Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore
Abstract
:1. Introduction
2. Results
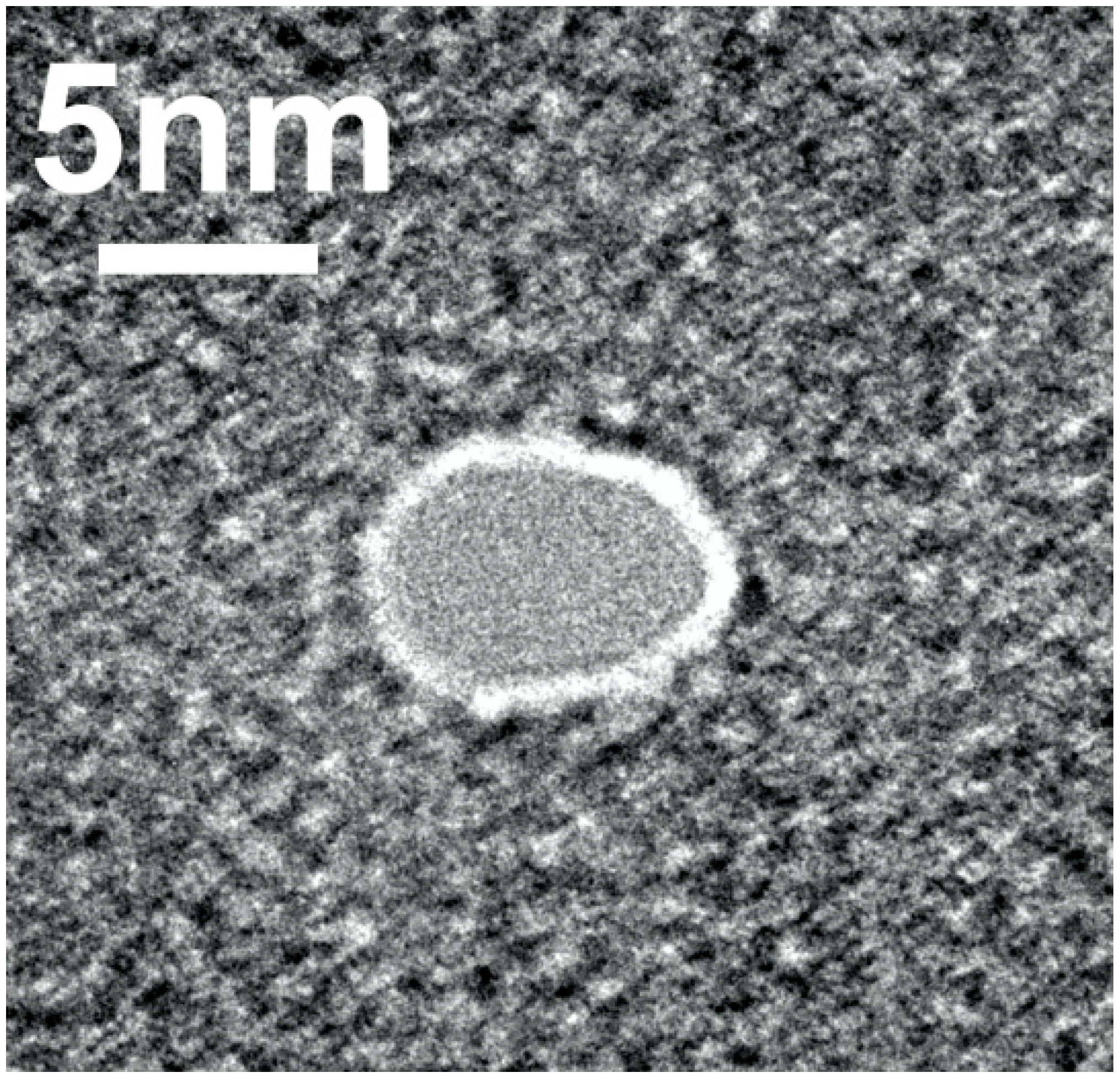
2.1. Nanopore Fabrication
2.2. Testing of Nanopore Performance
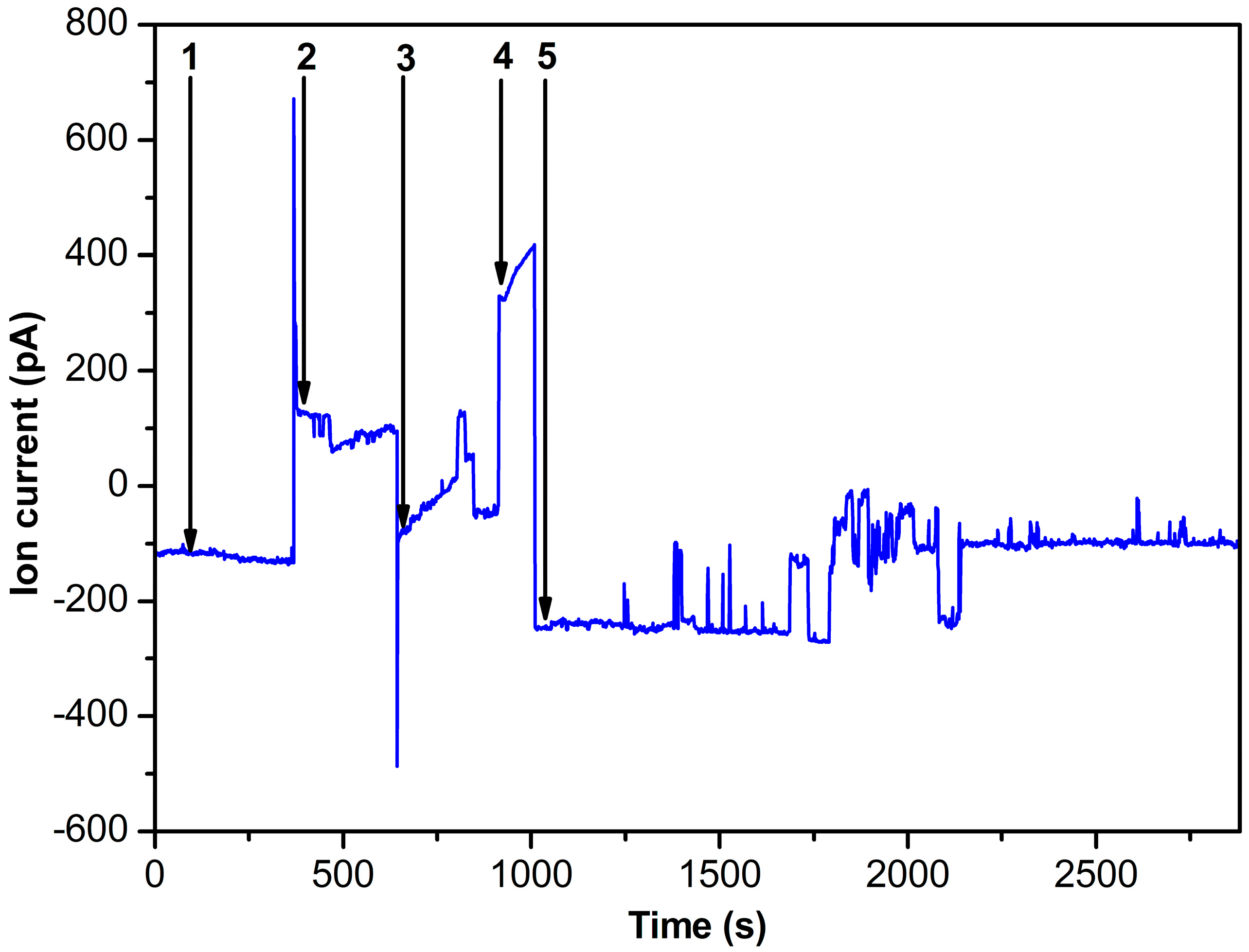
2.3. Nanopore Experiments
3. Discussion
4. Materials and Methods
4.1. Chemicals and Enzyme
4.2. Nanopore Fabrication and Characterization
4.3. Nanopore-Based Detector
4.4. Electrical Measurements
4.5. Spectrophotometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radmacher, M.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Direct observation of enzyme activity with the atomic force microscope. Science 1994, 265, 1577–1579. [Google Scholar] [CrossRef]
- Thomson, N.H.; Fritz, M.; Radmacher, M.; Cleveland, J.P.; Schmidt, C.F.; Hansma, P.K. Protein tracking and detection of protein motion using atomic force microscopy. Biophys. J. 1996, 70, 2421–2431. [Google Scholar] [CrossRef]
- Arnoldi, M.; Schäffer, T.E.; Fritz, M.; Radmacher, M. Direct observation of single catalytic events of chitosanase by atomic force microscopy. J. AZoNano—Online J. Nanotechnol. 2005, 1, 1–11. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Pleshakova, T.O.; Frantsuzov, P.A.; Krokhin, N.V.; Ziborov, V.S.; Archakov, A.I. Atomic force microscopy visualization and measurement of the activity and physicochemical properties of single monomeric and oligomeric enzymes. Biophysics 2011, 56, 892–896. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, S.; Liu, L.; Wu, H.-C. Measuring enzymatic activities with nanopores. ChemBioChem 2020, 21, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, Y.; Long, Y.-T.; Minteer, S.D.; Ying, Y.-L. Nanopore-based measurement of the interaction of P450cam monooxygenase and putidaredoxin at the single-molecule level. Faraday Discuss. 2022, 233, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Willems, K.; van Meervelt, V.; Wloka, C.; Maglia, G. Single-molecule nanopore enzymology. Philos. Trans. R. Soc. B 2017, 372, 20160230. [Google Scholar] [CrossRef] [PubMed]
- Wloka, C.; van Meervelt, V.; van Gelder, D.; Danda, N.; Jager, N.; Williams, C.P.; Maglia, G. Label-free and real-time detection of protein ubiquitination with a biological nanopore. ACS Nano 2017, 11, 4387–4394. [Google Scholar] [CrossRef]
- Pham, B.; Eron, S.J.; Hill, M.E.; Li, X.; Fahie, M.A.; Hardy, J.A.; Chen, M. A nanopore approach for analysis of caspase-7 activity in cell lysates. Biophys. J. 2019, 117, 844–855. [Google Scholar] [CrossRef]
- Fahie, M.A.; Pham, B.; Li, F.; Chen, M. A selective activity-based approach for analysis of enzymes with an OmpG nanopore. Methods Mol. Biol. 2021, 2186, 115–133. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Ivanov, Y.D.; Archakov, A.I. Atomic force microscopy revelation of molecular complexes in the multiprotein cytochrome P450 2B4-containing system. Proteomics 2004, 4, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebova, L.; Trinick, J. Titin: Properties and family relationships. Nat. Rev. Mol. Cell Biol. 2003, 4, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Berglund, G.I.; Carlsson, G.H.; Smith, A.T.; Szöke, H.; Henriksen, A.; Hajdu, J. The catalytic pathway of horseradish peroxidase at high resolution. Nature 2002, 417, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.I.; Bachmanova, G.I. Cytochrome P450 and Active Oxygen; Taylor & Francis: New York, NY, USA; Philadelphia, PA, USA, 1990; p. 275. [Google Scholar]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef]
- Kwok, H.; Briggs, K.; Tabard-Cossa, V. Nanopore fabrication by controlled dielectric breakdown. PLoS ONE 2014, 9, e92880. [Google Scholar] [CrossRef]
- Waugh, M.; Briggs, K.; Gunn, D.; Gibeault, M.; King, S.; Ingram, Q.; Jimenez, A.M.; Berryman, S.; Lomovtsev, D.; Andrzejewski, L.; et al. Solid-state nanopore fabrication by automated controlled breakdown. Nat. Protoc. 2020, 15, 122–143. [Google Scholar] [CrossRef]
- Nam, S.W.; Rooks, M.J.; Kim, K.-B.; Rossnagel, S.M. Ionic field effect transistors with sub-10 nm multiple nanopores. Nano Lett. 2009, 9, 2044–2048. [Google Scholar] [CrossRef]
- Metzler, D.E. Biochemistry: The Chemical Reactions of Living Cells; Academic Press: Oxford, UK, 1970. [Google Scholar]
- Rogozhin, V.V.; Kutuzova, G.D.; Ugarova, N.N. Inhibition of horseradish peroxidase by N-ethylamide of o-sulfobenzoylacetic acid. Russ. J. Bioorg. Chem. 2000, 26, 156–160. [Google Scholar] [CrossRef]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49, 69–86. [Google Scholar] [CrossRef]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- He, Y.; Tsutsui, M.; Zhou, Y.; Miao, X.-S. Solid-state nanopore systems: From materials to applications. NPG Asia Mater. 2021, 13, 48. [Google Scholar] [CrossRef]
- Esfandiar, A.; Radha, B.; Wang, F.C.; Yang, Q.; Hu, S.; Garaj, S.; Nair, R.R.; Geim, A.K.; Gopinadhan, K. Size effect in ion transport through angstrom-scale slits. Science 2017, 358, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Yazda, K.; Tahir, S.; Michel, T.; Loubet, B.; Manghi, M.; Bentin, J.; Picaud, F.; Palmeri, J.; Henn, F.; Jourdain, V. Voltage-activated transport of ions through single-walled carbon nanotubes. Nanoscale 2017, 9, 11976–11986. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, D.; Malyshev, G.; Ryzhkov, I.; Mozharov, A.; Shugurov, K.; Sharov, V.; Panov, M.; Tumkin, I.; Afonicheva, P.; Evstrapov, A.; et al. Focused ion beam milling based formation of nanochannels in silicon-glass microfluidic chips for the study of ion transport. Microfluid. Nanofluid. 2021, 25, 51. [Google Scholar] [CrossRef]
- Kowalczyk, S.W.; Grosberg, A.Y.; Rabin, Y.; Dekker, C. Modeling the conductance and DNA blockade of solid-state nanopores. Nanotechnology 2011, 22, 315101. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7) 2,20-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) as a Substrate Sigma Prod. No. P-6782. Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzymeactivity-assays/enzymatic-assay-of-peroxidase-abts-as-substrate (accessed on 18 February 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Ableev, A.N.; Shumov, I.D.; Ivanova, I.A.; Vaulin, N.V.; Lebedev, D.V.; Bukatin, A.S.; Mukhin, I.S.; Archakov, A.I. Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore. Int. J. Mol. Sci. 2023, 24, 15636. https://doi.org/10.3390/ijms242115636
Ivanov YD, Ableev AN, Shumov ID, Ivanova IA, Vaulin NV, Lebedev DV, Bukatin AS, Mukhin IS, Archakov AI. Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore. International Journal of Molecular Sciences. 2023; 24(21):15636. https://doi.org/10.3390/ijms242115636
Chicago/Turabian StyleIvanov, Yuri D., Alexander N. Ableev, Ivan D. Shumov, Irina A. Ivanova, Nikita V. Vaulin, Denis V. Lebedev, Anton S. Bukatin, Ivan S. Mukhin, and Alexander I. Archakov. 2023. "Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore" International Journal of Molecular Sciences 24, no. 21: 15636. https://doi.org/10.3390/ijms242115636
APA StyleIvanov, Y. D., Ableev, A. N., Shumov, I. D., Ivanova, I. A., Vaulin, N. V., Lebedev, D. V., Bukatin, A. S., Mukhin, I. S., & Archakov, A. I. (2023). Registration of Functioning of a Single Horseradish Peroxidase Macromolecule with a Solid-State Nanopore. International Journal of Molecular Sciences, 24(21), 15636. https://doi.org/10.3390/ijms242115636





