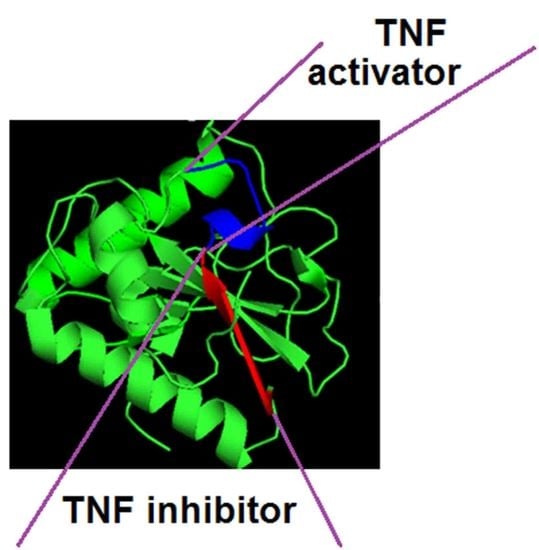
Short Peptides of Innate Immunity Protein Tag7 (PGLYRP1) Selectively Induce Inhibition or Activation of Tumor Cell Death via TNF Receptor
Abstract
1. Introduction
2. Results
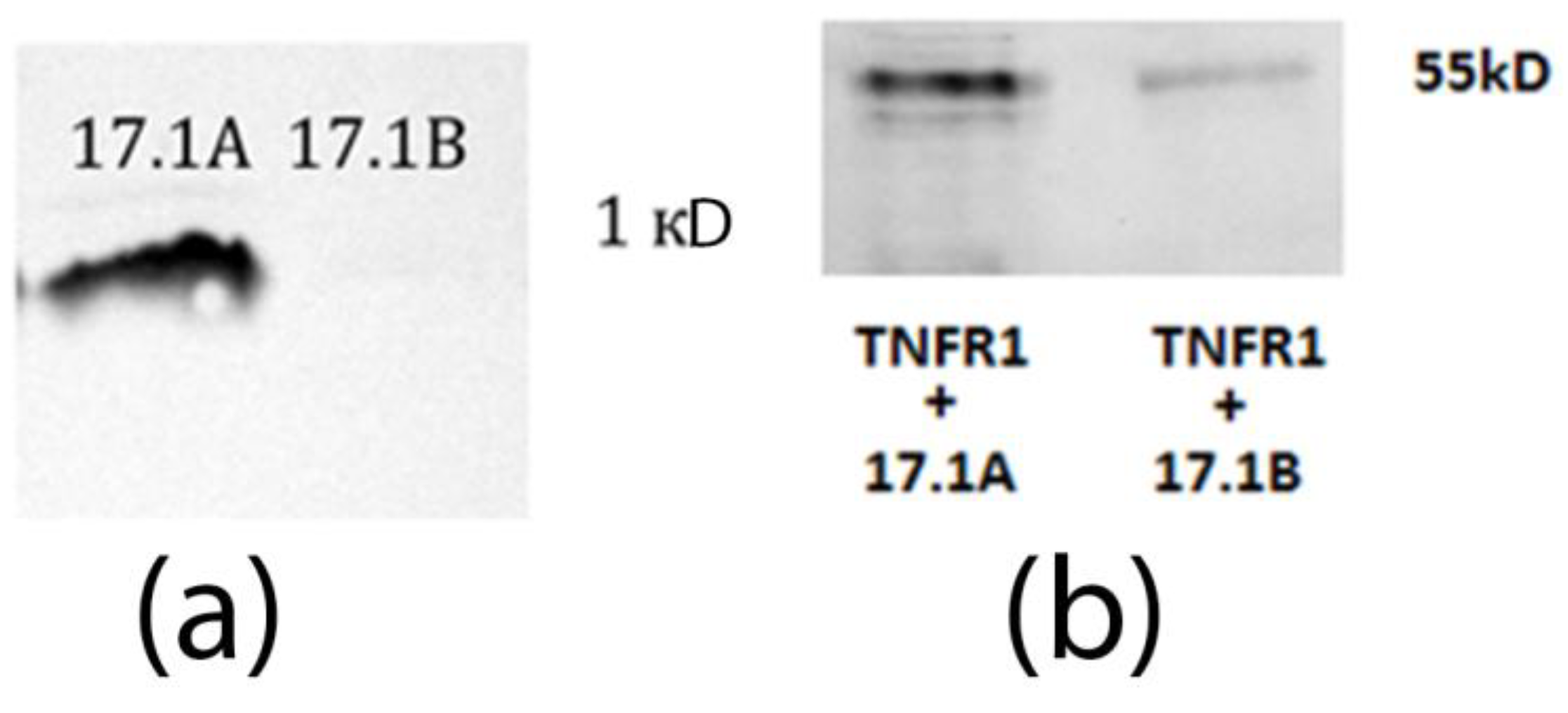
2.1. Peptides 17.1A and 17.1B Bind to TNFR1 on the Cell Surface
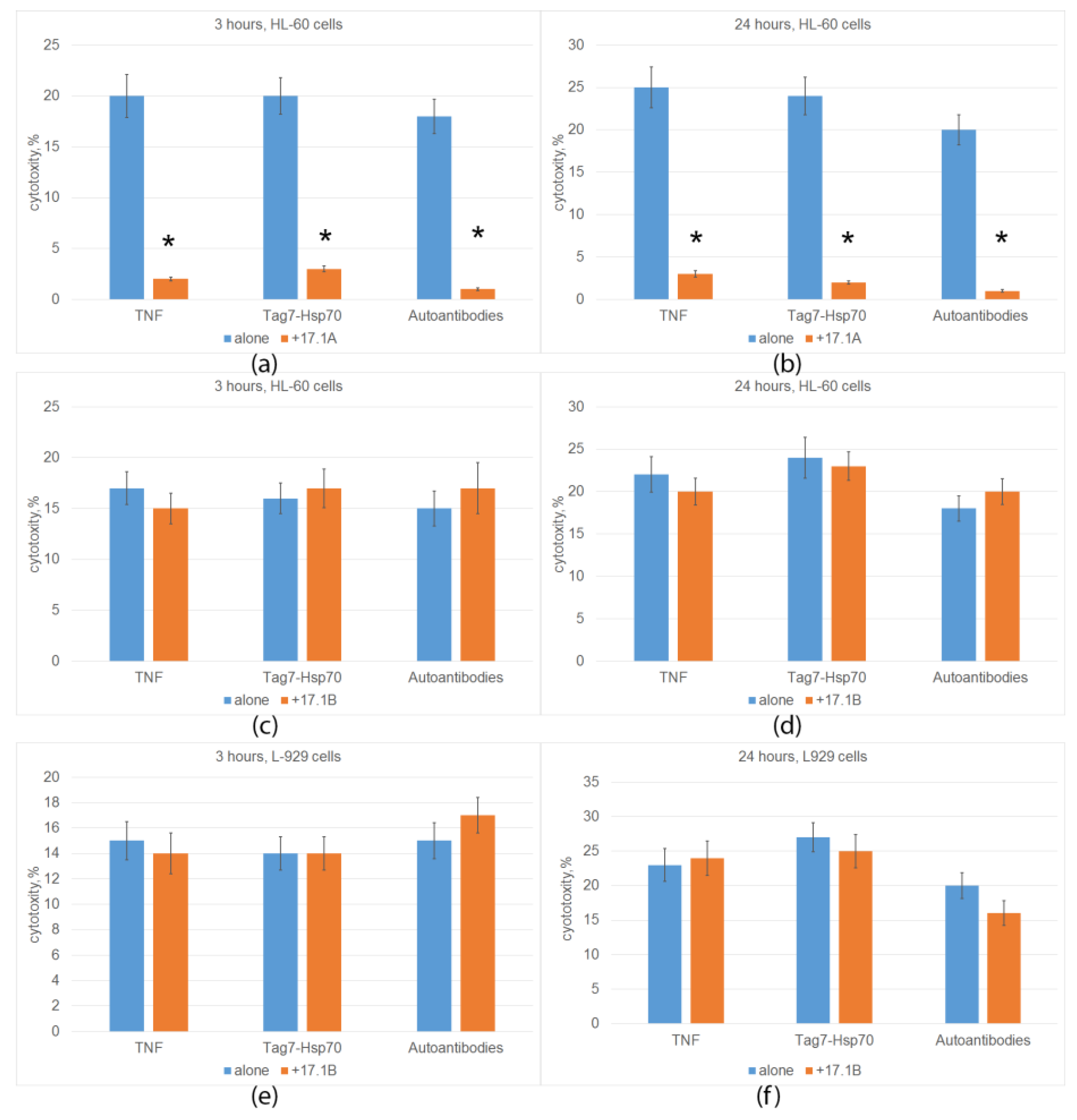
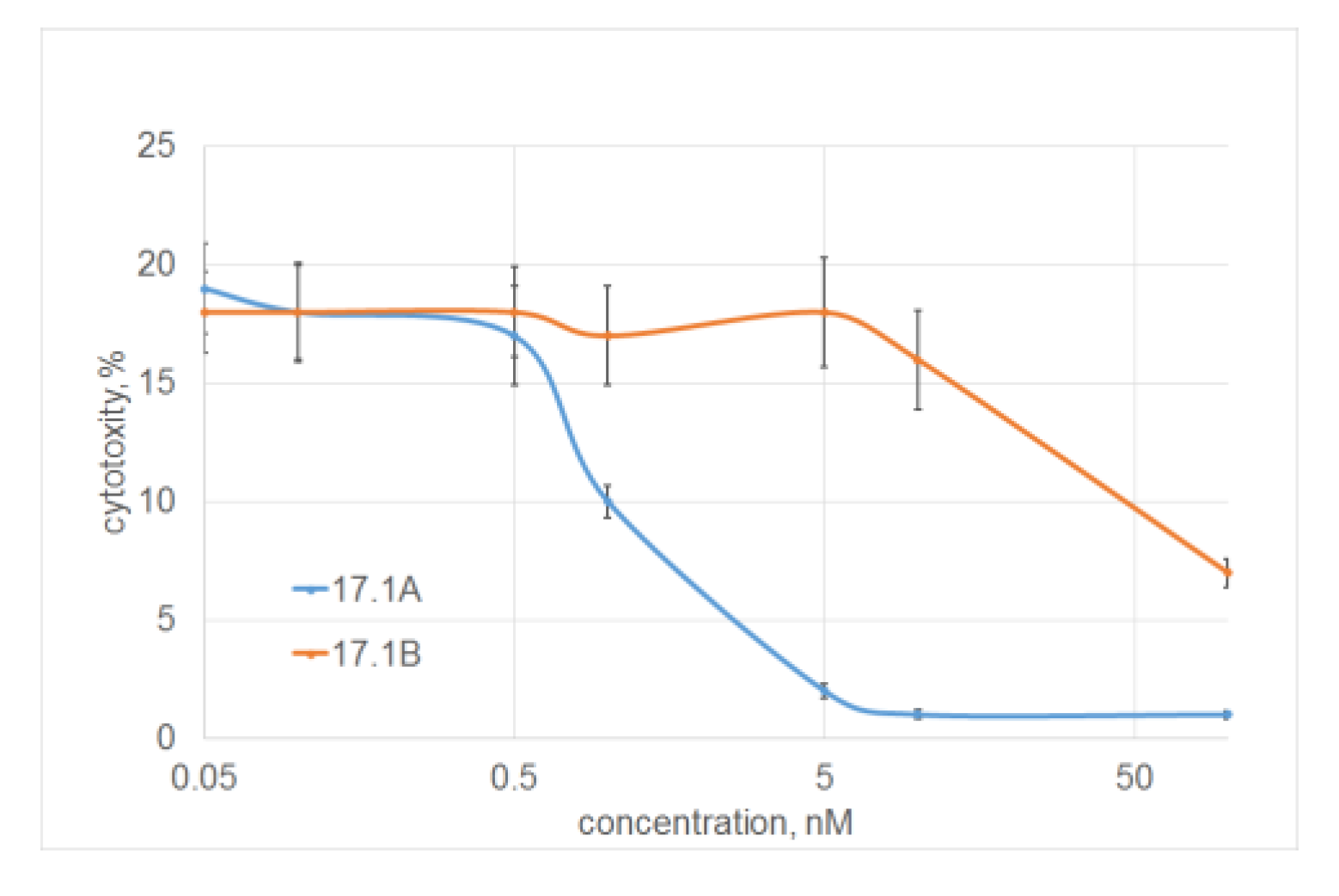
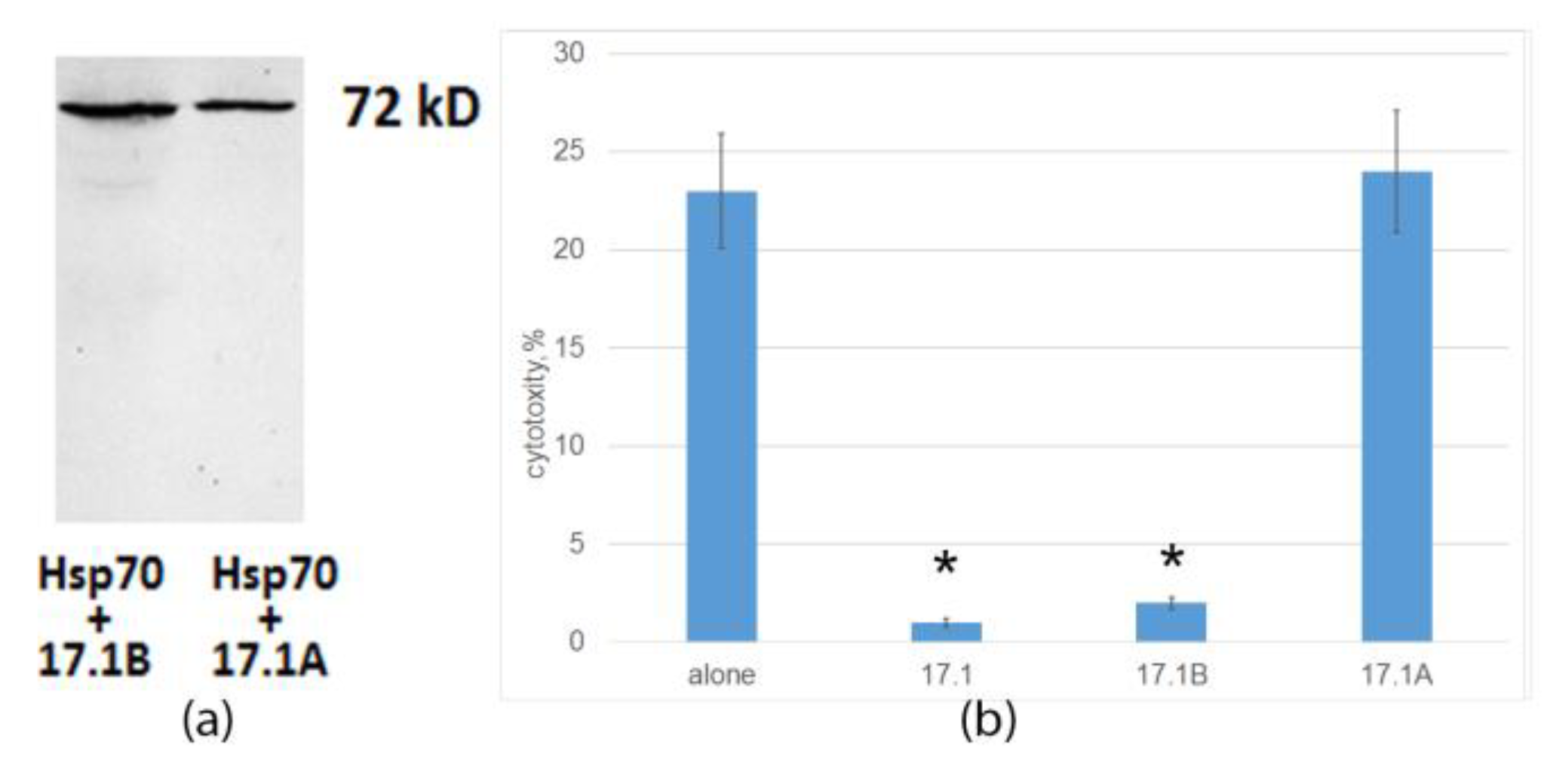
2.2. Peptide 17.1A Inhibits TNFR1-Dependent Cytotoxic Activity
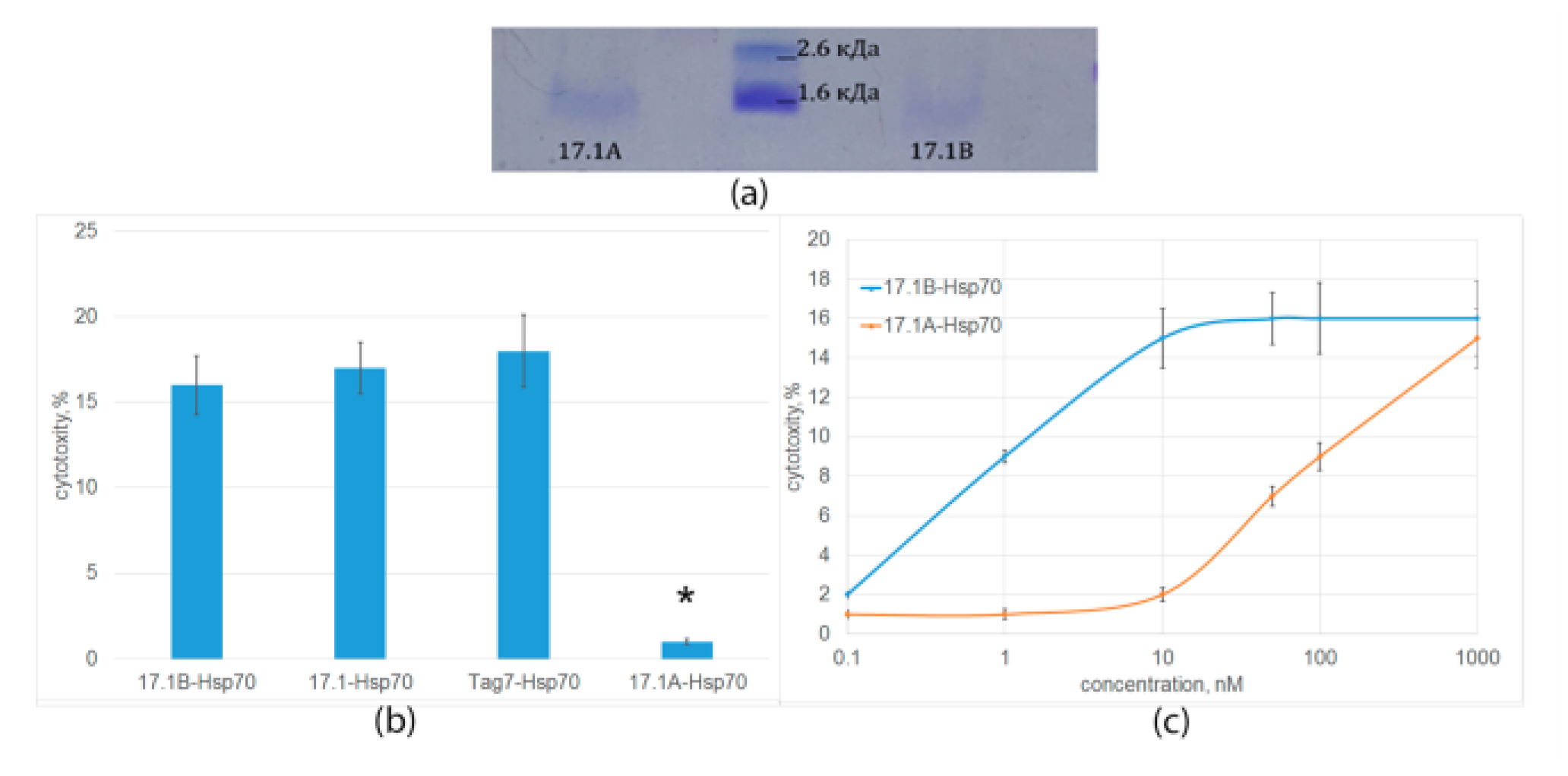
2.3. Peptide 17.1B Induces Cytotoxicity in Complex with Hsp 70
2.4. Peptide 17.1B Inhibits the Cytotoxic Activity of CD4+-T Lymphocytes
2.5. Determination of the Binding Constants of Peptides 17.1, 17.1A and 17.1B with TNFR1 and Hsp70
3. Discussion
4. Materials and Methods
4.1. Cell Cultivation and Sorting
4.2. Proteins and Antibodies
4.3. Peptides
4.4. Affinity Chromatography, Immunoadsorption and Immunoblotting
4.5. Cytotoxicity Assays
4.6. Microscale Thermophoresis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pegoretti, V.; Baron, W.; Laman, J.D.; Eisel, U.L.M. Selective Modulation of TNF-TNFRs Signaling: Insights for Multiple Sclerosis Treatment. Front. Immunol. 2018, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Apostolaki, M.; Armaka, M.; Victoratos, P.; Kollias, G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr. Dir. Autoimmun. 2010, 11, 1–26. [Google Scholar] [CrossRef]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem Cell 2021, 28, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, H.; Yu, M.; Yang, P.; Zhang, M.; Ba, H.; Yin, Y.; Wang, J.; Yin, B.; Zhou, X.; et al. Inhibition of transmembrane TNF-α shedding by a specific antibody protects against septic shock. Cell Death Dis. 2019, 10, 586. [Google Scholar] [CrossRef]
- Alshevskaya, A.; Lopatnikova, J.; Zhukova, J.; Chumasova, O.; Shkaruba, N.; Sizikov, A.; Evsegneeva, I.; Demina, D.; Nepomniashchikch, V.; Karaulov, A.; et al. The Influence of Severity and Disease Duration on TNF Receptors’ Redistribution in Asthma and Rheumatoid Arthritis. Cells 2022, 12, 5. [Google Scholar] [CrossRef]
- Ghamari, E.; Farnia, P.; Saif, S.; Marashian, M.; Ghanavi, J.; Tabarsi, P.; Farnia, P.; Velayati, A.A. Susceptibility to pulmonary tuberculosis: Host genetic deficiency in tumor necrosis factor alpha (TNF-α) gene and tumor necrosis factor receptor 2 (TNFR2). Int. J. Mycobacteriol. 2016, 5 (Suppl. S1), S136–S137. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front. Cell. Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Romanova, E.A.; Soshnikova, N.V.; Belogurov, A.A., Jr.; Lomakin, Y.A.; Sashchenko, L.P.; Yashin, D.V. Autoantibodies from SLE patients induce programmed cell death in murine fibroblast cells through interaction with TNFR1 receptor. Sci. Rep. 2020, 10, 11144. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Dukhanina, E.A.; Shatalov, Y.V.; Yashin, D.V.; Lukyanova, T.I.; Kabanova, O.D.; Romanova, E.A.; Khaidukov, S.V.; Galkin, A.V.; Gnuchev, N.V.; et al. Cytotoxic T lymphocytes carrying a pattern recognition protein Tag7 can detect evasive, HLA-negative but Hsp70-exposing tumor cells, thereby ensuring FasL/Fas-mediated contact killing. Blood 2007, 110, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Poznansky, S.A.; Yu, M.; Deng, K.; Fu, Q.; Markmann, J.F.; LeGuern, C. Leveraging the tolerogenic potential of TNF-α and regulatory B cells in organ transplantation. Front. Immunol. 2023, 14, 1173672. [Google Scholar] [CrossRef]
- Romanova, E.A.; Sharapova, T.N.; Telegin, G.B.; Minakov, A.N.; Chernov, A.S.; Ivanova, O.K.; Bychkov, M.L.; Sashchenko, L.P.; Yashin, D.V. A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death. Cells 2020, 9, 488. [Google Scholar] [CrossRef]
- Telegin, G.B.; Chernov, A.S.; Kazakov, V.A.; Romanova, E.A.; Sharapova, T.N.; Yashin, D.V.; Gabibov, A.G.; Sashchenko, L.P. A 8-mer Peptide of PGLYRP1/Tag7 Innate Immunity Protein Binds to TNFR1 Receptor and Inhibits TNFα-Induced Cytotoxic Effect and Inflammation. Front. Immunol. 2021, 12, 622471. [Google Scholar] [CrossRef]
- Duhr, S.; Braun, D. Optothermal molecule trapping by opposing fluid flow with thermophoretic drift. Phys. Rev. Lett. 2006, 97, 038103. [Google Scholar] [CrossRef]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Atretkhany, K.N.; Gogoleva, V.S.; Drutskaya, M.S.; Nedospasov, S.A. Distinct modes of TNF signaling through its two receptors in health and disease. J. Leukoc. Biol. 2020, 107, 893–905. [Google Scholar] [CrossRef]
- Freeman, A.J.; Kearney, C.J.; Silke, J.; Oliaro, J. Unleashing TNF cytotoxicity to enhance cancer immunotherapy. Trends Immunol. 2021, 42, 1128–1142. [Google Scholar] [CrossRef]
- Walczak, H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol. Rev. 2011, 244, 9–28. [Google Scholar] [CrossRef]
- Chédotal, H.; Narayanan, D.; Povlsen, K.; Gotfredsen, C.H.; Brambilla, R.; Gajhede, M.; Bach, A.; Clausen, M.H. Small-molecule modulators of tumor necrosis factor signaling. Drug Discov. Today 2023, 28, 103575. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.T.; Reyes, A.W.B.; Huy, T.X.N.; Arayan, L.T.; Min, W.; Lee, H.J.; Rhee, M.H.; Chang, H.H.; Kim, S. Activation of NF-kB-Mediated TNF-Induced Antimicrobial Immunity Is Required for the Efficient Brucella abortus Clearance in RAW 264.7 Cells. Front. Cell. Infect. Microbiol. 2017, 7, 437. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Cuomo, C.; Mignini, I.; Gasbarrini, A.; Papa, A. Focus on Anti-Tumour Necrosis Factor (TNF)-α-Related Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 8187. [Google Scholar] [CrossRef]
- Eng, G.P.; Bouchelouche, P.; Bartels, E.M.; Bliddal, H.; Bendtzen, K.; Stoltenberg, M. Anti-Drug Antibodies, Drug Levels, Interleukin-6 and Soluble TNF Receptors in Rheumatoid Arthritis Patients during the First 6 Months of Treatment with Adalimumab or Infliximab: A Descriptive Cohort Study. PLoS ONE 2016, 11, 0162316. [Google Scholar] [CrossRef] [PubMed]
- Yashin, D.V.; Sashchenko, L.P.; Georgiev, G.P. Mechanisms of Action of the PGLYRP1/Tag7 Protein in Innate and Acquired Immunity. Acta Nat. 2021, 13, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Gupta, D. Mammalian PGRPs: Novel antibacterial proteins. Cell. Microbiol. 2006, 8, 1059–1069. [Google Scholar] [CrossRef]
- Read, C.B.; Kuijper, J.L.; Hjorth, S.A.; Heipel, M.D.; Tang, X.; Fleetwood, A.J.; Dantzler, J.L.; Grell, S.N.; Kastrup, J.; Wang, C.; et al. Cutting Edge: Identification of neutrophil PGLYRP1 as a ligand for TREM-1. J. Immunol. 2015, 194, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Sharapova, T.N.; Romanova, E.A.; Ivanova, O.K.; Sashchenko, L.P.; Yashin, D.V. Cytokines TNFα, IFNγ and IL-2 Are Responsible for Signal Transmission from the Innate Immunity Protein Tag7 (PGLYRP1) to Cytotoxic Effector Lymphocytes. Cells 2020, 9, 2602. [Google Scholar] [CrossRef]
- Dukhanina, E.A.; Kabanova, O.D.; Lukyanova, T.I.; Shatalov, Y.V.; Yashin, D.V.; Romanova, E.A.; Gnuchev, N.V.; Galkin, A.V.; Georgiev, G.P.; Sashchenko, L.P. Opposite roles of metastasin (S100A4) in two potentially tumoricidal mechanisms involving human lymphocyte protein Tag7 and Hsp70. Proc. Natl. Acad. Sci. USA 2009, 106, 13963–13967. [Google Scholar] [CrossRef]
- Sashchenko, L.P.; Dukhanina, E.A.; Yashin, D.V.; Shatalov, Y.V.; Romanova, E.A.; Korobko, E.V.; Demin, A.V.; Lukyanova, T.I.; Kabanova, O.D.; Khaidukov, S.V.; et al. Peptidoglycan recognition protein tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J. Biol. Chem. 2004, 279, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Telegin, G.B.; Chernov, A.S.; Minakov, A.N.; Balmasova, I.P.; Romanova, E.A.; Sharapova, T.N.; Sashchenko, L.P.; Yashin, D.V. Short Peptides of Innate Immunity Protein Tag7 Inhibit the Production of Cytokines in CFA-Induced Arthritis. Int. J. Mol. Sci. 2022, 23, 12435. [Google Scholar] [CrossRef] [PubMed]
- Altin, J.G.; Pagler, E.B. A One-Step Procedure for Biotinylation and Chemical Cross-Linking of Lymphocyte Surface and Intracellular Membrane-Associated Molecules. Anal. Biochem. 1995, 224, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
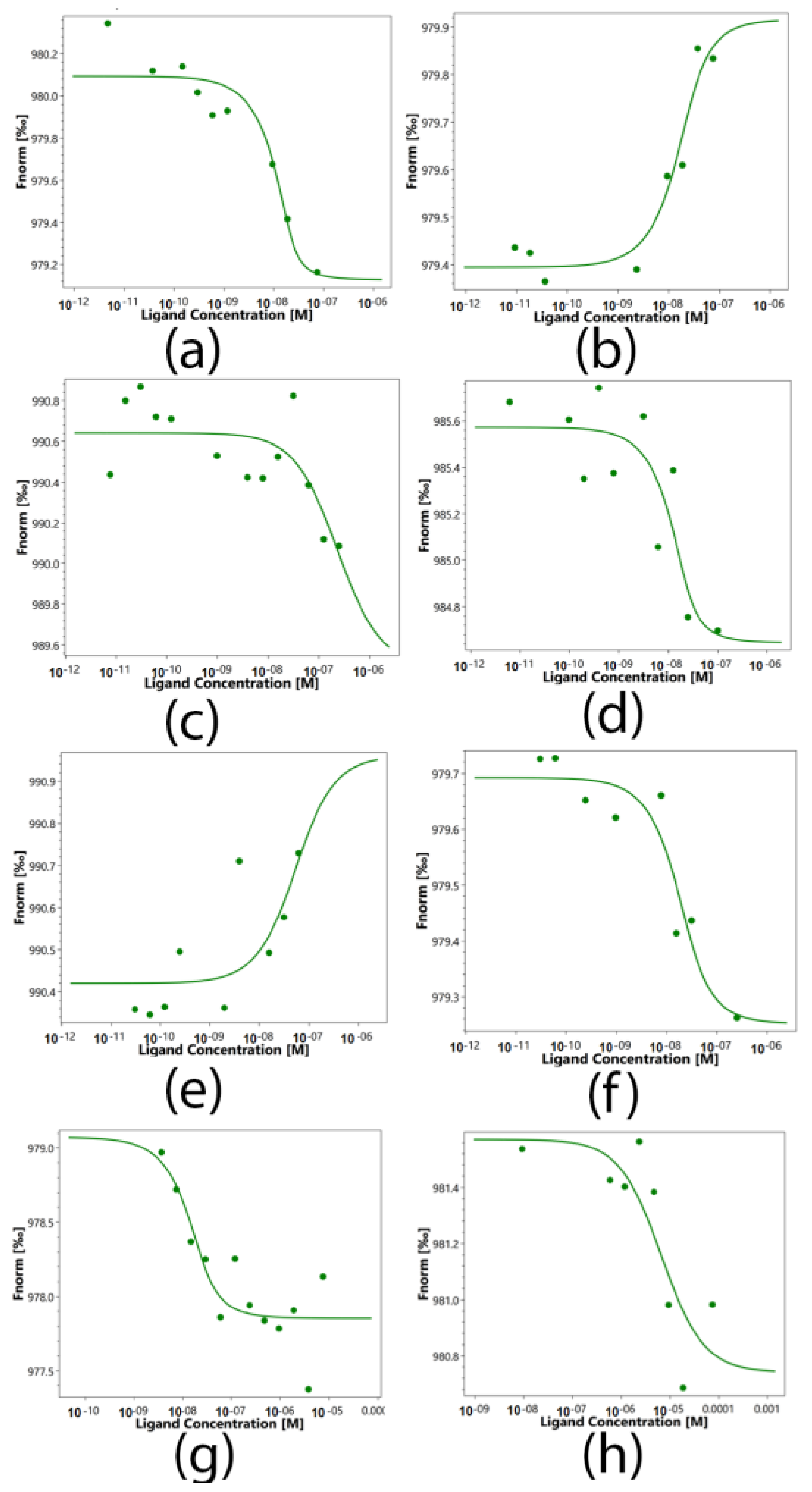
| Ligands | Kd, nM |
|---|---|
| Tag7-Hsp70 | 1.73 ± 0.3 |
| 17.1-Hsp70 | 7.16 ± 1 |
| 17.1A-Hsp70 | 222 ± 15 |
| 17.1B-Hsp70 | 3.15 ± 0.7 |
| Tag7-TNFR1 | 43.2 ± 5 |
| 17.1-TNFR1 | 8.84 ± 1 |
| 17.1A-TNFR1 | 5.44 ± 1 |
| 17.1B-TNFR1 | 6690 ± 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurkina, D.M.; Sharapova, T.N.; Romanova, E.A.; Yashin, D.V.; Sashchenko, L.P. Short Peptides of Innate Immunity Protein Tag7 (PGLYRP1) Selectively Induce Inhibition or Activation of Tumor Cell Death via TNF Receptor. Int. J. Mol. Sci. 2023, 24, 11363. https://doi.org/10.3390/ijms241411363
Yurkina DM, Sharapova TN, Romanova EA, Yashin DV, Sashchenko LP. Short Peptides of Innate Immunity Protein Tag7 (PGLYRP1) Selectively Induce Inhibition or Activation of Tumor Cell Death via TNF Receptor. International Journal of Molecular Sciences. 2023; 24(14):11363. https://doi.org/10.3390/ijms241411363
Chicago/Turabian StyleYurkina, Daria M., Tatiana N. Sharapova, Elena A. Romanova, Denis V. Yashin, and Lidia P. Sashchenko. 2023. "Short Peptides of Innate Immunity Protein Tag7 (PGLYRP1) Selectively Induce Inhibition or Activation of Tumor Cell Death via TNF Receptor" International Journal of Molecular Sciences 24, no. 14: 11363. https://doi.org/10.3390/ijms241411363
APA StyleYurkina, D. M., Sharapova, T. N., Romanova, E. A., Yashin, D. V., & Sashchenko, L. P. (2023). Short Peptides of Innate Immunity Protein Tag7 (PGLYRP1) Selectively Induce Inhibition or Activation of Tumor Cell Death via TNF Receptor. International Journal of Molecular Sciences, 24(14), 11363. https://doi.org/10.3390/ijms241411363