XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
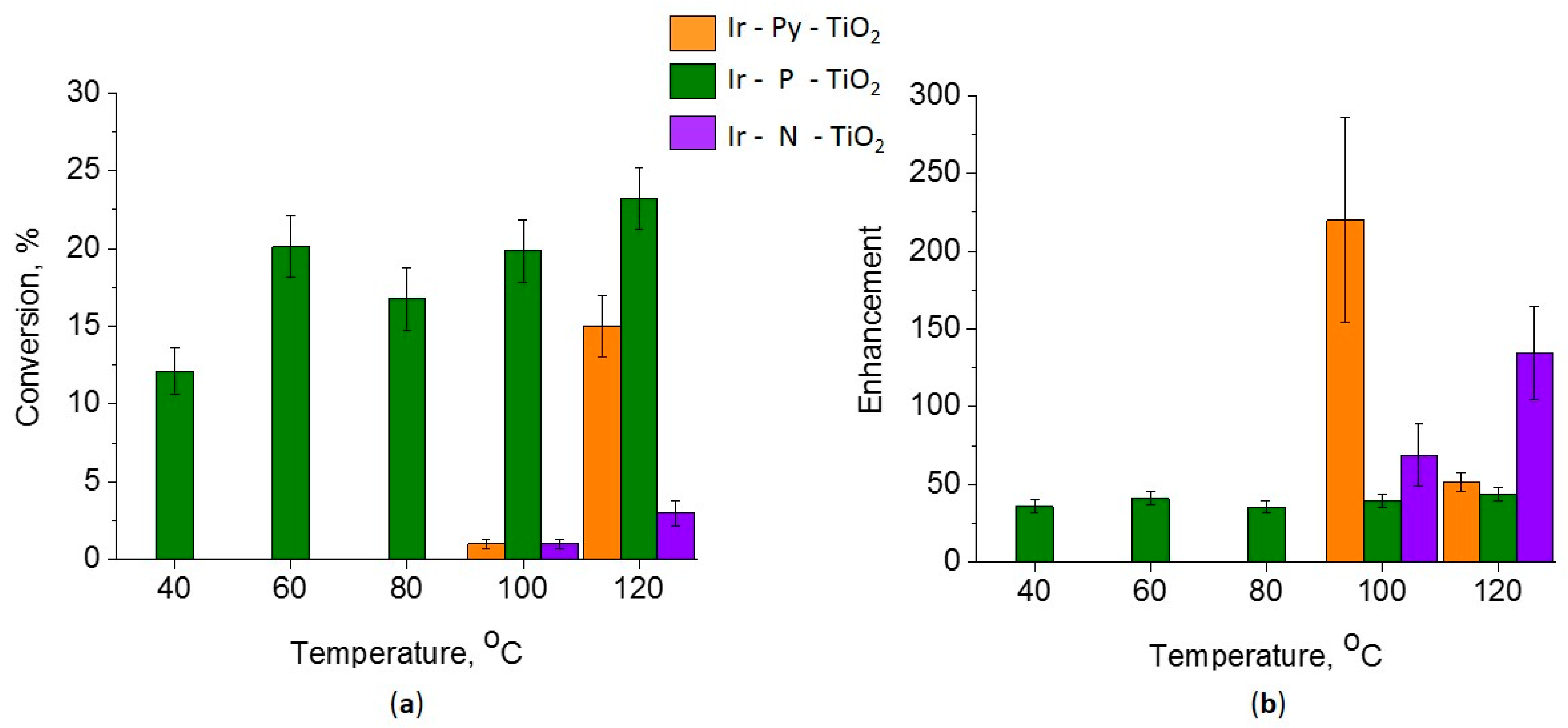
2.1. Catalytic Tests
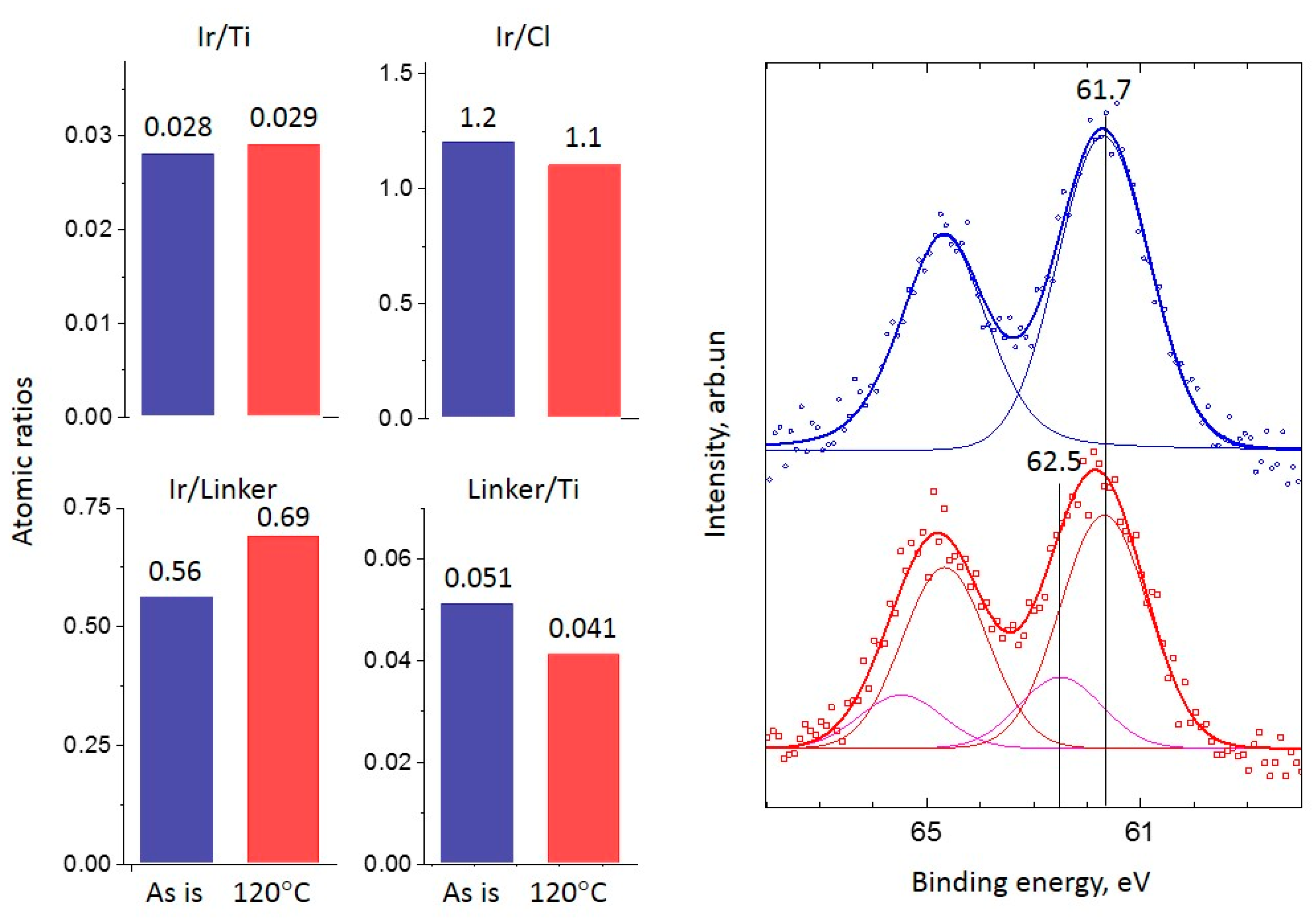
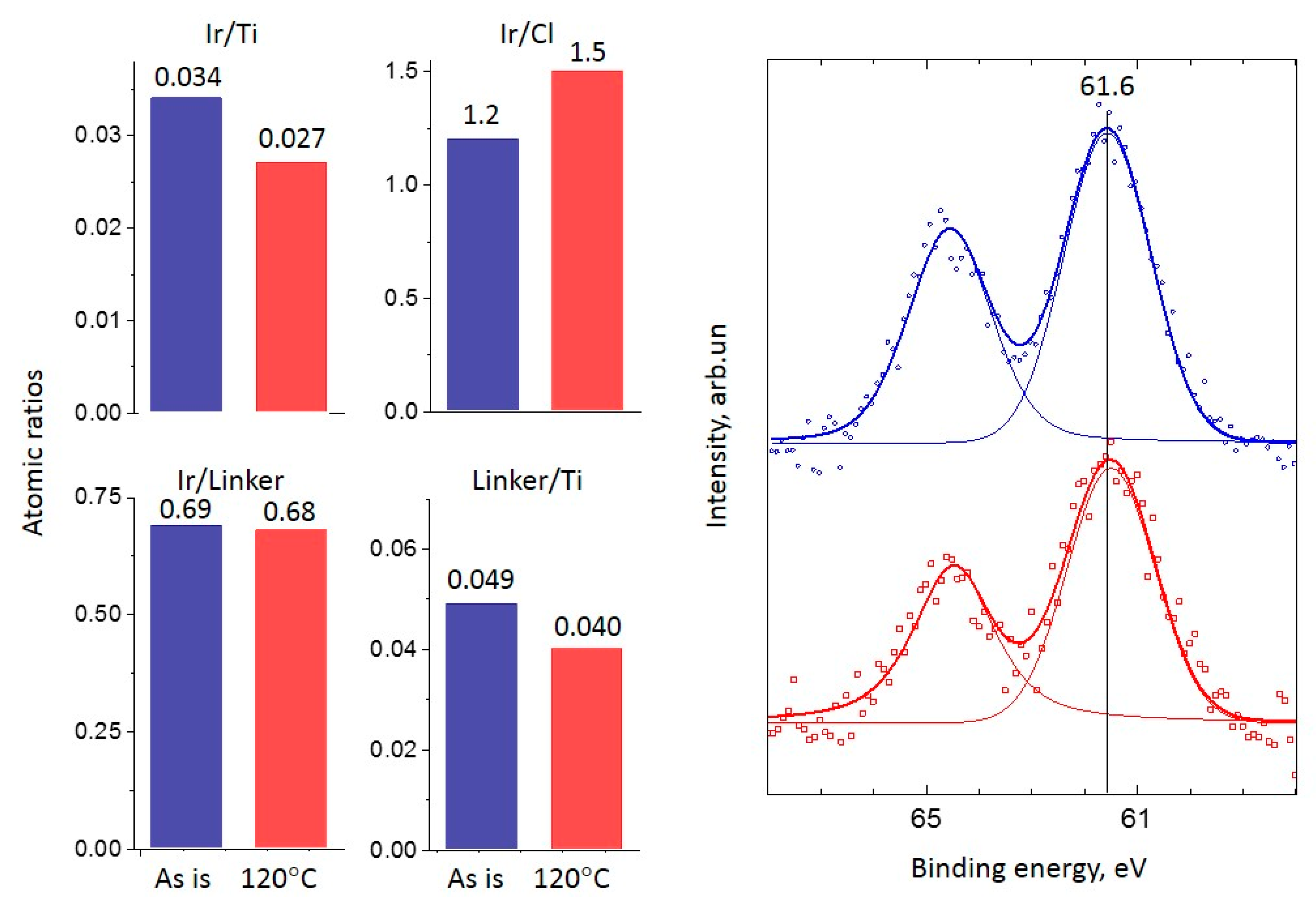
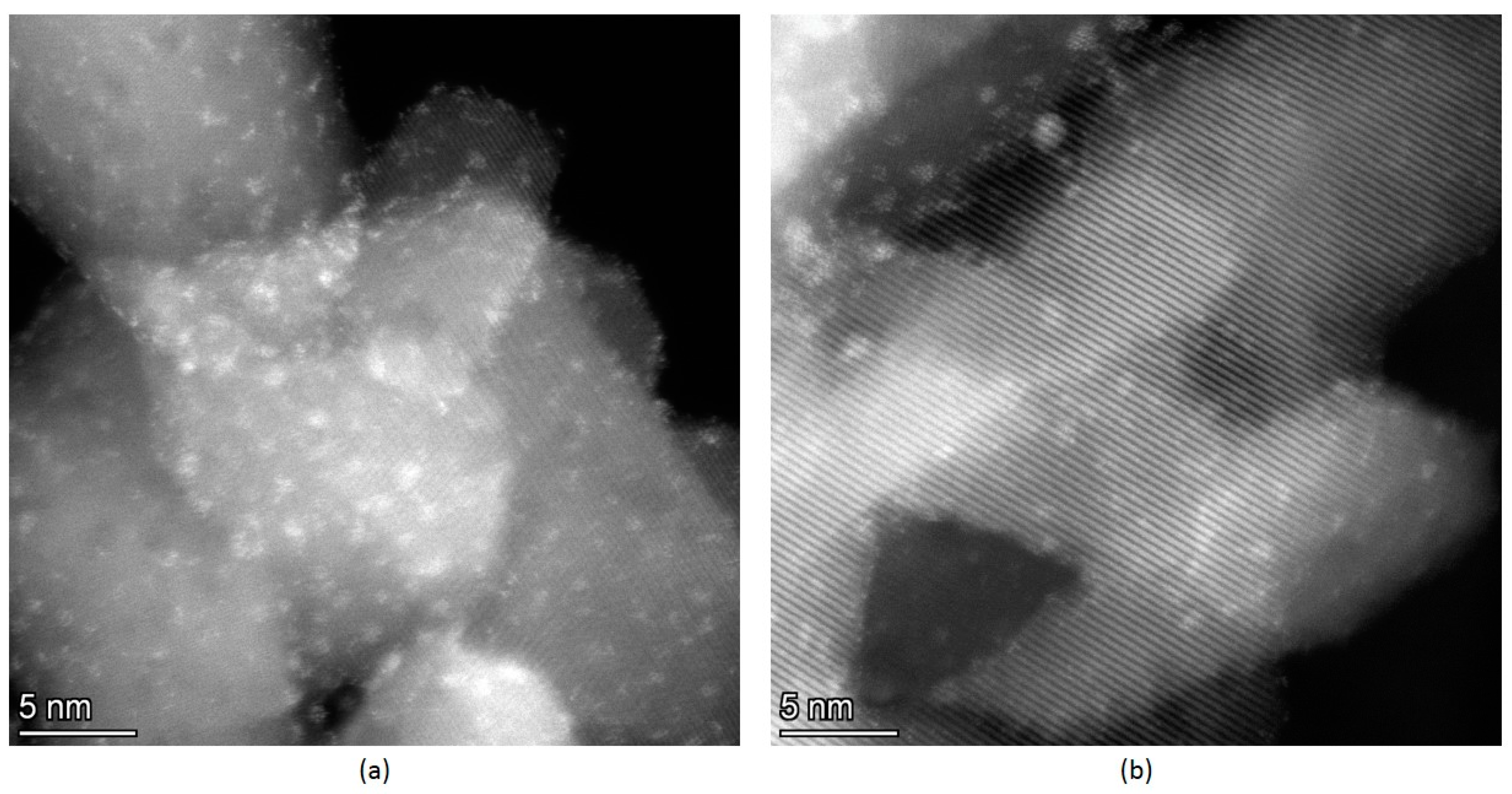
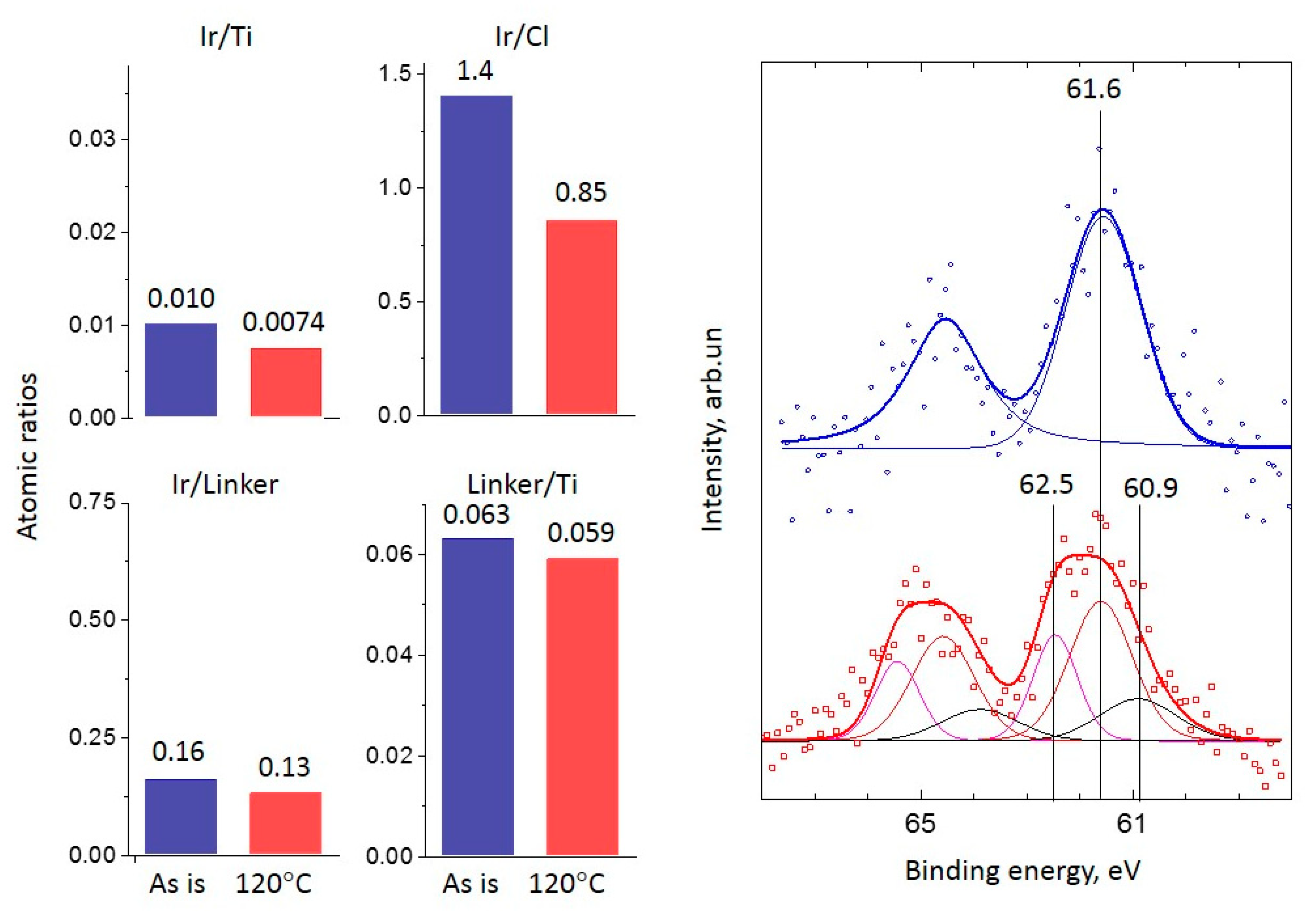
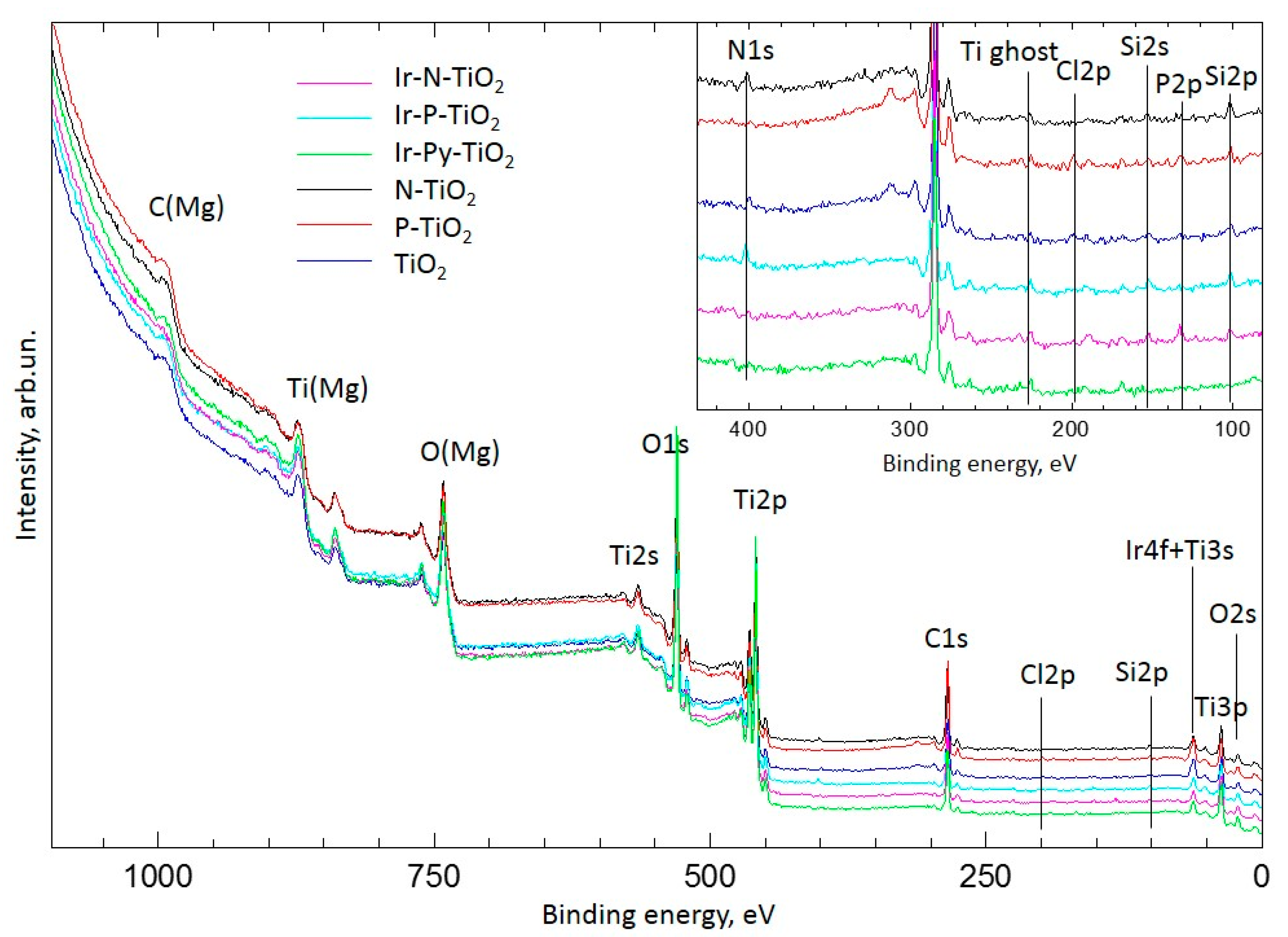
2.2. XPS Study
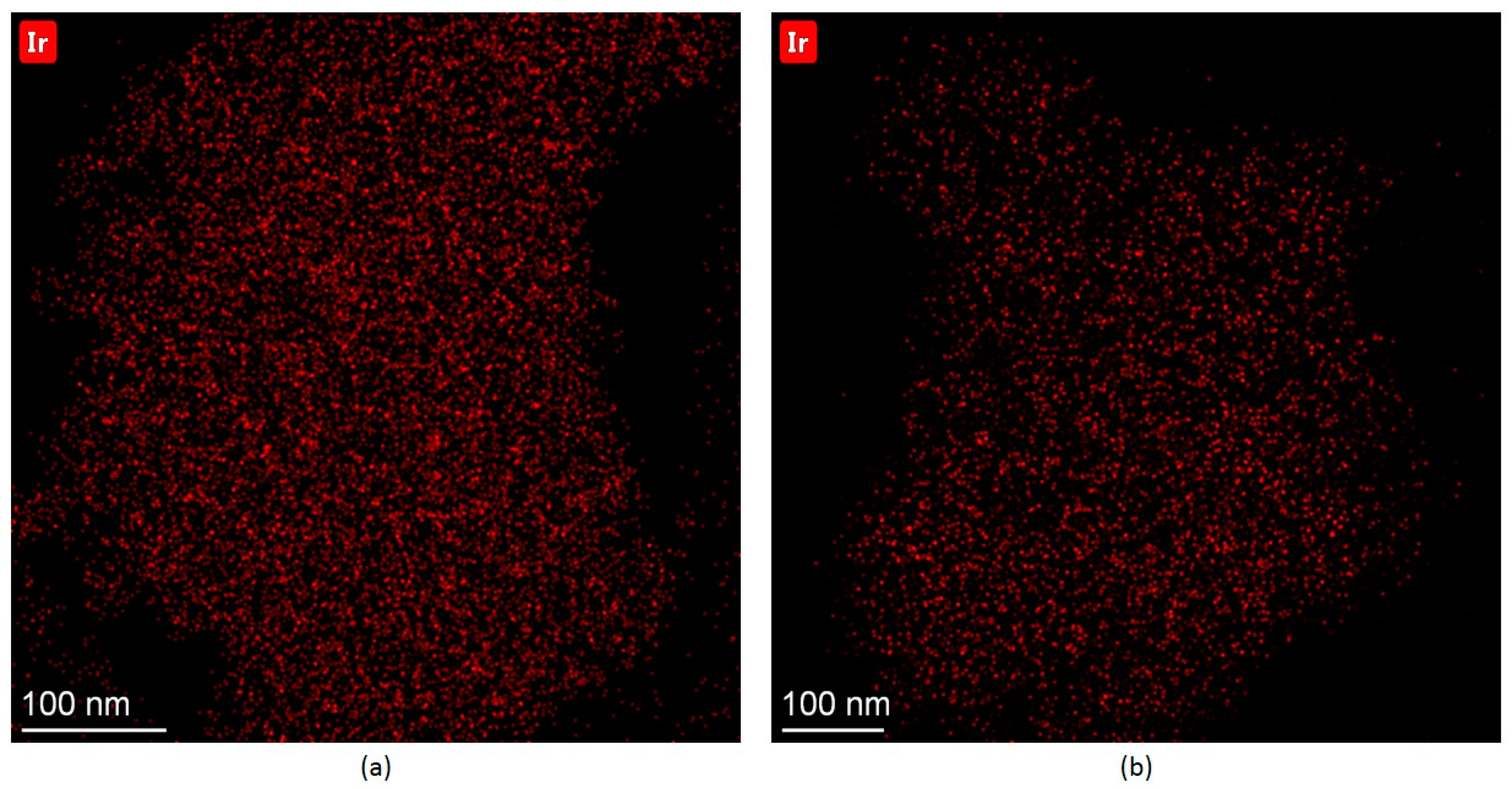
2.2.1. Ir-Py-TiO2
2.2.2. Ir-P-TiO2
2.2.3. Ir-N-TiO2
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motokura, K.; Ding, S.; Usui, K.; Kong, Y. Enhanced Catalysis Based on the Surface Environment of the Silica-Supported Metal Complex. ACS Catal. 2021, 11, 11985–12018. [Google Scholar] [CrossRef]
- Skovpin, I.V.; Sviyazov, S.V.; Burueva, D.B.; Kovtunova, L.M.; Nartova, A.V.; Kvon, R.I.; Bukhtiyarov, V.I.; Koptyug, I.V. Nonequilibrium nuclear spin states of ethylene during acetylene hydrogenetion with parahydrogen over immobilized iridium complexes. Dokl. Phys. Chem. 2023; In press. [Google Scholar]
- Kovtunov, K.V.; Salnikov, O.G.; Skovpin, I.V.; Chukanov, N.V.; Burueva, D.B.; Koptyug, I.V. Catalytic hydrogenation with parahydrogen: A bridge from homogeneous to heterogeneous catalysis. Pure Appl. Chem. 2020, 92, 1029–1046. [Google Scholar] [CrossRef]
- Skovpin, I.V.; Kovtunova, L.M.; Nartova, A.V.; Kvon, R.I.; Bukhtiyarov, V.I.; Koptyug, I.V. Anchored complexes of rhodium and iridium in the hydrogenation of alkynes and olefins with parahydrogen. Catal. Sci. Technol. 2022, 12, 3247–3253. [Google Scholar] [CrossRef]
- Lazaro, G.; Iglesias, M.; Femandez-Alvarez, F.J.; Sanz Miguel, P.J.; Perez-Torrente, J.J.; Oro, L.A. Synthesis of Poly(silyl ether)s by Rhodium(I)–NHC Catalyzed Hydrosilylation: Homogeneous versus Heterogeneous Catalysis. ChemCatChem 2013, 5, 1133–1141. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Li, M.; Han, J.; Liu, X.; Gong, J. Molecular heterogeneous catalysts derived from bipyridine-based organosilica nanotubes for C–H bond activation. Chem. Sci. 2017, 8, 4489–4496. [Google Scholar] [CrossRef]
- Esfandiari, M.; Havaei, G.; Zahiri, S.; Mohammadnezhad, G. Pincer complex immobilization onto different supports: Strategies and applications. Coord. Chem. Rev. 2022, 472, 214778. [Google Scholar] [CrossRef]
- Gutmann, T.; Ratajczyk, T.; Xu, Y.; Breitzke, H.; Grunberg, A.; Dillenberger, S.; Bommerich, U.; Trantzschel, T.; Bernarding, J.; Buntkowsky, G. Understanding the leaching properties of heterogenized catalysts: A combined solid-state and PHIP NMR study. Solid State Nucl. Magn. Reson. 2010, 38, 90–96. [Google Scholar] [CrossRef]
- Buljubasich, L.; Franzoni, M.B.; Münnemann, K. Parahydrogen Induced polarization by homogeneous catalysis: Theory and applications. Top. Curr. Chem. 2013, 338, 33–74. [Google Scholar] [CrossRef]
- Duckett, S.B.; Mewis, R.E. Application of Parahydrogen Induced Polarization Techniques in NMR Spectroscopy and Imaging. Acc. Chem. Res. 2012, 45, 1247–1257. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar] [CrossRef]
- Kirschning, A. (Ed.) Immobilized Catalysts; Springer: Berlin/Heidelberg, Germany, 2004; Volume 242. [Google Scholar]
- Can, L.; Yan, L. (Eds.) Bridging Heterogeneous and Homogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Conley, M.P.; Drost, R.M.; Baffert, M.; Gajan, D.; Elsevier, C.; Franks, W.T.; Oschkinat, H.; Veyre, L.; Zagdoun, A.; Rossini, A.; et al. A well-defined Pd hybrid material for the Z-selective semihydrogenation of alkynes characterized at the molecular level by DNP SENS. Chem. Eur. J. 2013, 19, 12234–12238. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis. Adv. Synth. Catal. 2006, 348, 1391–1412. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Morris, G.E. Some diolefin complexes of iridium(I) and a trans-influence series for the complexes [IrCl(cod)L]. J. Organomet. Chem. 1977, 135, 395–403. [Google Scholar] [CrossRef]
- Merckle, C.; Blumel, J. Improved rhodium hydrogenation catalysts immobilized on silica. Top. Catal. 2005, 34, 5–15. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Wang, Q.; Frenkel, A.I.; Finke, R.G. Development Plus Kinetic and Mechanistic Studies of a Prototype Supported-Nanoparticle Heterogeneous Catalyst Formation System in Contact with Solution: Ir(1,5-COD)Cl/γ-Al2O3 and Its Reduction by H2 to Ir(0)n/γ-Al2O3. J. Am. Chem. Soc. 2010, 132, 9701–9714. [Google Scholar] [CrossRef]
- Queffélec, C.; Schlindwein, S.H.; Gudat, D.; Silvestre, V.; Rodriguez-Zubiri, M.; Fayon, F.; Bujoli, B.; Wang, Q.; Boukherroub, R.; Szunerits, S. Wilkinson-type immobilized catalyst on diamond nanoparticles for alkene reduction. ChemCatChem 2017, 9, 432–439. [Google Scholar] [CrossRef]
- Quignard, F.; Choplin, A. Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2003; pp. 445–470. [Google Scholar]
- Price, P.M.; Clark, J.H.; Macquarrie, D.J. Modified silicas for clean technology. J. Chem. Soc. Dalton Trans. 2000, 101–110. [Google Scholar] [CrossRef]
- Copéret, C.; Comas-Vives, A.; Conley, M.P.; Estes, D.P.; Fedorov, A.; Mougel, V.; Nagae, H.; Núñez-Zarur, F.; Zhizhko, P.A. Surface Organometallic and Coordination Chemistry toward Single-Site Heterogeneous Catalysts: Strategies, Methods, Structures, and Activities. Chem. Rev. 2016, 116, 323–421. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stckle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992; pp. 1–296. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons Inc.: Chichester, UK, 1983. [Google Scholar]
- Fernando, N.K.; Cairns, A.B.; Murray, C.A.; Thompson, A.L.; Dickerson, J.L.; Garman, E.F.; Ahmed, N.; Ratcliff, L.E.; Regoutz, A. Structural and electronic effects of X-ray irradiation on prototypical [M(COD)Cl]2 catalysts. J. Phys. Chem. A 2021, 125, 7473–7488. [Google Scholar] [CrossRef]
- Kvon, R.I.; Nartova, A.V.; Kovtunova, L.M.; Bukhtiyarov, V.I. Comparative XPS Study of the Composition and Electronic State of Iridium in Bulk and Immobilized Binuclear [Ir(COD)Cl]2 Complexes. J. Struct. Chem. 2023, 64, 270–275. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-ray photoelectron spectra of Ir, IrO2 and IrCl3 revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, T.; Ren, P.; Cao, D.; Lin, Y.; Wang, L.; Zhang, B.; Xiang, X. Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics. Catalysts 2020, 10, 69. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Lee, C.-Y.; Chiu, H.-T. Enhanced Photocatalysis from Truncated Octahedral Bipyramids of Anatase TiO2 with Exposed {001}/{101} Facets. ACS Omega 2018, 3, 10225–10232. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F.; Beck, W.; Bartunik, H.D. X-ray photoelectron and Mössbauer spectroscopy of triphenylphosphine–iridium complexes. J. Chem. Soc. Dalton Trans. 1973, 17, 1828–1829. [Google Scholar] [CrossRef]
- Cotton, F.A.; Lahuerta, P.; Sanau, M.; Schwotzer, W. Air oxidation of Ir2(Cl)2(COD)2 revisited. The structures of [Ir(μ2-Cl)(COD)]2(ruby form) and its oxidation product, Ir2Cl2(COD)2(μ2-OH)2(μ2-O). Inorganica Chimica Acta 1986, 120, 153–157. [Google Scholar] [CrossRef]
- Heroguel, F.; Gebert, D.; Detwiler, M.D.; Zemlyanov, D.Y.; Baudouin, D.; Coperet, C. Dense and narrowly distributed silica-supported rhodium and iridium nanoparticles: Preparation via surface organometallic chemistry and chemisorption stoichiometry. J. Catal. 2014, 316, 260–269. [Google Scholar] [CrossRef]
- Nartova, A.V.; Matveev, A.V.; Mashukov, M.Y.; Belotserkovskii, V.A.; Sankova, N.N.; Kudinov, V.Y.; Okunev, A.G. iOk Platform for Automatic Search and Analysis of Objects in Images Using Artificial Intelligence in the Study of Supported Catalysts. Kinet. Catal. 2023, 64, 458–465. [Google Scholar] [CrossRef]

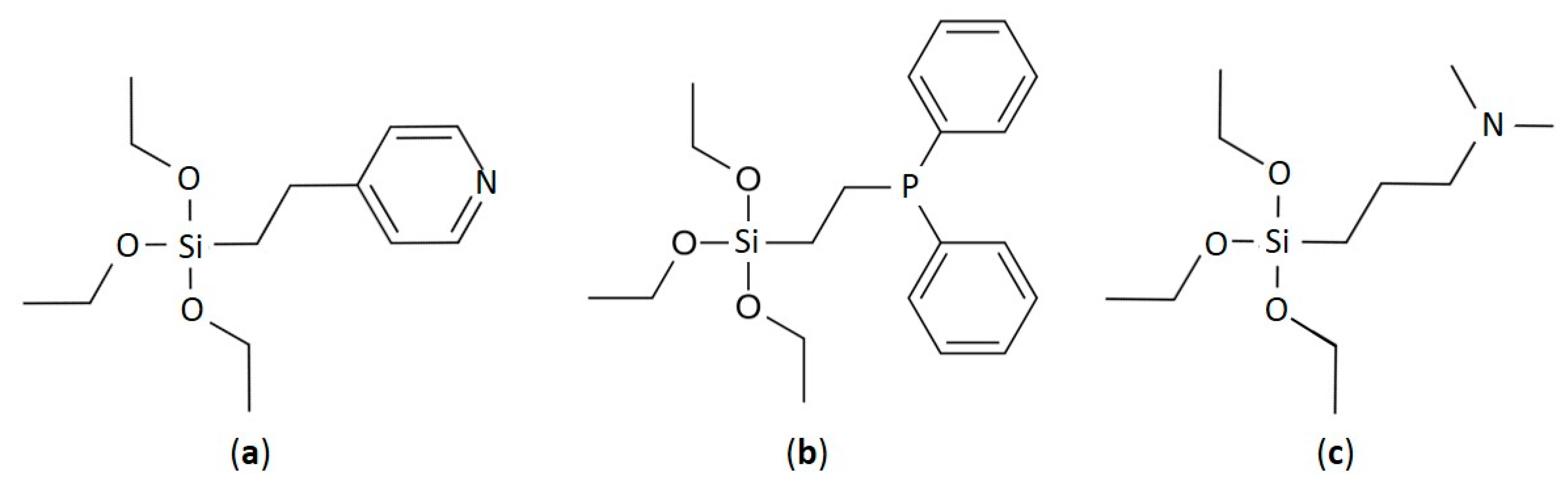
| Sample | Linker | Complex Compound |
|---|---|---|
| Ir-Py-TiO2 | C2H4Py | [Ir(COD)Cl]2 |
| Ir-P-TiO2 | C2H4P(Ph)2 | [Ir(COD)Cl]2 |
| Ir-N-TiO2 | C3H6N(CH3)2 | [Ir(COD)Cl]2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartova, A.V.; Kvon, R.I.; Kovtunova, L.M.; Skovpin, I.V.; Koptyug, I.V.; Bukhtiyarov, V.I. XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation. Int. J. Mol. Sci. 2023, 24, 15643. https://doi.org/10.3390/ijms242115643
Nartova AV, Kvon RI, Kovtunova LM, Skovpin IV, Koptyug IV, Bukhtiyarov VI. XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation. International Journal of Molecular Sciences. 2023; 24(21):15643. https://doi.org/10.3390/ijms242115643
Chicago/Turabian StyleNartova, Anna V., Ren I. Kvon, Larisa M. Kovtunova, Ivan V. Skovpin, Igor V. Koptyug, and Valerii I. Bukhtiyarov. 2023. "XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation" International Journal of Molecular Sciences 24, no. 21: 15643. https://doi.org/10.3390/ijms242115643
APA StyleNartova, A. V., Kvon, R. I., Kovtunova, L. M., Skovpin, I. V., Koptyug, I. V., & Bukhtiyarov, V. I. (2023). XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation. International Journal of Molecular Sciences, 24(21), 15643. https://doi.org/10.3390/ijms242115643






