Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation
Abstract
:1. Introduction
2. Results
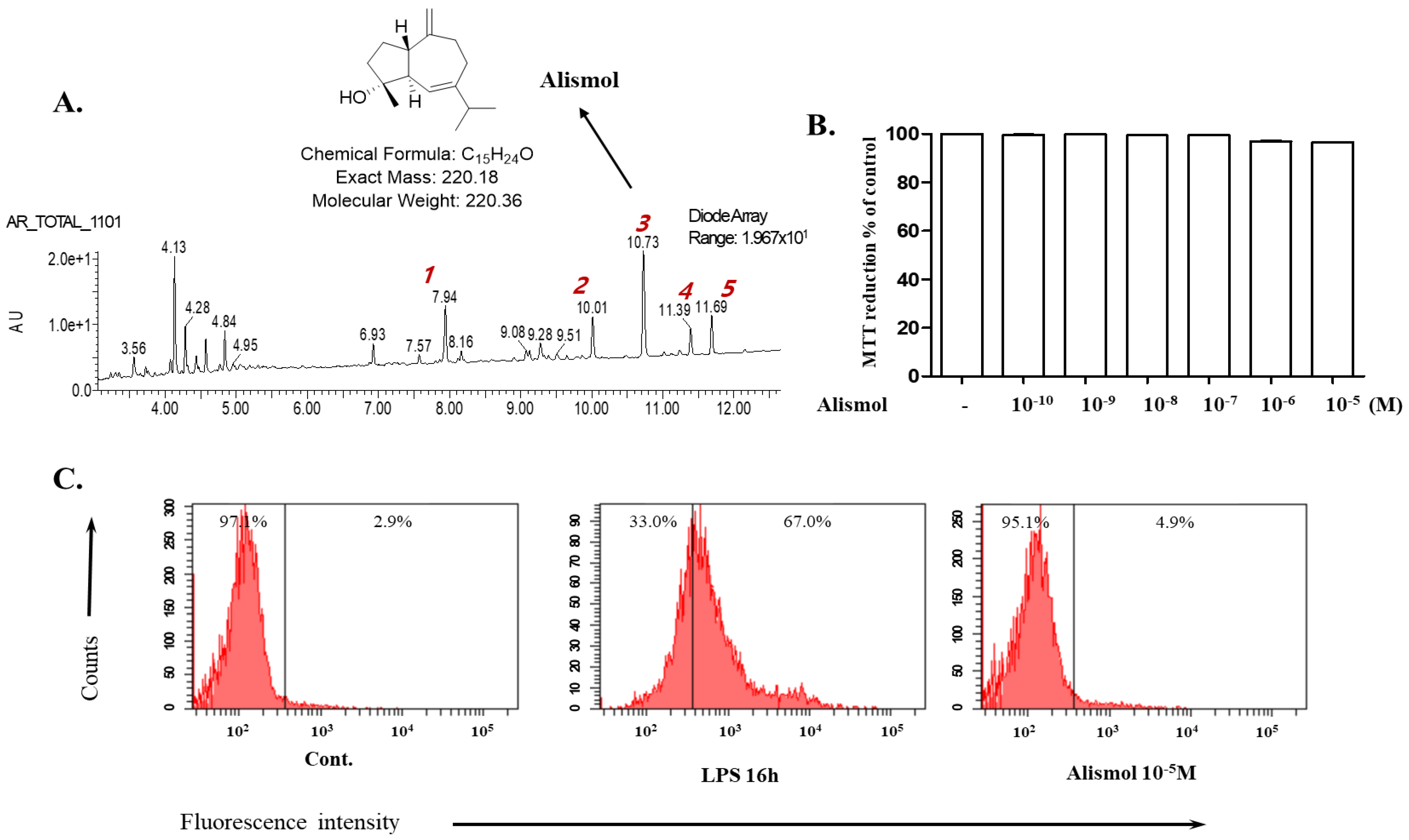
2.1. Alismol Purified from the Ethanol Extract of the Tuber of Alisma orientale Juzepzuk
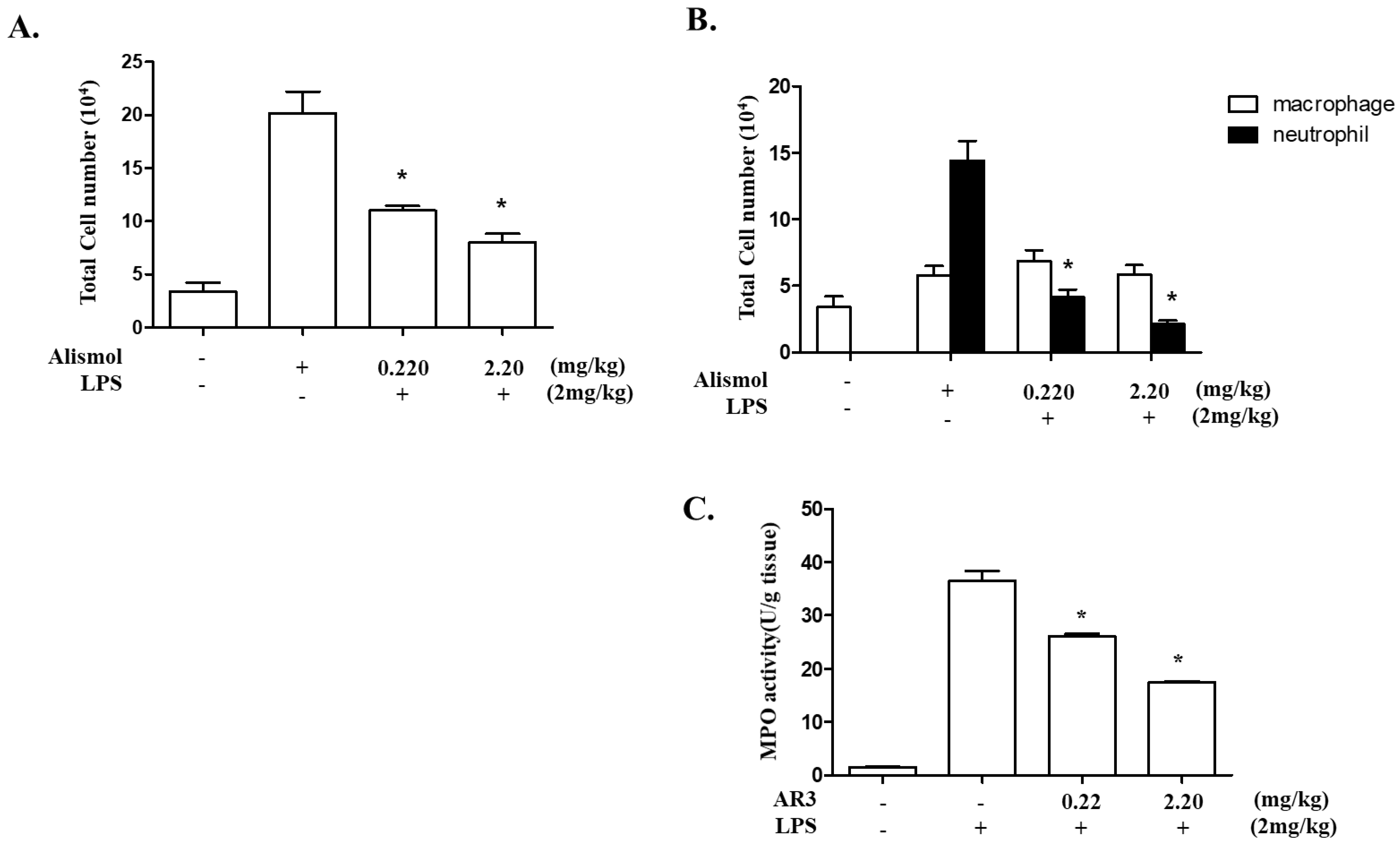
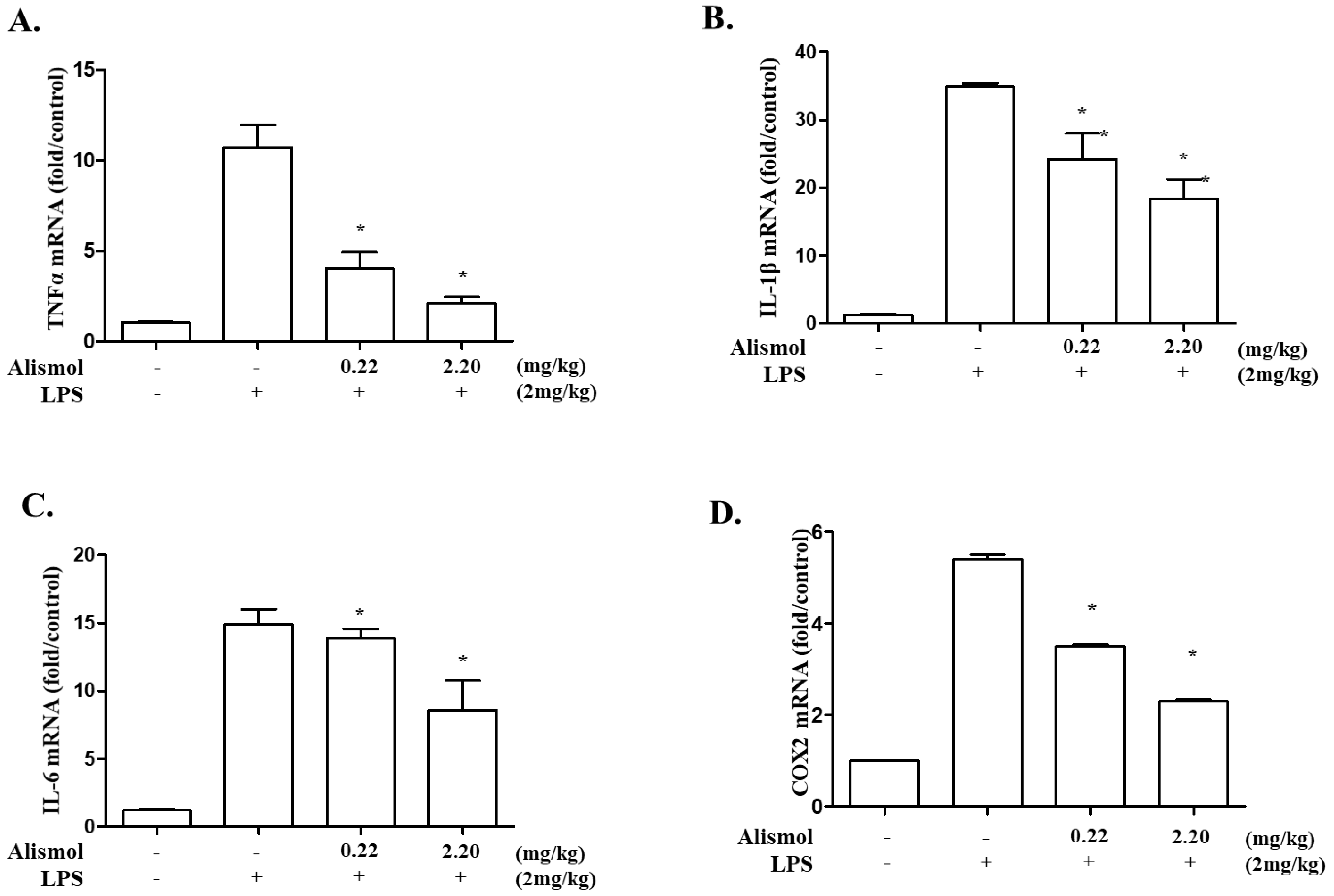
2.2. Alismol Decreases Lung Inflammation in an LPS-Induced ALI Mouse Model
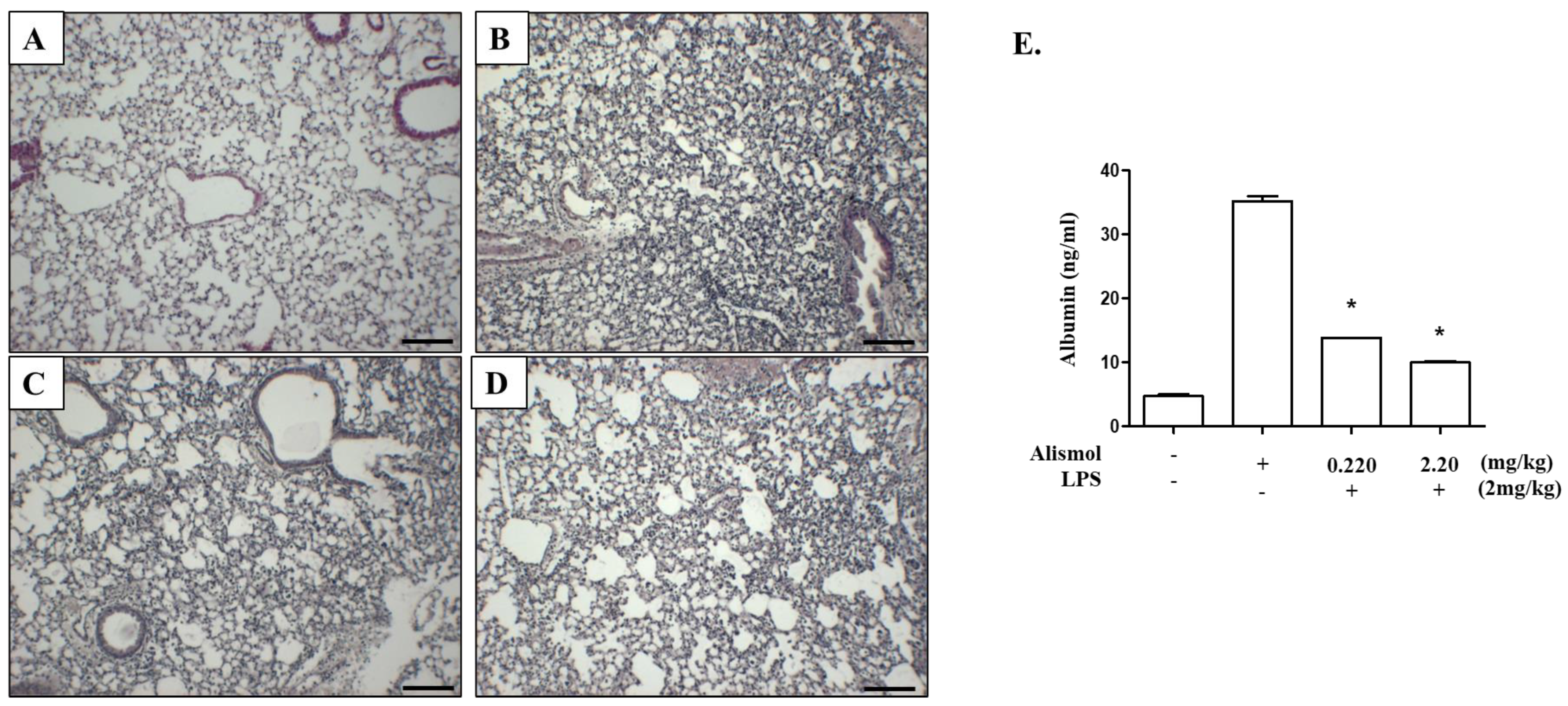
2.3. Alismol Protects Mice from ALI-Associated Lung Damage
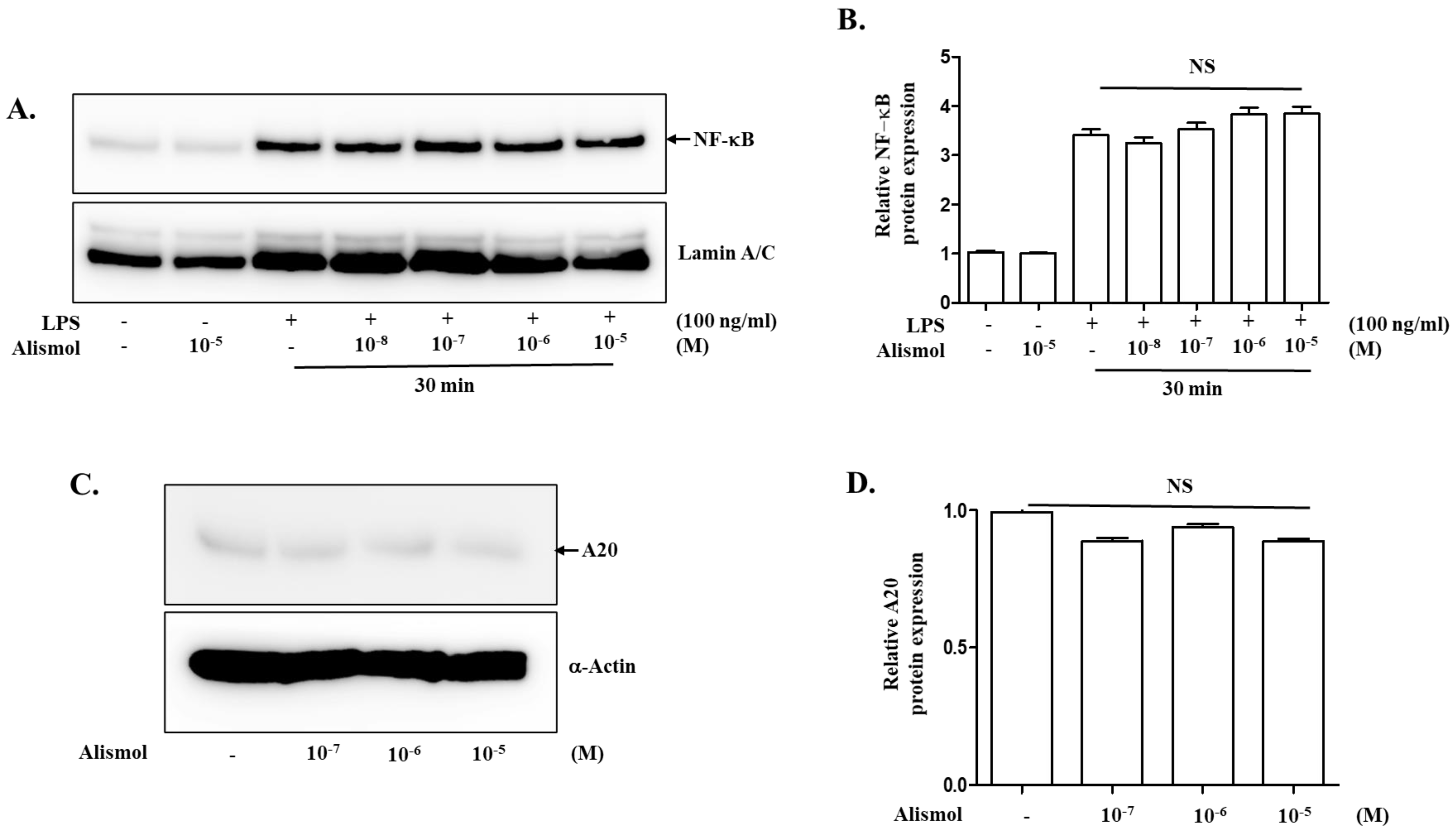
2.4. The Anti-Inflammatory Effect of Alismol Is Associated with Nrf2 Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Isolation of Alismol
4.2. Reagents and Antibodies
4.3. Cell Culture
4.4. Measurement of Cytotoxicity
4.5. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.6. Acute Lung Injury (ALI) Mouse Model and Lung Analyses
4.7. Measurement of Pro-Inflammatory Cytokines and Serum Albumin in BALF
4.8. Myeloperoxidase (MPO) Activity
4.9. Western Blot Analysis
4.10. Ubiquitination of Nrf2
4.11. Isolation of Total RNA and Real-Time Quantitative RT-PCR
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mokra, D. Acute Lung Injury—From Pathophysiology to Treatment. Physiol. Res. 2021, 69, S353–S366. [Google Scholar] [CrossRef]
- Salomão, R.; Ferreira, B.; Salomão, M.; Santos, S.; Azevedo, L.; Brunialti, M. Sepsis: Evolving concepts and challenges. Braz. J. Med. Biol. Res. 2019, 52, e8595. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef]
- Millar, M.W.; Fazal, F.; Rahman, A. Therapeutic Targeting of NF-κB in Acute Lung Injury: A Double-Edged Sword. Cells 2022, 11, 3317. [Google Scholar] [CrossRef]
- Lang, J.D.; McArdle, P.J.; O’Reilly, P.J.; Matalon, S. Oxidant-Antioxidant Balance in Acute Lung Injury. Chest 2002, 122, 314S–320S. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Kan, Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- de la Vega, M.R.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.N.; Zhang, D.D. Role of Nrf2 and Autophagy in Acute Lung Injury. Curr. Pharmacol. Rep. 2016, 2, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.; Ding, D.; Yang, J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front. Pharmacol. 2022, 13, 1039022. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chen, H.; Zhao, Y.-Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158, 373–387. [Google Scholar] [CrossRef]
- Kim, K.H.; Kwun, M.J.; Choi, J.Y.; Ahn, K.S.; Oh, S.R.; Lee, Y.G.; Christman, J.W.; Sadikot, R.T.; Han, C.W.; Joo, M. Therapeutic Effect of the Tuber of Alisma orientale on Lipopolysaccharide-Induced Acute Lung Injury. Evid. Based Complement. Altern. Med. 2013, 2013, 863892. [Google Scholar]
- Han, C.W.; Kwun, M.J.; Kim, K.H.; Choi, J.Y.; Oh, S.R.; Ahn, K.S.; Lee, J.H.; Joo, J.M. Ethanol extract of Alismatis Rhizoma reduces acute lung in-flammation by suppressing NF-κB and activating Nrf2. J. Ethnopharmacol. 2013, 146, 402–410. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, K.H.; Kim, E.J.; Choi, J.-Y.; Kim, S.-J.; Jeong, S.-I.; Kim, J.-I.; Joo, M. Anti-inflammatory activity of the decoction of Forsythia suspensa (Thunb.) Vahl is related to Nrf2 and A20. J. Ethnopharmacol. 2018, 227, 97–104. [Google Scholar] [CrossRef]
- Lyu, J.H.; Kim, K.H.; Kim, H.W.; Cho, S.I.; Ha, K.T.; Choi, J.Y.; Han, C.W.; Jeong, H.-S.; Lee, H.-K.; Ahn, K.-S.; et al. Dangkwisoo-san, an herbal medicinal formula, ameliorates acute lung inflammation via activation of Nrf2 and suppression of NF-κB. J. Ethnopharmacol. 2012, 140, 107–116. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2010, 17, 293–307. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.Y.; Ahn, S.; Won, R.; Kim, S.-J.; Jeong, S.-I.; Lee, J.J.; Kim, J.-I.; Choi, J.-Y.; Joo, M. The methanol extract of Guettarda speciosa Linn. Ameliorates acute lung injury in mice. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef] [PubMed]
- Gye, Y.P.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L797–L805. [Google Scholar]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2020, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, K.; King, L.S.; Aggarwal, N.R.; De Gorordo, A.; D’Alessio, F.R.; Kubo, K. Acute lung injury review. Intern. Med. 2009, 48, 621–630. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Momtazi, G.; Lambrecht, B.N.; Naranjo, J.R.; Schock, B.C. Regulators of A20 (TNFAIP3): New drug-able targets in inflammation. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L456–L469. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, O.S.; Jin, H.-G.; Woo, E.-R.; Kim, Y.S.; Kim, H.P. The Rhizomes of Alisma orientale and Alisol Derivatives Inhibit Allergic Response and Experimental Atopic Dermatitis. Biol. Pharm. Bull. 2012, 35, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. Studies on Alismatis Rhizoma. I. Anti-allergic effects of methanol extract and six terpene components from Alismatis Rhizoma (dried rhizome of Alisma orientale). Biol. Pharm. Bull. 1997, 20, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef]
- Cippitelli, M.; Sica, A.; Viggiano, V.; Ye, J.; Ghosh, P.; Birrer, M.J.; Young, H.A. Negative Transcriptional Regulation of the Interferon-γ Promoter by Glucocorticoids and Dominant Negative Mutants of c-Jun. J. Biol. Chem. 1995, 270, 12548–12556. [Google Scholar] [CrossRef]
- Nakayama, A.; Kawasaki, H.; Jin, C.; Munekata, E.; Taira, K.; Yokoyama, K.K. Transcriptional regulation of interferon gamma gene by p300 co-activator. Nucleic Acids Res. Suppl. 2001, 1, 89–90. [Google Scholar] [CrossRef]
- De Araujo-Souza, P.S.; Hanschke, S.C.H.; Viola, J.P.B. Epigenetic control of interferon-gamma expression in CD8 T cells. J. Immunol. Res. 2015, 2015, 849573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Kim, S.; Kwun, M.J.; Lee, J.Y.; Oh, S.-R.; Choi, J.-Y.; Joo, M. Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation. Int. J. Mol. Sci. 2023, 24, 15573. https://doi.org/10.3390/ijms242115573
Kim KH, Kim S, Kwun MJ, Lee JY, Oh S-R, Choi J-Y, Joo M. Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation. International Journal of Molecular Sciences. 2023; 24(21):15573. https://doi.org/10.3390/ijms242115573
Chicago/Turabian StyleKim, Kyun Ha, Soyeon Kim, Min Jung Kwun, Ji Yeon Lee, Sei-Ryang Oh, Jun-Yong Choi, and Myungsoo Joo. 2023. "Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation" International Journal of Molecular Sciences 24, no. 21: 15573. https://doi.org/10.3390/ijms242115573
APA StyleKim, K. H., Kim, S., Kwun, M. J., Lee, J. Y., Oh, S.-R., Choi, J.-Y., & Joo, M. (2023). Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation. International Journal of Molecular Sciences, 24(21), 15573. https://doi.org/10.3390/ijms242115573




