Genetic Abnormalities in Extramedullary Multiple Myeloma
Abstract
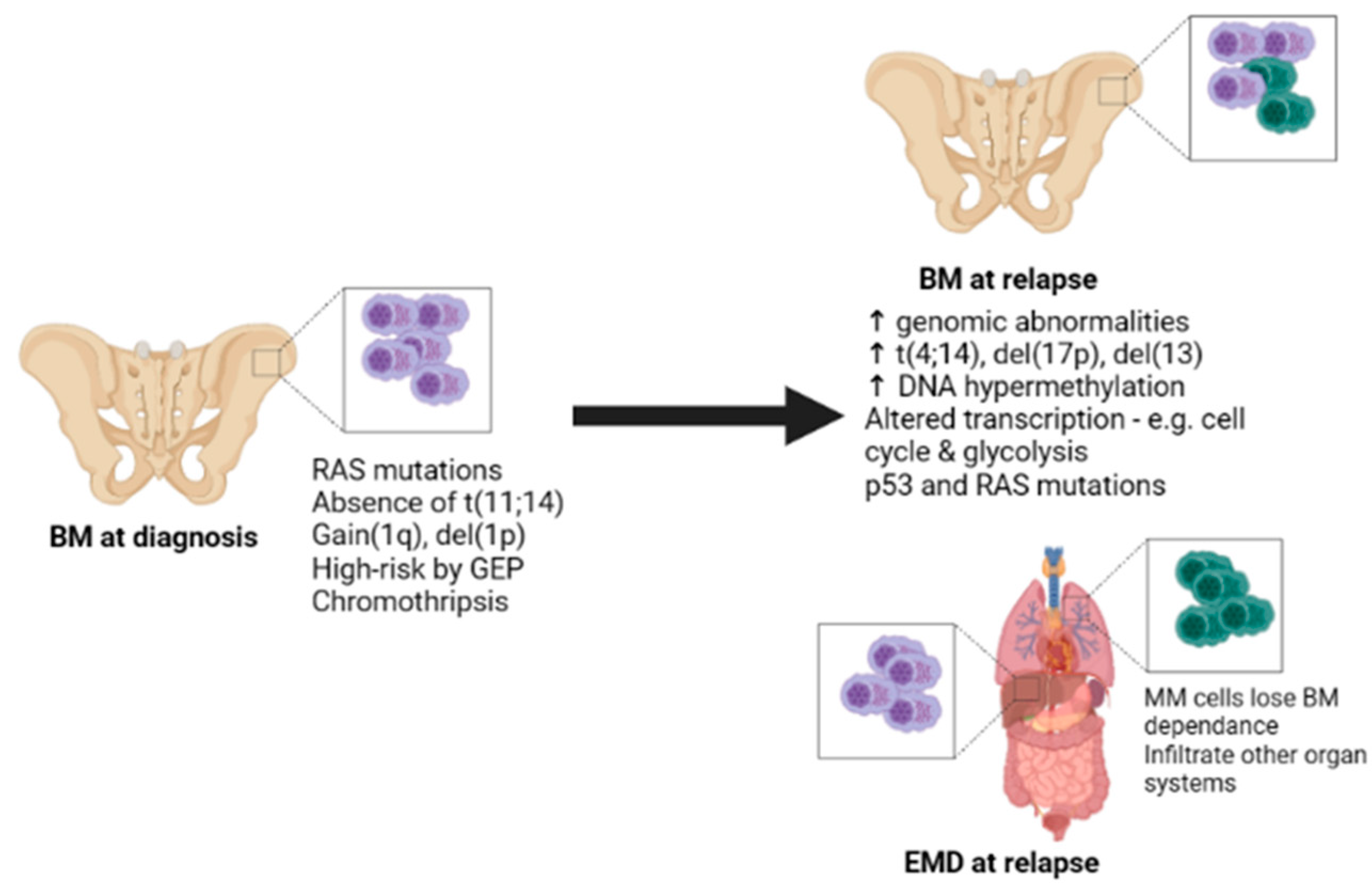
1. Introduction
2. Cytogenetic Abnormalities
3. Altered Gene Expression
4. Mutational Landscape
5. Epigenetic Changes
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.J.; Iacobelli, S.; Koster, L.; Nahi, H.; Stoppa, A.M.; Masszi, T.; Caillot, D.; Lenhoff, S.; Udvardy, M.; et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: A study from the Chronic Malignancies Working Party of the EBMT. Haematologica 2018, 103, 890–897. [Google Scholar] [CrossRef]
- Pour, L.S.S.; Greslikova, H.; Kupska, R.; Majkova, P.; Zahradova, L.; Sandecka, V.; Adam, Z.; Krejci, M.; Kuglik, P.; Hajek, R. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica 2014, 99, 360–364. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Blade, J.; Beksac, M.; Caers, J.; Jurczyszyn, A.; von Lilienfeld-Toal, M.; Moreau, P.; Rasche, L.; Rosinol, L.; Usmani, S.Z.; Zamagni, E.; et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022, 12, 45. [Google Scholar] [CrossRef]
- Batsukh, K.L.S.; Min, G.J.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Kim, H.J.; Lee, S.; et al. Distinct Clinical Outcomes between Paramedullary and Extramedullary Lesions in Newly Diagnosed Multiple Myeloma. Immune Netw. 2017, 17, 250–260. [Google Scholar] [CrossRef]
- Black, H.; Glavey, S. Gene expression profiling as a prognostic tool in multiple myeloma. Cancer Drug Resist. 2021, 4, 1008–1018. [Google Scholar] [CrossRef]
- Saxe, D.; Seo, E.J.; Bergeron, M.B.; Han, J.Y. Recent advances in cytogenetic characterization of multiple myeloma. Int. J. Lab. Hematol. 2018, 41, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Billecke, L.; Murga Penas, E.M.; May, A.M.; Engelhardt, M.; Nagler, A.; Leiba, M.; Schiby, G.; Kroger, N.; Zustin, J.; Marx, A.; et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br. J. Haematol. 2013, 161, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.B.; Kortuem, K.M.; Kurdoglu, A.; Izatt, T.; Aldrich, J.; Reiman, R.; Phillips, L.; Baker, A.; Shi, C.X.; Schmidt, J.; et al. Extramedullary myeloma whole genome sequencing reveals novel mutations in Cereblon, proteasome subunit G2 and the glucocorticoid receptor in multi drug resistant disease. Br. J. Haematol. 2013, 161, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, S.; Paszekova, H.; Besse, L.; Sedlarikova, L.; Kubaczkova, V.; Almasi, M.; Pour, L.; Hajek, R. Extramedullary relapse of multiple myeloma defined as the highest risk group based on deregulated gene expression data. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2015, 159, 288–293. [Google Scholar] [CrossRef]
- Qu, X.; Chen, L.; Qiu, H.; Lu, H.; Wu, H.; Qiu, H.; Liu, P.; Guo, R.; Li, J. Extramedullary manifestation in multiple myeloma bears high incidence of poor cytogenetic aberration and novel agents resistance. Biomed. Res. Int. 2015, 2015, 787809. [Google Scholar] [CrossRef]
- Besse, L.S.L.; Greslikova, H.; Kupska, R.; Almasi, M.; Penka, M.; Jelinek, T.; Pour, L.; Adam, Z.; Kuglik, P.; Krejci, M.; et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur. J. Hematol. 2016, 97, 93–100. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Sirdesai, S.; Chen, M.; Khong, T.; Spencer, A. Circulating Tumour DNA Analysis for Tumour Genome Characterisation and Monitoring Disease Burden in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 1858. [Google Scholar] [CrossRef]
- Yao, Q.; Morgan, G.J.; Chim, C.S. Distinct promoter methylation profile reveals spatial epigenetic heterogeneity in 2 myeloma patients with multifocal extramedullary relapses. Clin. Epigenetics 2018, 10, 158. [Google Scholar] [CrossRef]
- Smetana, J.; Oppelt, J.; Stork, M.; Pour, L.; Kuglik, P. Chromothripsis 18 in multiple myeloma patient with rapid extramedullary relapse. Mol. Cytogenet. 2018, 11, 7. [Google Scholar] [CrossRef]
- Liu, Y.; Jelloul, F.; Zhang, Y.; Bhavsar, T.; Ho, C.; Rao, M.; Lewis, N.E.; Cimera, R.; Baik, J.; Sigler, A.; et al. Genetic Basis of Extramedullary Plasmablastic Transformation of Multiple Myeloma. Am. J. Surg. Pathol. 2020, 44, 838–848. [Google Scholar] [CrossRef]
- Long, X.; Xu, Q.; Lou, Y.; Li, C.; Gu, J.; Cai, H.; Wang, D.; Xu, J.; Li, T.; Zhou, X.; et al. The utility of non-invasive liquid biopsy for mutational analysis and minimal residual disease assessment in extramedullary multiple myeloma. Br. J. Haematol. 2020, 189, e45–e48. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Kim, S.J.; Hong, Y.; Jo, A.; Kim, N.; Kim, H.J.; Lee, H.O.; Kim, K.; Park, W.Y. Alterations in the Transcriptional Programs of Myeloma Cells and the Microenvironment during Extramedullary Progression Affect Proliferation and Immune Evasion. Clin. Cancer Res. 2020, 26, 935–944. [Google Scholar] [CrossRef]
- Kriegova, E.; Fillerova, R.; Minarik, J.; Savara, J.; Manakova, J.; Petrackova, A.; Dihel, M.; Balcarkova, J.; Krhovska, P.; Pika, T.; et al. Whole-genome optical mapping of bone-marrow myeloma cells reveals association of extramedullary multiple myeloma with chromosome 1 abnormalities. Sci. Rep. 2021, 11, 14671. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shi, Y.; Chen, Z.; Zhang, J.; Zhu, Y.; Guo, R.; Zhang, R.; Shi, Q.; Li, J.; Chen, L. Characteristics and prognostic value of extramedullary chromosomal abnormalities in extramedullary myeloma. Chin. Med. J. 2022, 135, 2500–2502. [Google Scholar] [CrossRef]
- Chen, T.; Sun, Z.; Cui, Y.; Ji, J.; Li, Y.; Qu, X. Identification of long noncoding RNA NEAT1 as a key gene involved in the extramedullary disease of multiple myeloma by bioinformatics analysis. Hematology 2023, 28, 2164449. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, J.; Li, Y.; Cui, Y.; Fan, L.; Li, J.; Qu, X. Identification of evolutionary mechanisms of myelomatous effusion by single-cell RNA sequencing. Blood Adv. 2023. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myelome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef]
- Misund, K.; Hofste Op Bruinink, D.; Coward, E.; Hoogenboezem, R.M.; Rustad, E.H.; Sanders, M.A.; Rye, M.; Sponaas, A.M.; van der Holt, B.; Zweegman, S.; et al. Clonal evolution after treatment pressure in multiple myeloma: Heterogenous genomic aberrations and transcriptomic convergence. Leukemia 2022, 36, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Mikhael, J.R.; Dingli, D.; Roy, V.; Reeder, C.B.; Buadi, F.K.; Hayman, S.R.; Dispenzieri, A.; Fonseca, R.; Sher, T.; Kyle, R.A.; et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin. Proc. 2013, 88, 360–376. [Google Scholar] [CrossRef]
- Varga, C.; Xie, W.; Laubach, J.; Ghobrial, I.M.; O’Donnell, E.K.; Weinstock, M.; Paba-Prada, C.; Warren, D.; Maglio, M.E.; Schlossman, R.; et al. Development of extramedullary myeloma in the era of novel agents: No evidence of increased risk with lenalidomide-bortezomib combinations. Br. J. Haematol. 2015, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Bink, K.; Haralambieva, E.; Kremer, M.; Ott, G.; Beham-Schmid, C.; de Leval, L.; Peh, S.C.; Laeng, H.R.; Jutting, U.; Hutzler, P.; et al. Primary extramedullary plasmacytoma: Similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica 2008, 93, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Qazilbash, M.H.; Saliba, R.M.; Ahmed, B.; Parikh, G.; Mendoza, F.; Ashraf, N.; Hosing, C.; Flosser, T.; Weber, D.M.; Wang, M.; et al. Deletion of the short arm of chromosome 1 (del 1p) is a strong predictor of poor outcome in myeloma patients undergoing an autotransplant. Biol. Blood Marrow Transpl. 2007, 13, 1066–1072. [Google Scholar] [CrossRef]
- Hanamura, I. Multiple myeloma with high-risk cytogenetics and its treatment approach. Int. J. Hematol. 2022, 115, 762–777. [Google Scholar] [CrossRef]
- Neuse, C.J.; Lomas, O.C.; Schliemann, C.; Shen, Y.J.; Manier, S.; Bustoros, M.; Ghobrial, I.M. Genome instability in multiple myeloma. Leukemia 2020, 34, 2887–2897. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, K.H.; Rustad, E.H.; Derkach, A.; Zheng-Lin, B.; Yellapantula, V.; Diamond, B.; Hultcrantz, M.; Ziccheddu, B.; Boyle, E.M.; Blaney, P.; et al. Copy number signatures predict chromothripsis and clinical outcomes in newly diagnosed multiple myeloma. Nat. Commun. 2021, 12, 5172. [Google Scholar] [CrossRef]
- Magrangeas, F.; Avet-Loiseau, H.; Munshi, N.C.; Minvielle, S. Chromothripsis identifies a rare and aggressive entity among newly diagnosed multiple myeloma patients. Blood 2011, 118, 675–678. [Google Scholar] [CrossRef]
- Shaughnessy, J.D., Jr.; Zhan, F.; Burington, B.E.; Huang, Y.; Colla, S.; Hanamura, I.; Stewart, J.P.; Kordsmeier, B.; Randolph, C.; Williams, D.R.; et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2007, 109, 2276–2284. [Google Scholar] [CrossRef]
- Kuiper, R.; Broyl, A.; de Knegt, Y.; van Vliet, M.H.; van Beers, E.H.; van der Holt, B.; el Jarari, L.; Mulligan, G.; Gregory, W.; Morgan, G.; et al. A gene expression signature for high-risk multiple myeloma. Leukemia 2012, 26, 2406–2413. [Google Scholar] [CrossRef]
- Van Beers, E.H.; Huigh, D.; Bosman, L.; de Best, L.; Kuiper, R.; Spaan, M.; van Duin, M.; Sonneveld, P.; Dumee, B.; van Vliet, M.H. Analytical Validation of SKY92 for the Identification of High-Risk Multiple Myeloma. J. Mol. Diagn. 2021, 23, 120–129. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Heuck, C.; Mitchell, A.; Szymonifka, J.; Nair, B.; Hoering, A.; Alsayed, Y.; Waheed, S.; Haider, S.; Restrepo, A.; et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012, 97, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Ahmann, G.J.; Henderson, K.; Santana-Davila, R.; Greipp, P.R.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Kumar, S.; Rajkumar, S.V.; et al. Clinical implication of centrosome amplification in plasma cell neoplasm. Blood 2006, 107, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Braggio, E.; Mulligan, G.; Bryant, B.; Remstein, E.; Valdez, R.; Dogan, A.; Fonseca, R. The centrosome index is a powerful prognostic marker in myeloma and identifies a cohort of patients that might benefit from aurora kinase inhibition. Blood 2008, 111, 1603–1609. [Google Scholar] [CrossRef]
- Walker, B.A.; Boyle, E.M.; Wardell, C.P.; Murison, A.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Johnson, D.C.; Kaiser, M.F.; Melchor, L.; et al. Mutational Spectrum, Copy Number Changes, and Outcome: Results of a Sequencing Study of Patients with Newly Diagnosed Myeloma. J. Clin. Oncol. 2015, 33, 3911–3920. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, K.K.; Escure, G.; Demonchy, J.; Willaume, A.; Van de Wyngaert, Z.; Farhat, M.; Chauvet, P.; Facon, T.; Quesnel, B.; Manier, S. Deregulation and Targeting of TP53 Pathway in Multiple Myeloma. Front. Oncol. 2018, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.M.K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.E.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; Samur, M.; et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 2018, 132, 587–597. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Kang, J.; Amatangelo, M.; Polonskaia, A.; Katz, M.; Chiu, H.; Couto, S.; Wang, M.; Ren, Y.; Ortiz, M.; et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia 2020, 34, 1197–1201. [Google Scholar] [CrossRef]
- Sarah Gooding, N.A.-P.; Towfic, F.; Ortiz Estévez, M.; Philip, P.; Chamberlain, K.-T.T.; Flynt, E.; Hirst, M.; Rozelle, D.; Dhiman, P.; Neri, P.; et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 2021, 137, 232–237. [Google Scholar] [CrossRef]
- De Haart, S.J.; Willems, S.M.; Mutis, T.; Koudijs, M.J.; van Blokland, M.T.; Lokhorst, H.M.; de Weger, R.A.; Minnema, M.C. Comparison of intramedullary myeloma and corresponding extramedullary soft tissue plasmacytomas using genetic mutational panel analyses. Blood Cancer J. 2016, 6, e426. [Google Scholar] [CrossRef]
- Choi, M.; Kipps, T.; Kurzrock, R. ATM Mutations in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2016, 15, 1781–1791. [Google Scholar] [CrossRef]
- Austen, B.; Barone, G.; Reiman, A.; Byrd, P.J.; Baker, C.; Starczynski, J.; Nobbs, M.C.; Murphy, R.P.; Enright, H.; Chaila, E.; et al. Pathogenic ATM mutations occur rarely in a subset of multiple myeloma patients. Br. J. Haematol. 2008, 142, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Caprio, C.; Sacco, A.; Giustini, V.; Roccaro, A.M. Epigenetic Aberrations in Multiple Myeloma. Cancers 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Fung, T.K.; Cheung, W.C.; Liang, R.; Kwong, Y.L. SOCS1 and SHP1 hypermethylation in multiple myeloma: Implications for epigenetic activation of the Jak/STAT pathway. Blood 2004, 103, 4630–4635. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Skouri, N.; Ben Ali, C.; Safra, I.; Abdelkefi, A.; Ladeb, S.; Mrad, K.; Ben Othman, T.; Ben Ahmed, M. Abnormal repression of SHP-1, SHP-2 and SOCS-1 transcription sustains the activation of the JAK/STAT3 pathway and the progression of the disease in multiple myeloma. PLoS ONE 2017, 12, e0174835. [Google Scholar] [CrossRef]
- Farre, L.; Sanz, G.; Ruiz-Xiville, N.; Castro de Moura, M.; Martin-Tejera, J.F.; Goncalves-Ribeiro, S.; Martinez-Iniesta, M.; Calaf, M.; Luis Mosquera, J.; Martin-Subero, J.I.; et al. Extramedullary multiple myeloma patient-derived orthotopic xenograft with a highly altered genome: Combined molecular and therapeutic studies. Dis. Model Mech. 2021, 14, dmm048223. [Google Scholar] [CrossRef]
- Venney, D.; Greenfield, G.; Preston, P.; Mohd-Sarip, A.; Mills, K.I. Chapter 11—Epigenetic targeted therapies in hematological malignancies. In Epigenet. Cancer Therapy, 2nd ed.; Gray, S.G., Ed.; Academic Press: Boston, MA, USA, 2023; pp. 213–236. [Google Scholar]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
| Study/Reference | Patient Cohort Description | Sample Type(s) | Methodologies | Results Summary |
|---|---|---|---|---|
| Billecke et al., 2013 [12] | 36 MM patients, 17 with EMD at diagnosis or relapse; 11 bone-related and 6 extraosseous | BM | FISH | High incidence of del(17p) in both EMD groups compared to non-EMD. |
| Egan et al., 2013 [13] | One relapsed, refractory patient with extraosseous EMD | EMD | WES, WGS, RNA-seq | Highly altered genome revealed. Of note were mutations in ATM, KRAS, NFKB2, and PSMG2. First report of CRBN mutation in EMD and low CRBN gene expression. |
| Sevickova et al., 2015 [14] | 18 patients, 9 with ‘high-risk’ * MM and 9 with EMD | ‘High-risk’ patients; BM EMD patients; paired BM and EMD cells | Quantitative PCR | Four GEP-70 genes deregulated in EMD BM vs. high-risk MM BM samples. Within patients with EMD, nine genes were deregulated in EMD tissue compared to BM. |
| Qu et al., 2015 [15] | Retrospective study of 300 patients, 41 of which had EMD at diagnosis or progression | BM | FISH | Del(17p13) and amp(1q21) associated with EMD. |
| Besse et al., 2016 [16] | 31 EMD patients either at MM diagnosis or relapse, 15 bone-related, 16 extraosseous | Paired BM and EMD | FISH | In unrelated samples, higher incidence of t(4;14) in EMD compared to BM. In paired samples, gain(1q) was frequent in BM and EMD. |
| Mithraprabhu et al., 2018 [17] | One patient with relapse extraossesous EMD | Paired EMD and PB (several time-points) | WES | ctDNA can be used to track EMD progression and clonal evolution. Both spatial and temporal heterogeneity observed. NRAS mutation observed at clonal and subclonal levels. |
| Yao et al., 2018 [18] | Two MM patients with relapse EMD | Diagnostic BM, paired relapse (BM and EMD), and relapse PB for one patient | Methylation-specific PCR | SHP1 methylation detected in both patients only at relapse. Evidence of spatial methylation heterogeneity in MM/EMD. |
| Smetana et al., 2018 [19] | One MM patient with EMD at relapse | BM (diagnostic) | Array-CGH, Targeted NGS | Patient presented with huge chromothripsis of chromosome 18 and mutations in NRAS, RAF1, TP53, CUX1 and POU4F1 before progression to EMD. |
| Liu et al., 2020 [20] | 10 patients with EMD, 4 at diagnosis and 6 at relapse | BM & EMD, paired where possible | FISH, Targeted NGS, SNP microarray | Gain(1q21) and del(1p32) common in BM and EMD lesions. High prevalence of RAS mutations |
| Long et al., 2020 [21] | 10 MM patients without EMD, 8 MM patients with EMD | Paired BM, EMD & plasma | Targeted NGS | ctDNA may be used for mutational characterization of EMD. Evidence of spatial heterogeneity between BM and EMD/ctDNA. |
| Ryu et al., 2020 [22] | 15 MM patients, 5 with EMD | BM and EMD, paired samples for three patients | scRNA-seq, WES | RAS pathway mutations common in BM and EMD samples. Transcriptional alterations observed in the cell cycle, glycolysis, oxidative phosphorylation, proteasome, and antigen presentation upon EMD progression. Upregulated IL-6 signalling in EMD. |
| Kriegova et al., 2021 [23] | 11 newly diagnosed MM patients, 4 with bone-related EMD | BM | Whole-genome optical mapping | Large intrachromosomal rearrangements within chromosome 1 detected in all EMD patients. |
| Xia et al., 2022 [24] | 30 patients with EMD; 19 bone-related and 11 extraosseous | Paired BM and EMD | FISH | Higher frequency of genomic aberrations in EMD tissue vs. BM. Higher prevalence of gain(1q) and P53 deletion in EMD, and higher in bon-related EMD compared to extraosseous. |
| Chen et al., 2023 [25] | 3 relapsed MM patients with bone-related EMD | Paired BM (diagnostic) and EMD | RNA-seq, scRNA-seq, microarray | Identified lncRNA NEAT1 as upregulated in EMD and confirmed its association with aggressive disease in vitro. |
| Sun et al., 2023 [26] | 3 patients with EMD at progression (specifically ME) | Matched BM, PB, and EMD | scRNA-seq | Determined transcriptome changes associated with EMD progression, specifically plasma cell proliferation and migration. Identified LILRB4 upregulation in ME compared to BM and confirmed effects in vitro. |
| Risk Classification | Criteria |
|---|---|
| Standard-Risk | Trisomies t(11;14) t(6;14) |
| High-Risk | High-risk genetic abnormalities:
|
| R-ISS Stage III High plasma cell S-phase GEP: High risk signatures | |
| Double-hit MM: Any two high-risk factors Triple-hit MM: Any three high-risk factors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAvera, R.; Quinn, J.; Murphy, P.; Glavey, S. Genetic Abnormalities in Extramedullary Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 11259. https://doi.org/10.3390/ijms241411259
McAvera R, Quinn J, Murphy P, Glavey S. Genetic Abnormalities in Extramedullary Multiple Myeloma. International Journal of Molecular Sciences. 2023; 24(14):11259. https://doi.org/10.3390/ijms241411259
Chicago/Turabian StyleMcAvera, Roisin, John Quinn, Philip Murphy, and Siobhan Glavey. 2023. "Genetic Abnormalities in Extramedullary Multiple Myeloma" International Journal of Molecular Sciences 24, no. 14: 11259. https://doi.org/10.3390/ijms241411259
APA StyleMcAvera, R., Quinn, J., Murphy, P., & Glavey, S. (2023). Genetic Abnormalities in Extramedullary Multiple Myeloma. International Journal of Molecular Sciences, 24(14), 11259. https://doi.org/10.3390/ijms241411259





