Circulome. Cancer Metastasis Rev. 2017, 36, 235–248. [Google Scholar] [CrossRef]
- Shen, Y.; Lai, Y.; Xu, D.; Xu, L.; Song, L.; Zhou, J.; Song, C.; Wang, J. Diagnosis of Thyroid Neoplasm Using Support Vector Machine Algorithms Based on Platelet RNA-Seq. Endocrine 2021, 72, 758–783. [Google Scholar] [CrossRef]
- Sol, N.; in ‘t Veld, S.G.J.G.; Vancura, A.; Tjerkstra, M.; Leurs, C.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Zwaan, K.; et al. Tumor-Educated Platelet RNA for the Detection and (Pseudo)Progression Monitoring of Glioblastoma. Cell Rep. Med. 2020, 1, 100101. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, K.; Supernat, A.; Best, M.G.; In ’t Veld, S.G.J.G.; Łapińska-Szumczyk, S.; Łojkowska, A.; Różański, R.; Żaczek, A.J.; Jassem, J.; Würdinger, T.; et al. ImPlatelet Classifier: Image-Converted RNA Biomarker Profiles Enable Blood-Based Cancer Diagnostics. Mol. Oncol. 2021, 15, 2688–2701. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Liu, C.; Zhang, B.; Ma, L. Tumor-Educated Platelets as a Promising Biomarker for Blood-Based Detection of Renal Cell Carcinoma. Front. Oncol. 2022, 12, 689. [Google Scholar] [CrossRef] [PubMed]
| R-ISS Stage | Criteria | |
|---|---|---|
| I | Sβ2M 1 | <3.5 mg/L |
| Serum albumin | ≥3.5 g/dL | |
| Chromosomal abnormalities by iFISH 2 | Standard risk | |
| LDH 3 | Normal (<upper normal limit) | |
| II | Not R-ISS stage I or III | |
| III | Sβ2M and either Chromosomal abnormalities by FISH or LDH | ≥5.5 mg/L *High risk High (>upper normal limit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Bazou, D.; Santos-Martinez, M.J. Bleeding and Thrombosis in Multiple Myeloma: Platelets as Key Players during Cell Interactions and Potential Use as Drug Delivery Systems. Int. J. Mol. Sci. 2023, 24, 15855. https://doi.org/10.3390/ijms242115855
Kulkarni A, Bazou D, Santos-Martinez MJ. Bleeding and Thrombosis in Multiple Myeloma: Platelets as Key Players during Cell Interactions and Potential Use as Drug Delivery Systems. International Journal of Molecular Sciences. 2023; 24(21):15855. https://doi.org/10.3390/ijms242115855
Chicago/Turabian StyleKulkarni, Anushka, Despina Bazou, and Maria José Santos-Martinez. 2023. "Bleeding and Thrombosis in Multiple Myeloma: Platelets as Key Players during Cell Interactions and Potential Use as Drug Delivery Systems" International Journal of Molecular Sciences 24, no. 21: 15855. https://doi.org/10.3390/ijms242115855
APA StyleKulkarni, A., Bazou, D., & Santos-Martinez, M. J. (2023). Bleeding and Thrombosis in Multiple Myeloma: Platelets as Key Players during Cell Interactions and Potential Use as Drug Delivery Systems. International Journal of Molecular Sciences, 24(21), 15855. https://doi.org/10.3390/ijms242115855






