2.1. Onion Peel Extract Characterization
The present study aimed to compare the performance of free OP extract to the performance of microencapsulated OP extract as natural UV filters to reduce the utilization of synthetic UV filters. OP extract was obtained by solid–liquid extraction and was characterized regarding its biological properties. The results regarding the antioxidant capacity and the quantification of the main phenolic compounds of the onion peel extract are presented in
Table 1.
It is important to note that the TPC (total phenolic content) value obtained is within the range of the literature values. Nevertheless, it is closer to the higher values, demonstrating a high phenolic content in this extract [
30,
31,
32]. Furthermore, the antioxidant capacity of the OP extract was also evaluated by the DPPH assay and ABTS assay. The obtained results appear in the range of the literature; however, they are slightly lower than those obtained for the same extraction method [
7]. The results of these analyses depend on the extraction method, the solvent used, and the variety of onion used, which may lead to significant variations in the results obtained among authors. Additionally, it is critical to note that the value of the half-maximal inhibitory concentration (IC
50), which represents the concentration of the extract needed to inhibit 50% of the DPPH radical present in the solution, is inferior to 50 mg L
−1, which means this extract is considered a very strong antioxidant [
33].
The main phenolic compounds identified by HPLC-DAD were quercetin and resveratrol, which is in accordance with the literature, although present in smaller concentrations. The literature also refers to the presence of kaempferol; however, this compound was not detected in the studied OP extract [
7].
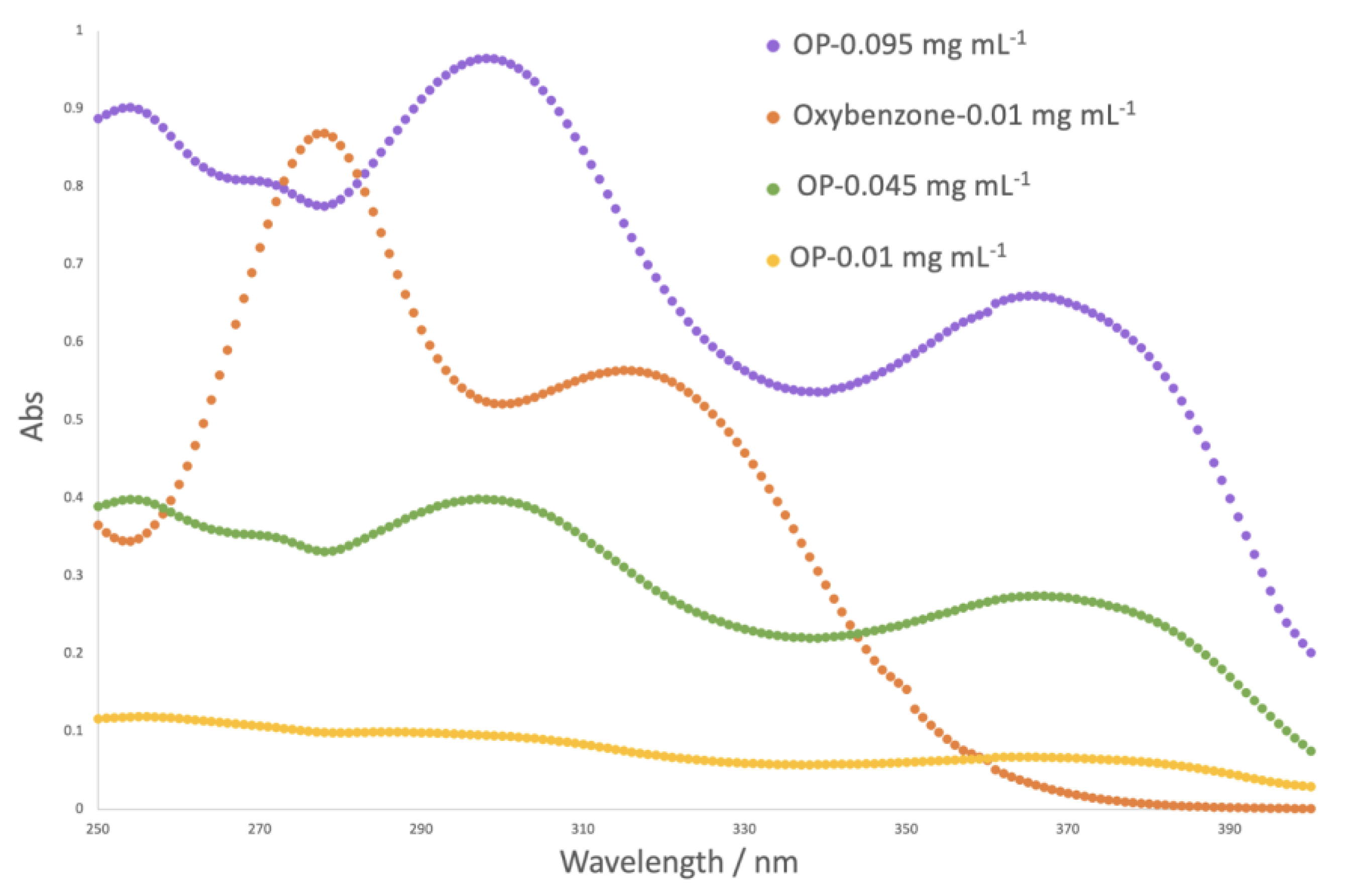
The sun protection factor (SPF) is a numerical indicator of a sunscreen’s protection level against UV rays that damage the skin. The higher the SPF of a product, the more effective it is at preventing sun damage. The sun protection factor was analyzed for different concentrations of onion peel (OP) extract and was compared to a synthetic UV filter serving as positive control (oxybenzone) (
Table 2). The absorbance spectra are illustrated in
Figure 1.
For the same concentration, the protection factor for the OP extract solution was inferior to the one obtained for the positive control. However, it is important to note that the maximum concentration of synthetic UV filter is legislated, which does not happen for the phenolic extract. The phenolic extract is a mixture of compounds, and not all contain photoprotective capacity. In order to achieve the same level of protection, a higher concentration of OP extract is needed. For higher concentrations, the results reveal higher values of SPF, demonstrating the high potential of the OP extract to be used as a natural UV filter.
Figure 1 also demonstrates that OP extract might potentially provide protection against a broader range of UV radiation, as it absorbs radiation between 350 and 390 nm, which oxybenzone (synthetic UV filter) does not absorb.
2.2. Microencapsulation
OP extract was microencapsulated by molecular inclusion, using β-cyclodextrin as the coating agent. The two most important aspects when microencapsulating bioactive compounds are particle size and encapsulation efficiency [
21].
Particles with diameters within nanometers have a higher tendency to aggregate due to the type of interaction established and to their high surface area. Furthermore, nanoparticles might be able to infiltrate the blood and cause cytotoxicity [
5]. Particle size also influences the release of the core material. Materials entrapped in smaller particles have enhanced accessibility to the external phase, which might result in a faster release by diffusion, a lower drug loading, and water might penetrate the particle more easily. On the other hand, smaller particles might better adhere to the skin due to better binding per unit of particle mass compared to the binding presented for larger particles [
34].
Encapsulation efficiency measures the amount of extract that was successfully entrapped in the microcapsule, and so the highest encapsulation efficiency possible is desired.
To achieve microparticles that contain the desired characteristics for cosmetic applications, the produced microparticles were optimized regarding these two factors.
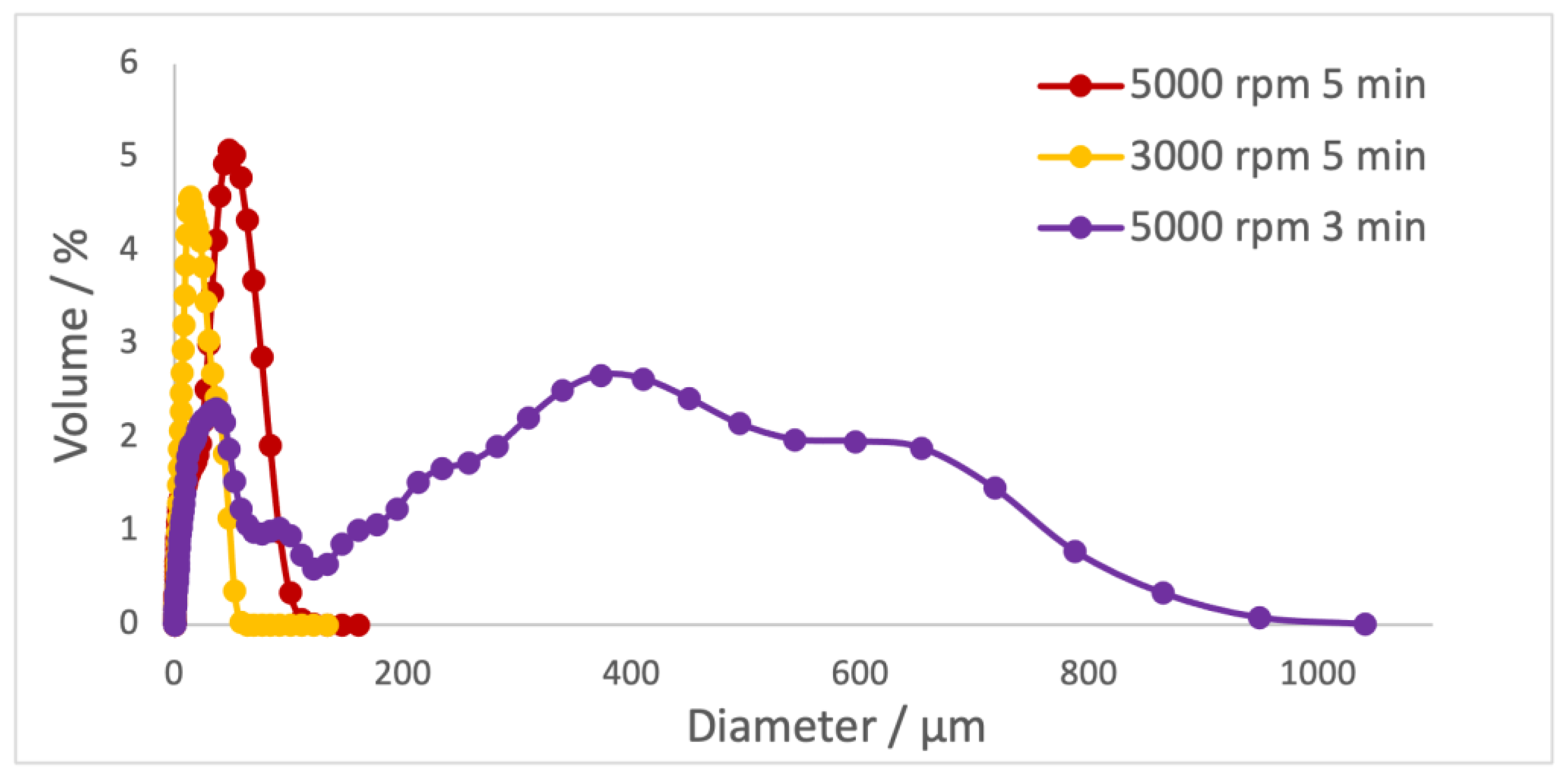
Firstly, the effect of the rotation velocity and time of rotation on the size of the microparticles was evaluated (
Table 3 and
Figure 2).
Decreasing the rotation speed leads to a decrease in the particle size. Reducing the rotation time reveals an increase in the diameter of the particles and an increase in the polydispersity index. The optimum outcome would be particles with a larger diameter and a small polydispersity (5000 rpm/5 min) to guarantee a homogeneous distribution of the extract in microparticles and minimize the risk of microparticles infiltrating the blood and causing cytotoxicity.
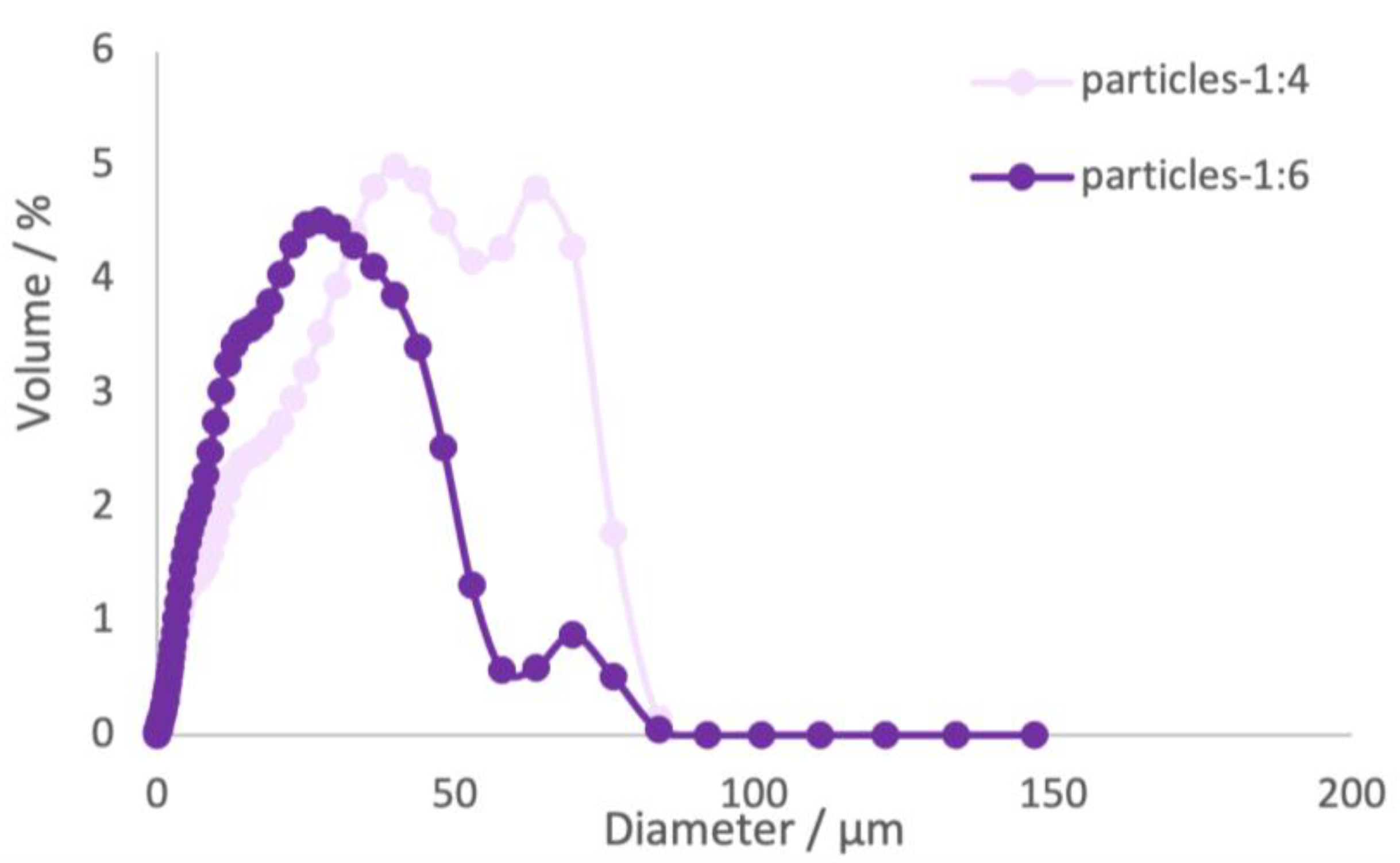
Furthermore, the effect of the loading capacity in the microparticles was studied. The loading capacity is the amount of encapsulated material per weight unit of microparticle. Therefore, the microencapsulation protocol was followed for different ratios of mass of extract/mass of β-cyclodextrin, 1:4, 1:6, and 1:10 (
w/
w). The size of the microparticles and the encapsulation efficiency were evaluated for each sample (
Table 4 and
Figure 3).
The sample at the ratio 1:10 (
w/
w) did not produce a sufficient amount of sample to be analyzed by Coulter counter. Furthermore, this sample presented a low encapsulation efficiency, which led to the exclusion of this sample for further analyses and evaluations. The samples 1:4 and 1:6 exhibited a bimodal distribution of particle sizes, as illustrated in
Figure 3, suggesting the formation of aggregates of microparticles during the batch production. The mean diameter values obtained for sample 1:4 was found to be higher than the values obtained for sample 1:6, measuring 31 μm and 21 μm, respectively. This difference can be associated with the higher concentration of material verified in sample 1:4. According to [
35], the mean diameters of powders can be influenced by various factors, such as the nature of the wall materials, the type of core material, concentration of wall materials, stirring speed and polymer molecular weight. For samples 1:4 and 1:6, the PDI values were higher than 1. These findings indicate that the samples exhibited a wide range of particle sizes and a greater degree of heterogeneity [
35,
36]. The higher PDI value obtained for sample 1:6 compared to sample 1:4, suggests a greater degree of heterogeneity in the particle size distribution. This suggests that the particles in sample 1:6 vary to a larger extent in terms of their sizes, resulting in a broader size distribution compared to sample 1:4.
Encapsulation efficiency measures the amount of extract successfully entrapped in the microcapsule and is one of the most important characteristics when evaluating encapsulation processes. The encapsulation efficiency was determined as presented in
Section 3.5.2. The results show that this polymer is more efficient in the encapsulation process when presenting a high loading capacity. Akdeniz, Sumnu [
30] microencapsulated phenolic extracts from onion skin using maltodextrin combined with gum Arabic, casein or whey protein concentrate as coating material. They found that the encapsulation efficiency also increased with the decrease in dextrin ratio, and also when combining maltodextrin with casein. On the other hand, for the combination of maltodextrin with gum Arabic and whey protein concentrate, the encapsulation efficiency increased with the increment in maltodextrin ratio in the coating material. Their highest efficiency was for the combination of maltodextrin with casein, with the lowest amount of dextrin, and presented a value of approximately 90%, which is in concordance with the results for this study.
Ultimately, the antioxidant capacity of the microencapsulated extract was assessed and subsequently compared with that of the non-encapsulated extract (
Table 5).
The results reveal that the microencapsulated extract exhibits superior antioxidant capacity compared to the non-microencapsulated extract, a finding that is consistent with the existing literature [
37,
38]. Moreover, the sample with the ratio of 1 g
extract to 4 g
coating agent demonstrates better antioxidant capacity than the sample with the ratio of 1 g
extract to 6 g
coating agent.
After optimizing the protocol to obtain the desired microparticles, OP extract microparticles and the β-CD powder (coating agent) were analyzed by scanning electron microscopy (SEM) in order to examine their morphology (
Figure 4).
The results revealed that the microparticles present an irregular shape, with some similarities with the β-CD powder morphology, in accordance with findings by several other authors for microparticles produced using β-CD powder as the coating agent [
39,
40]. The results also demonstrated that microparticles are porous. Porosity may indicate that one of the phenomena that allows the release of the OP extract is passing through the pores.
In order to evaluate the thermal stability of the microparticles, two analyses were performed: thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). TGA and DSC have been considered effective tests to study alterations in physical and chemical properties of the material caused by temperature [
41]. TGA analysis was performed in both β-CD powder and microparticles containing the OP extract (
Figure 5).
TGA analysis revealed two thermal events of mass loss. The first event, in the range of 20 and 105 °C, is due to the evaporation of adsorbed water on the microparticles’ surface. The second event from 250 to 400 °C corresponds to the decomposition and depolymerization of the microparticle constituents. MP-OP exhibits a lower degree of weight loss at 100 °C, suggesting that it possesses a lower moisture content than β-CD.
Moreover, from 105 to 300 °C, the findings indicate that MP-OP shows lower thermal stability when compared to β-CD. Consequently, caution should be exercised when using microparticles in applications or processes that involve temperatures exceeding this threshold.
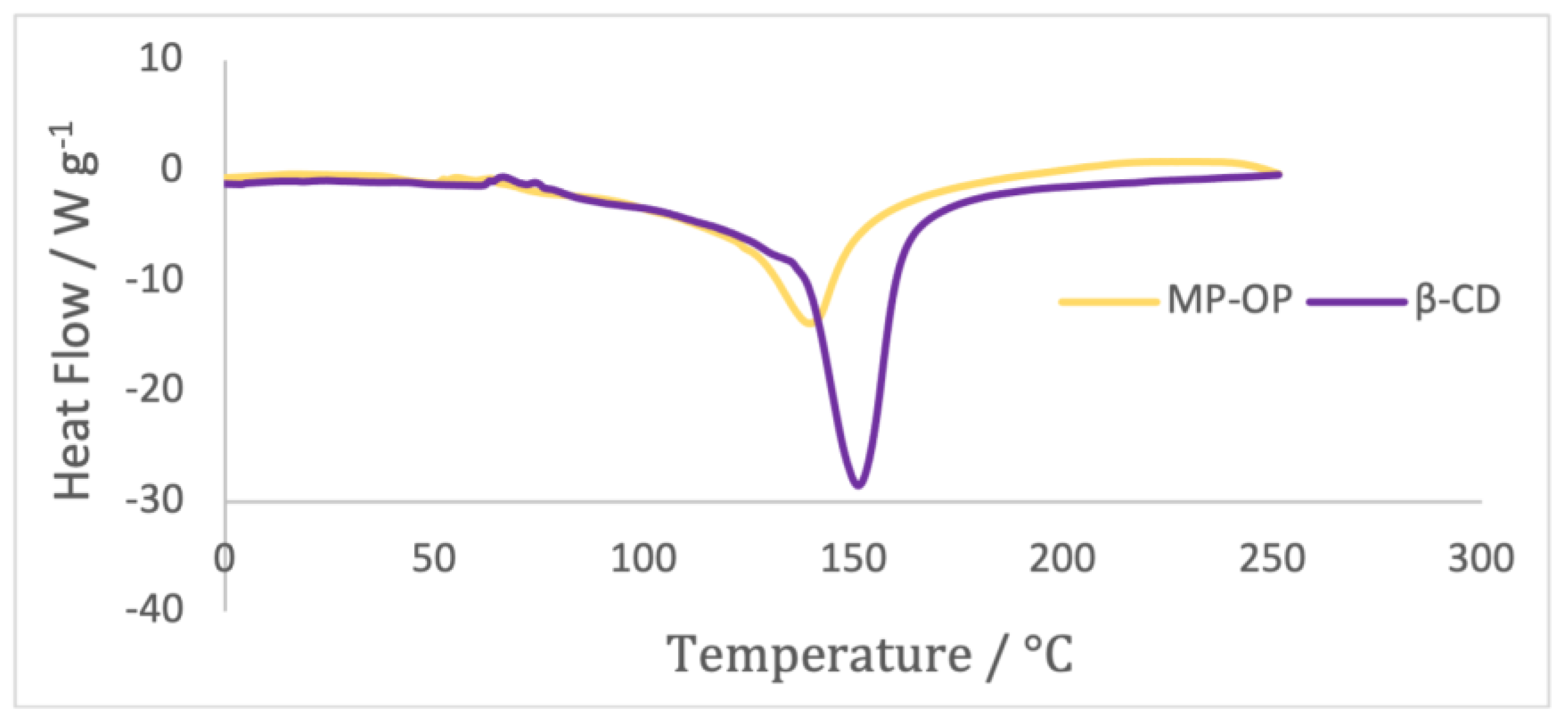
Differential scanning calorimetry (DSC) is a widely employed and routine method for confirming the formation of complexes in the solid state [
42]. DSC analysis was performed in both β-CD powder and microparticles containing the OP extract (
Figure 6).
MP-OP and β-CD exhibited a broad endothermic event with a peak observed at approximately 140 °C for MP-OP and around 151 °C for β-CD. The endothermic peak of MP-OP shifted left by several degrees compared to β-CD, presumably due to the interaction of OP extract with β-CD molecules.
The shift or disappearance of the endothermic melting peak observed on a DSC spectrum for a guest molecule typically indicates successful encapsulation or combination [
43]. Therefore, the obtained results provide evidence of successful encapsulation of onion peel extract within β-cyclodextrin.
In order to evaluate the performance as a photoprotection agent of the microencapsulated extract and compare it with the performance of the non-encapsulated OP extract, the SPF value was calculated (
Table 6).
Microparticles are characterized by having a controlled release of the trapped compound; in this case, there is a controlled release of the OP extract. It is expected that, for solutions of the same concentration of OP, the solution of the microencapsulation extract presents a smaller amount of free extract, as a significant amount should still be entrapped at the time of the analysis. A smaller amount of free extract should lead to an inferior protection factor, as demonstrated by the results.
2.3. Sunscreen Formulations
Five oil-in-water formulations were produced (
Figure 7). As a negative control, a formulation without additives was made. Synthetic UV filter oxybenzone was added at a concentration of 5% to the positive control (PC) formulation, and it was chosen since it is one of the most widely used synthetic UV filters in commercial sunscreens [
44]. To study the effect of OP extract in the formulations and compare its photoprotective performance to the performance of the synthetic UV filter, a formulation containing 5% of OP extract (OPE), non-encapsulated, was produced. In order to evaluate microencapsulation as a protection technique, a formulation containing 5% of microencapsulated OP extract (MP-OPE) was also developed. Finally, a formulation (MIX) containing a mixture of 1.6% of each of the three additives (oxybenzone, non-encapsulated, and microencapsulated OP extract) was produced to perform a more thorough evaluation and comparison.
The stability of the formulations was evaluated over a 5-week time span. All formulations maintained organoleptic qualities, such as color and smell, unaltered over the course of the study. NC and PC formulations had a beige color, while the other formulations presented a brownish color due to the presence of the OP extract. MP-OPE and MIX formulations were darker than the OPE formulation since it was necessary to use a larger amount of microparticles (also brownish) to achieve the same percentage of OP extract used in OPE formulation. In terms of aroma, NC and PC were odorless, while the rest presented a light onion aroma. No creams demonstrated any changes in smell over the time of the study. Regarding their appearance/consistency, MP-OPE and MIX formulations had small visible particles, while the remaining formulations were completely homogeneous.
To evaluate the formulations’ physical stability, a centrifuge assay was performed (
Figure 8). The centrifuge test revealed that none of the formulations presented phase separation, confirming the stability of the emulsions. Nevertheless, the undissolved granules were deposited at the bottom of the MIX formulation falcon.
The formulations were also subjected to a thermal stability test to simulate the long-term shelf life with temperature changes between 4 and 50 °C. The results revealed no changes in color, texture, smell, or mass, indicating the emulsions presented high long-term stability and maintained their properties regardless of temperature changes.
Skin pH is within the range of 4 to 6, and any cosmetic formulation produced with the intent of topical application should present pH values within this range [
45]. The pH of the formulations was determined for three different analysis times: week of production, two weeks after production and four weeks after production (
Figure 9).
Results reveal that the pH of all formulations slightly decreases over time. Nevertheless, all formulations’ pH values remain within the ideal range for topical application. Furthermore, it is demonstrated that the presence of OP extract in the formulations acidifies them, which is expected since phenolic compounds are weak acids [
7].
Antioxidants react with free radicals, neutralizing these and preventing the oxidation of the formulations [
46]. Synthetic antioxidants are present in cosmetic formulations in low concentrations, for example, butylated hydroxytoluene (BHT) is used in a concentration of 0.50% [
47]. To study OP extract as an antioxidant, it would be necessary to produce a formulation with 0.50% of OP extract and compare it with a positive control formulation containing a synthetic antioxidant. However, the scope of this study was to evaluate the behavior of OP extract as an alternative UV filter and not as an antioxidant, and so no formulation containing only 0.50% of OP extract was produced. Nevertheless, the antioxidant capacity of the formulations was evaluated by the DPPH assay, in order to elucidate the behavior of phenolic extract in formulations (
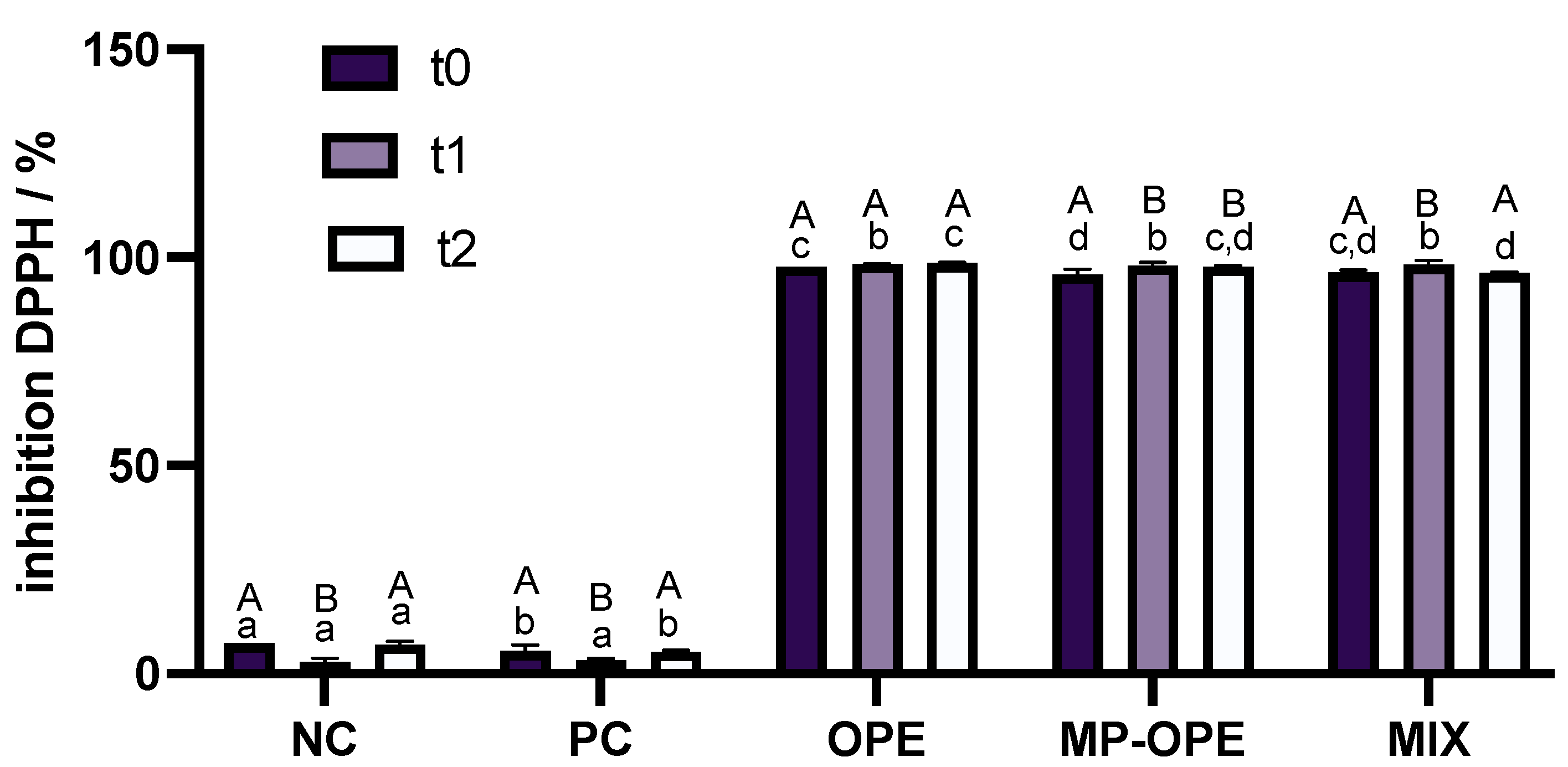
Figure 10).
The results reveal that neither NC nor PC present antioxidant capacity, while the formulations containing the phenolic extract are able to inhibit almost completely the DPPH radical, presenting very strong antioxidant capacity. These observations are in accordance with the anticipated results, since NC and PC contain no antioxidants in their composition, and hence no oxidation was prevented. Phenolic compounds are known for their antioxidant capacity [
14], and formulations containing the extract were expected to be able to postpone and reduce oxidation, which was demonstrated by the results.
Synthetic antioxidants’ concentration on cosmetic formulations are limited by legislation. As previously stated, agricultural by-product extracts are constituted by several other bioactive compounds, such as vitamins, which allows their use in higher concentrations, increasing the antioxidant power of the formulations.
The sun protection factor (SPF) was calculated to evaluate the photoprotection capacity of the formulations over time (
Table 7).
The results reveal that NC presents no protection factor against UV radiation, which was expected since no additive/UV filter/extract was added to this formulation. PC formulation presented the highest value of SPF.
In terms of the formulations containing OP extract, the analysis is more complex. In the week of production of the sunscreens, the formulation containing microencapsulated extract (MP-OPE) presented a higher protection factor than both the OPE and MIX formulations. For the second analysis time, two weeks after production, and for the final analysis time, four weeks after production, MIX emulsion presents the highest SPF value. OPE formulation always presents the lowest protection factor, which may be justified by the fast degradation of the OP extract while not protected. Considering the variation in the protection factor, it is possible to further discuss the results presented above. The results reveal that between t0 and t2, the MP-OPE formulation presents a constant decrease in the SPF value, which may be justified by the controlled release of the OP extract when microencapsulated. Extract release from the microparticles is expected to peak in the first few hours, followed by a constant release of the extract from that point on. Considering that the first analysis was performed two days after production of the cream (t0), the microparticles in the formulation should be releasing OP extract at a constant rate, leading to a proportional decrease in the SPF value with time. On the other hand, the OPE formulation contains no encapsulated extract, leading to a greater decline in the value of the SPF between the first two analyses. At t1 (two weeks after production), the majority of the OP extract had already been degraded. The existence of a smaller amount of extract in the formulation between t1 and t2 (four weeks after production) leads to a less abrupt decrease in the protection factor value.
These results reveal that microencapsulation protects the extract from degradation. When incorporated in cosmetic formulations, the microencapsulated extract enables sunscreens to maintain their photoprotection for longer periods, increasing sunscreen shelf life. MIX and PC formulations demonstrated the best results, presenting almost no decline in the SPF value. The results suggest that the OP extract, either free or microencapsulated, presents itself as a prospective natural UV filter to substitute synthetic UV filters or combined with these to decrease the use and concentration of the latter.
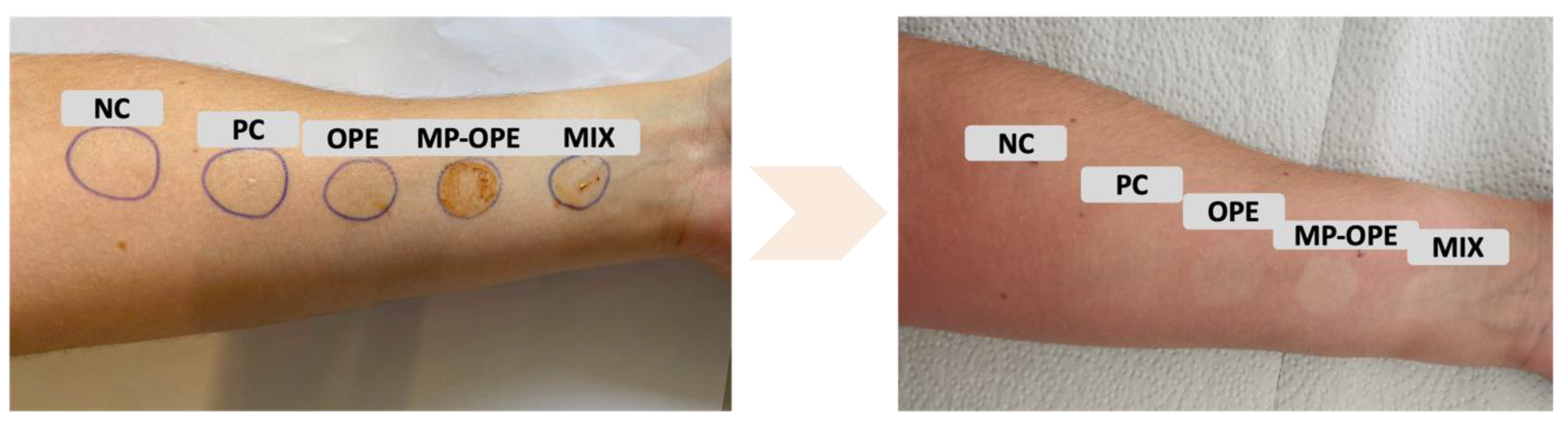
Finally, the photoprotection capacity of the formulations was further evaluated by studying a volunteer’s skin reactivity to the formulations after one hour of sunlight exposure (
Figure 11). The volunteer had healthy skin.
As expected, the skin area to which the NC formulation was applied displayed the greatest irritation, as this formulation had no additive to protect against UV radiation. All the other formulations demonstrated protection against UV radiation, which is in concordance with the results obtained by the SPF value test. Visually, it appears that formulations containing OP extract provide higher protection. MP-OPE and MIX formulations presented the best results, with the skin area where they were applied appearing less tanned and irritated than the rest.
Figure 11 shows that the OPE formulation presents higher protection than the PC, but protects less against UV radiation than the MP-OPE and MIX formulations. The results reveal that by microencapsulating the extracts, protection of the OP extract is successfully accomplished, preserving the photoprotection capacity of the extract and enhancing its protection against UV radiation.
The results of this test appear to contrast with the obtained SPF values. However, SPF only evaluates photoprotection in the UVB region. OP extract is a mixture of compounds that, due to their synergy, absorb UV radiation in other regions of the UV radiation spectrum, providing higher protection than what the SPF value reveals.
Even though these are preliminary studies, they suggest that OP extract, either non-encapsulated or microencapsulated, might be used as a source of photoprotection for more sustainable and environmentally friendly sunscreens.