Delivery and Transcriptome Assessment of an In Vitro Three-Dimensional Proximal Tubule Model Established by Human Kidney 2 Cells in Clinical Gelatin Sponges
Abstract
1. Introduction
2. Results
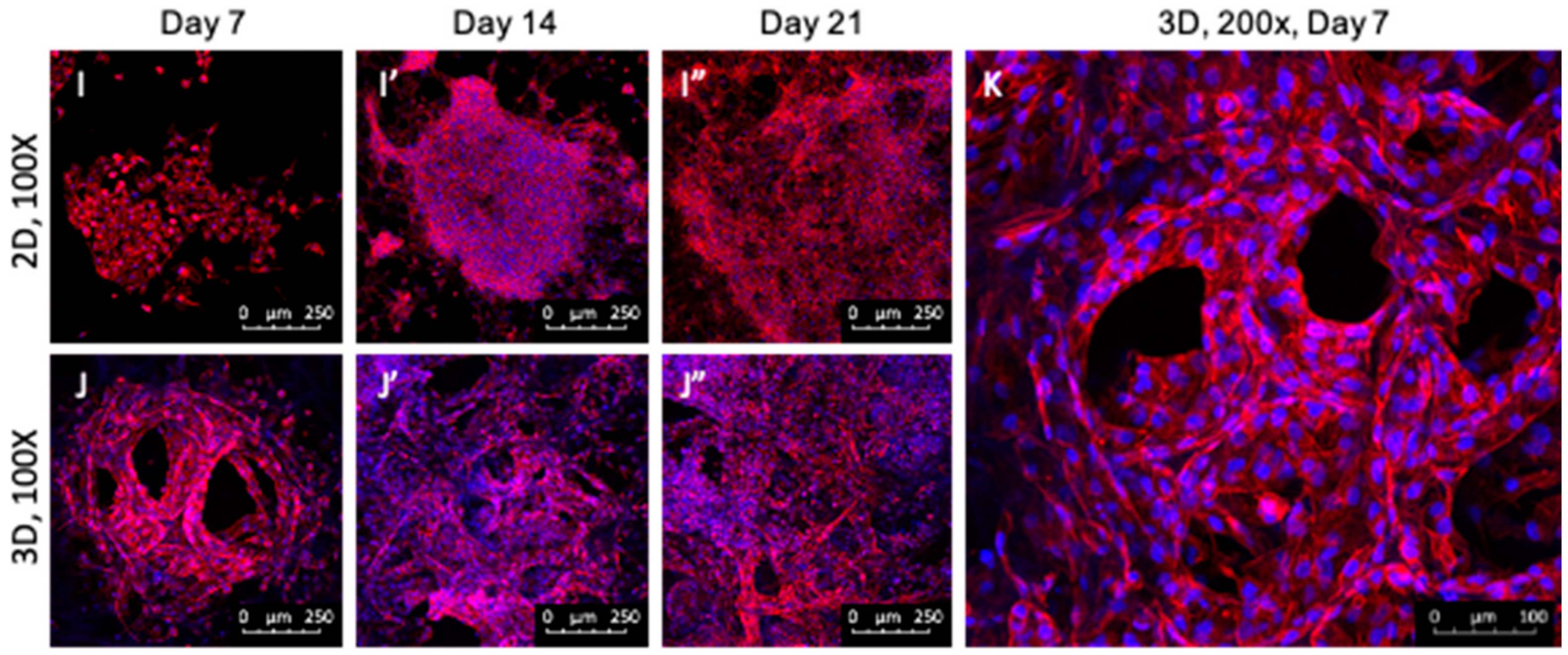
2.1. Growth Pattern and Morphology of HK-2 Cells in the 2D and 3D Culture
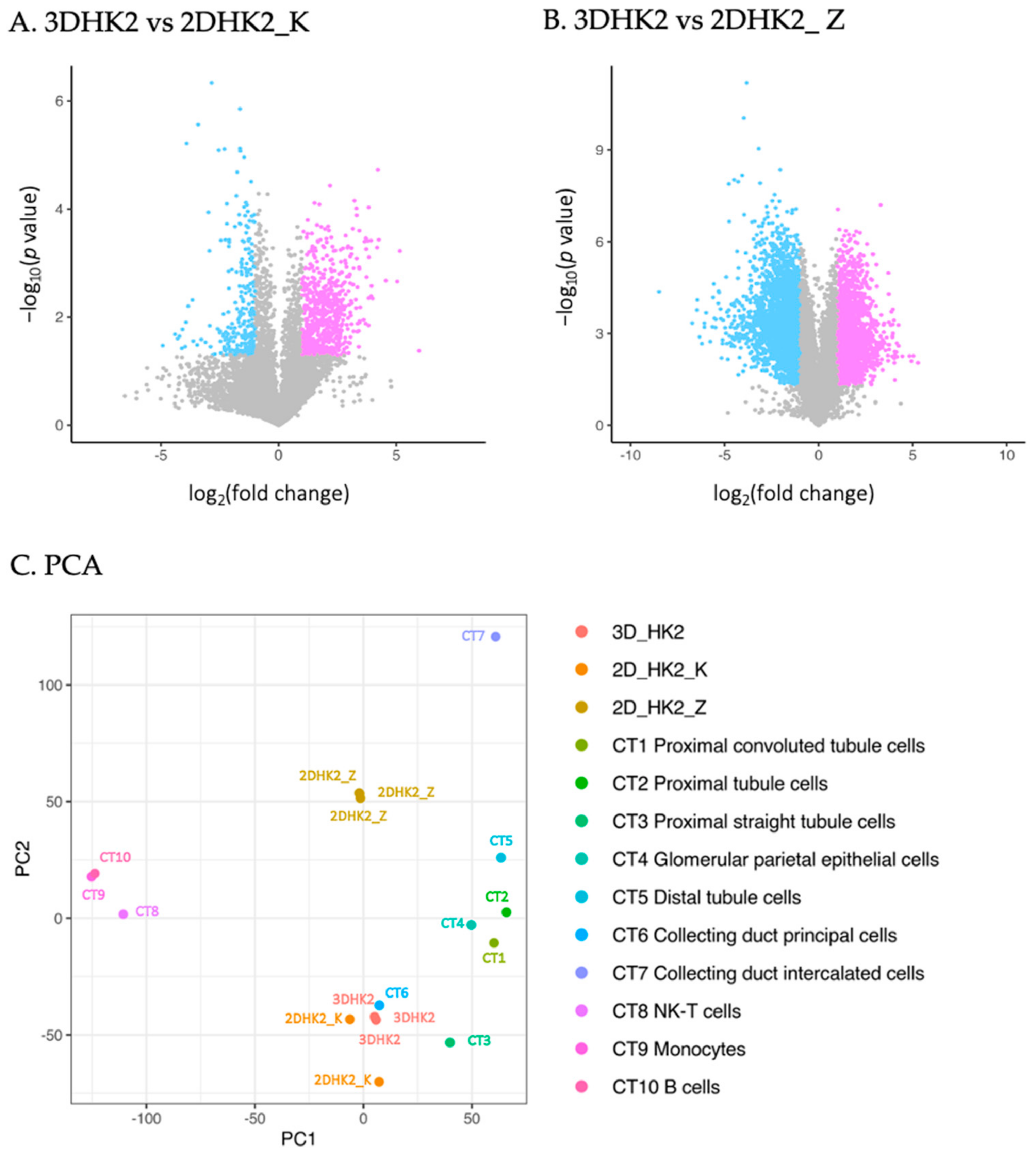
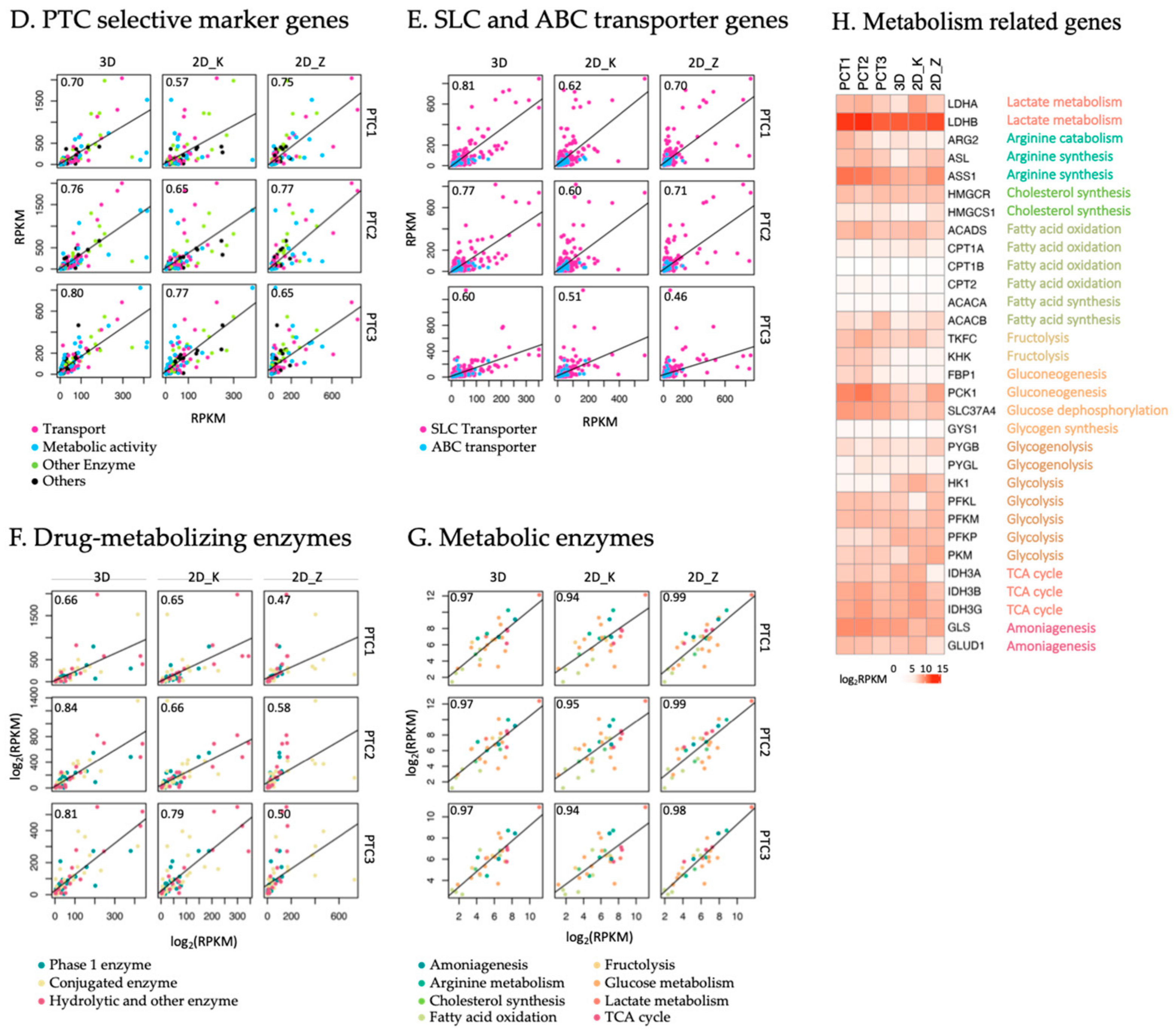
2.2. Comparative Transcriptomic Analysis of HK-2 Cells and Human Native PTCs
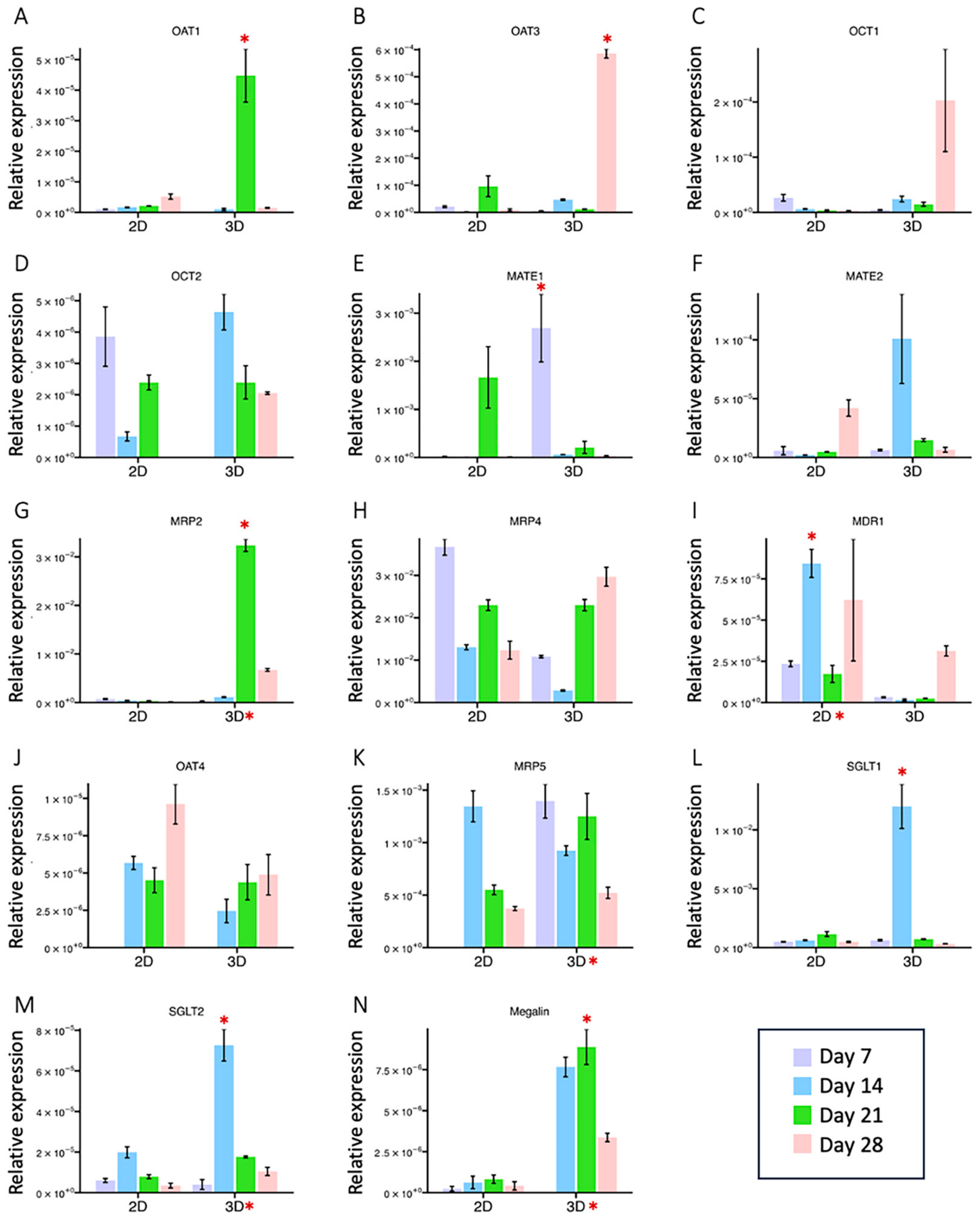
2.3. Temporal Gene Expression Analysis of HK-2 Cells in 2D and 3D Culture
2.4. The Presence of Proximal Tubule Markers in the 2D vs. 3D Model
3. Discussion
3.1. HK-2 Cells Cultured in 3D Gelatin Sponges Displayed Long-Term Growth and Developed Tubule-like Structure
3.2. HK-2 Cells Cultured in 3D Gelatin Sponges Recaptured Transcriptional Patterns of Human PTCs
3.3. HK-2 Cells Cultured in 3D Gelatin Sponges Restored or Maintained More Key PTC Markers and Transporters
4. Materials and Methods
4.1. HK-2 Cell Culture in 2D and 3D Models
4.2. Cell Viability Assay
4.3. Immunohistochemistry Analysis
4.4. RNA Extraction for RNA-seq Analysis
4.5. Library Construction and Sequencing
4.6. Transcriptome Comparison Analysis
4.7. RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nigam, S.K.; Wu, W.; Bush, K.T.; Hoenig, M.P.; Blantz, R.C.; Bhatnagar, V. Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters. Clin. J. Am. Soc. Nephrol. 2015, 10, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2022, 37, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, N.P.; Moe, O.W. Proximal tubule function and response to acidosis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Lafont, F.; Burkhardt, J.K.; Simons, K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature 1994, 372, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Washio, I.; Matsuoka, N.; Kubo, H.; Staub, A.Y.; Nakamichi, N.; Ishiguro, N.; Kato, Y.; Nakanishi, T.; Tamai, I. Usefulness of kidney slices for functional analysis of apical reabsorptive transporters. Sci. Rep. 2017, 7, 12814. [Google Scholar] [CrossRef]
- Izzedine, H.; Launay-Vacher, V.; Isnard-Bagnis, C.; Deray, G. Drug-induced Fanconi’s syndrome. Am. J. Kidney Dis. 2003, 41, 292–309. [Google Scholar] [CrossRef]
- Perazella, M.A. Renal vulnerability to drug toxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 1275–1283. [Google Scholar] [CrossRef]
- Hall, A.M.; Bass, P.; Unwin, R.J. Drug-induced renal Fanconi syndrome. QJM 2014, 107, 261–269. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D View of Colorectal Cancer Models in Predicting Therapeutic Responses and Resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Khundmiri, S.J.; Chen, L.; Lederer, E.D.; Yang, C.R.; Knepper, M.A. Transcriptomes of Major Proximal Tubule Cell Culture Models. J. Am. Soc. Nephrol. 2021, 32, 86–97. [Google Scholar] [CrossRef]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef]
- Clark, J.Z.; Chen, L.; Chou, C.L.; Jung, H.J.; Lee, J.W.; Knepper, M.A. Representation and relative abundance of cell-type selective markers in whole-kidney RNA-Seq data. Kidney Int. 2019, 95, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Turhan, A.G.; Hwang, J.W.; Chaker, D.; Tasteyre, A.; Latsis, T.; Griscelli, F.; Desterke, C.; Bennaceur-Griscelli, A. iPSC-Derived Organoids as Therapeutic Models in Regenerative Medicine and Oncology. Front. Med. 2021, 8, 728543. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Secker, P.F.; Luks, L.; Schlichenmaier, N.; Dietrich, D.R. RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix. ALTEX 2018, 35, 223–234. [Google Scholar] [CrossRef]
- DesRochers, T.M.; Suter, L.; Roth, A.; Kaplan, D.L. Bioengineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS ONE 2013, 8, e59219. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jeon, H. 3D cell cultures toward quantitative high-throughput drug screening. Trends. Pharmacol. Sci. 2022, 43, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C. Cell culture: In vitro model system and a promising path to in vivo applications. J. Histotechnol. 2023, 46, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.A.; Stafford, R.M.; Petrasovits, B.A.; Boone, M.A.; Valentovic, M.A. Establishment of HK-2 Cells as a Relevant Model to Study Tenofovir-Induced Cytotoxicity. Int. J. Mol. Sci. 2017, 18, 531. [Google Scholar] [CrossRef]
- Gildea, J.J.; Shah, I.; Weiss, R.; Casscells, N.D.; McGrath, H.E.; Zhang, J.; Jones, J.E.; Felder, R.A. HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertension 2010, 56, 505–511. [Google Scholar] [CrossRef]
- Jenkinson, S.E.; Chung, G.W.; van Loon, E.; Bakar, N.S.; Dalzell, A.M.; Brown, C.D. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflug. Arch. 2012, 464, 601–611. [Google Scholar] [CrossRef]
- Bajaj, P.; Chowdhury, S.K.; Yucha, R.; Kelly, E.J.; Xiao, G. Emerging Kidney Models to Investigate Metabolism, Transport, and Toxicity of Drugs and Xenobiotics. Drug Metab. Dispos. 2018, 46, 1692–1702. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Li, C.; Dou, Y.; Li, Y.; Thote, T.; Wang, D.A.; Ge, Z. Cells behave distinctly within sponges and hydrogels due to differences of internal structure. Tissue Eng. Part A 2013, 19, 2166–2175. [Google Scholar] [CrossRef]
- Liao, J.; Yu, Z.; Chen, Y.; Bao, M.; Zou, C.; Zhang, H.; Liu, D.; Li, T.; Zhang, Q.; Li, J.; et al. Single-cell RNA sequencing of human kidney. Sci. Data 2020, 7, 4. [Google Scholar] [CrossRef]
- Zhang, L.; Sang, X.; Han, Y.; Abulitibu, A.; Elken, M.; Mao, Z.; Kang, S.; Yang, W.; Lu, C. The expression of apoptosis related genes in HK-2 cells overexpressing PPM1K was determined by RNA-seq analysis. Front. Genet. 2022, 13, 1004610. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.A.; Clemencon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Aspects Med. 2013, 34, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Seal, R.L.; Braschi, B.; Gray, K.; Jones, T.E.M.; Tweedie, S.; Haim-Vilmovsky, L.; Bruford, E.A. Genenames.org: The HGNC resources in 2023. Nucleic Acids Res. 2023, 51, D1003–D1009. [Google Scholar] [CrossRef]
- Lewis, S.; Chen, L.; Raghuram, V.; Khundmiri, S.J.; Chou, C.L.; Yang, C.R.; Knepper, M.A. “SLC-omics” of the kidney: Solute transporters along the nephron. Am. J. Physiol. Cell Physiol. 2021, 321, C507–C518. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Sadighi, A.; Ryu, R.; Akhlaghi, F. Physicochemical Properties, Biotransformation, and Transport Pathways of Established and Newly Approved Medications: A Systematic Review of the Top 200 Most Prescribed Drugs vs. the FDA-Approved Drugs Between 2005 and 2016. Clin. Pharmacokinet. 2019, 58, 1281–1294. [Google Scholar] [CrossRef]
- Morrissey, K.M.; Stocker, S.L.; Wittwer, M.B.; Xu, L.; Giacomini, K.M. Renal Transporters in Drug Development. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 503–529. [Google Scholar] [CrossRef]
- Pelis, R.M.; Wright, S.H. SLC22, SLC44, and SLC47 transporters--organic anion and cation transporters: Molecular and cellular properties. Curr. Top. Membr. 2014, 73, 233–261. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Bossennec, M.; Di Roio, A.; Caux, C.; Menetrier-Caux, C. MDR1 in immunity: Friend or foe? Oncoimmunology 2018, 7, e1499388. [Google Scholar] [CrossRef] [PubMed]
- Ekaratanawong, S.; Anzai, N.; Jutabha, P.; Miyazaki, H.; Noshiro, R.; Takeda, M.; Kanai, Y.; Sophasan, S.; Endou, H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J. Pharmacol. Sci. 2004, 94, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.B.; Fenton, R.A.; Rieg, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469. [Google Scholar] [CrossRef]
- Launay-Vacher, V.; Izzedine, H.; Karie, S.; Hulot, J.S.; Baumelou, A.; Deray, G. Renal tubular drug transporters. Nephron Physiol. 2006, 103, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wijnholds, J.; Mol, C.A.; van Deemter, L.; de Haas, M.; Scheffer, G.L.; Baas, F.; Beijnen, J.H.; Scheper, R.J.; Hatse, S.; De Clercq, E.; et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc. Natl. Acad. Sci. USA 2000, 97, 7476–7481. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Nielsen, R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2007, 158, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Muthukrishnan, A. Expression of cytokeratin 18 and 19 in oral potentially malignant disorders: A systematic review. J. Ind. Acad. Oral Med. Radiol. 2014, 26, 173–177. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Domański, L.; Bober, J.; Safranow, K.; Pawlik, A.; Kwiatkowski, S.; Ciechanowski, K. Gamma-glutamyl transpeptidase as the marker of kidney graft function. Adv. Clin. Exp. Med. 2014, 23, 947–952. [Google Scholar] [CrossRef]
- Tiong, H.Y.; Huang, P.; Xiong, S.; Li, Y.; Vathsala, A.; Zink, D. Drug-induced nephrotoxicity: Clinical impact and preclinical in vitro models. Mol. Pharm. 2014, 11, 1933–1948. [Google Scholar] [CrossRef]
- Wieser, M.; Stadler, G.; Jennings, P.; Streubel, B.; Pfaller, W.; Ambros, P.; Riedl, C.; Katinger, H.; Grillari, J.; Grillari-Voglauer, R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am. J. Physiol. Renal Physiol. 2008, 295, F1365–F1375. [Google Scholar] [CrossRef]
- Vesey, D.A.; Qi, W.; Chen, X.; Pollock, C.A.; Johnson, D.W. Isolation and primary culture of human proximal tubule cells. Methods Mol. Biol. 2009, 466, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Connors, D.; Barber, L.; Jayachandra, S.; Hanumegowda, U.M.; Adams, S.P. Multiplexed assay panel of cytotoxicity in HK-2 cells for detection of renal proximal tubule injury potential of compounds. Toxicol. In Vitro 2009, 23, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Oo, Z.Y.; Chang, S.Y.; Huang, P.; Eng, K.G.; Zeng, J.L.; Kaestli, A.J.; Gopalan, B.; Kandasamy, K.; Tasnim, F.; et al. An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol. Res. 2013, 2, 352–365. [Google Scholar] [CrossRef]
- Lu, Y.T.; Ma, X.L.; Xu, Y.H.; Hu, J.; Wang, F.; Qin, W.Y.; Xiong, W.Y. A Fluorescent Glucose Transport Assay for Screening SGLT2 Inhibitors in Endogenous SGLT2-Expressing HK-2 Cells. Nat. Prod. Bioprospect. 2019, 9, 13–21. [Google Scholar] [CrossRef]
- Handl, J.; Capek, J.; Majtnerova, P.; Bacova, J.; Rousar, T. The effect of repeated passaging on the susceptibility of human proximal tubular HK-2 cells to toxic compounds. Physiol. Res. 2020, 69, 731–738. [Google Scholar] [CrossRef]
- Sasaki, H.; Sugiyama, M.; Sasaki, N. Establishment of renal proximal tubule cell lines derived from the kidney of p53 knockout mice. Cytotechnology 2019, 71, 45–56. [Google Scholar] [CrossRef]
- Khetan, S.; Burdick, J.A. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials 2010, 31, 8228–8234. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Christensen, E.I.; Wagner, C.A.; Kaissling, B. Uriniferous tubule: Structural and functional organization. Compr. Physiol. 2012, 2, 805–861. [Google Scholar] [CrossRef]
- Schuh, C.D.; Polesel, M.; Platonova, E.; Haenni, D.; Gassama, A.; Tokonami, N.; Ghazi, S.; Bugarski, M.; Devuyst, O.; Ziegler, U.; et al. Combined Structural and Functional Imaging of the Kidney Reveals Major Axial Differences in Proximal Tubule Endocytosis. J. Am. Soc. Nephrol. 2018, 29, 2696–2712. [Google Scholar] [CrossRef]
- Faria, J.; Ahmed, S.; Gerritsen, K.G.F.; Mihaila, S.M.; Masereeuw, R. Kidney-based in vitro models for drug-induced toxicity testing. Arch. Toxicol. 2019, 93, 3397–3418. [Google Scholar] [CrossRef]
- Brosseau, N.; Ramotar, D. The human organic cation transporter OCT1 and its role as a target for drug responses. Drug Metab. Rev. 2019, 51, 389–407. [Google Scholar] [CrossRef]
- Franke, R.M.; Kosloske, A.M.; Lancaster, C.S.; Filipski, K.K.; Hu, C.; Zolk, O.; Mathijssen, R.H.; Sparreboom, A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-D-glucosaminidase. Clin. Cancer Res. 2010, 16, 4198–4206. [Google Scholar] [CrossRef]
- Fu, Y.; Breljak, D.; Onishi, A.; Batz, F.; Patel, R.; Huang, W.; Song, P.; Freeman, B.; Mayoux, E.; Koepsell, H.; et al. Organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol. Renal Physiol. 2018, 315, F386–F394. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Bi, Y.; Yu, H.; Wei, J.; Zhang, Y.; Han, L.; Zhang, Y. Potent Inhibitors of Organic Anion Transporters 1 and 3 From Natural Compounds and Their Protective Effect on Aristolochic Acid Nephropathy. Toxicol. Sci. 2020, 175, 279–291. [Google Scholar] [CrossRef]
- Fardel, O.; Jigorel, E.; Le Vee, M.; Payen, L. Physiological, pharmacological and clinical features of the multidrug resistance protein 2. Biomed. Pharmacother. 2005, 59, 104–114. [Google Scholar] [CrossRef]
- Ritter, C.A.; Jedlitschky, G.; Meyer zu Schwabedissen, H.; Grube, M.; Kock, K.; Kroemer, H.K. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab. Rev. 2005, 37, 253–278. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef]
- Sauzay, C.; White-Koning, M.; Hennebelle, I.; Deluche, T.; Delmas, C.; Imbs, D.C.; Chatelut, E.; Thomas, F. Inhibition of OCT2, MATE1 and MATE2-K as a possible mechanism of drug interaction between pazopanib and cisplatin. Pharmacol. Res. 2016, 110, 89–95. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yung, E.; Pintilie, M.; Muaddi, H.; Chaib, S.; Yeung, M.; Fusciello, M.; Sykes, J.; Pitcher, B.; Hagenkort, A.; et al. MATE2 Expression Is Associated with Cancer Cell Response to Metformin. PLoS ONE 2016, 11, e0165214. [Google Scholar] [CrossRef]
- Powell, D.R.; DaCosta, C.M.; Gay, J.; Ding, Z.M.; Smith, M.; Greer, J.; Doree, D.; Jeter-Jones, S.; Mseeh, F.; Rodriguez, L.A.; et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E117–E130. [Google Scholar] [CrossRef] [PubMed]
- Notenboom, S.; Wouterse, A.C.; Peters, B.; Kuik, L.H.; Heemskerk, S.; Russel, F.G.; Masereeuw, R. Increased apical insertion of the multidrug resistance protein 2 (MRP2/ABCC2) in renal proximal tubules following gentamicin exposure. J. Pharmacol. Exp. Ther. 2006, 318, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Nieskens, T.T.; Peters, J.G.; Schreurs, M.J.; Smits, N.; Woestenenk, R.; Jansen, K.; van der Made, T.K.; Roring, M.; Hilgendorf, C.; Wilmer, M.J.; et al. A Human Renal Proximal Tubule Cell Line with Stable Organic Anion Transporter 1 and 3 Expression Predictive for Antiviral-Induced Toxicity. AAPS J. 2016, 18, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Anders, S.; Reyes, A.; Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012, 22, 2008–2017. [Google Scholar] [CrossRef]
- Bullard, J.H.; Purdom, E.; Hansen, K.D.; Dudoit, S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinform. 2010, 11, 94. [Google Scholar] [CrossRef]
- Tarazona, S.; Furio-Tari, P.; Turra, D.; Pietro, A.D.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research R Package Version 1.9.12; Northwestern University: Evanston, IL, USA, 2019; Available online: https://CRAN.R-project.org/package=psych (accessed on 4 June 2023).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]



| Data Sources | Cells | Culture Method | Medium | Culture Period |
|---|---|---|---|---|
| From this study (3DHK2) | HK-2 | 3D: Gelatin sponge | KSF Medium | 14 days |
| Khundmiri et al. [13] (2DHK2_K) | HK-2 | 2D: Transwell | KSF Medium | 95–98% confluence |
| Zhang et al. [31] (2DHK2_Z) | HK-2 | 2D: 25 cm2 flask | DMEM medium with 10% FBS | NS |
| Liao et al. [30] (scRNA-seq_L) | Human kidney cells | NA | NA | NA |
| Gene | Sequence or TaqMan® Assay IDs |
|---|---|
| GAPDH | Forward primer 5′-GGCGCTGAGTACGTCGTGGAG-3′ Reverse primer 5′-ATTGCTGATGATCTTGAGGCTGTTG-3′ |
| MATE1 | Forward primer 5′-TGATCAGGAACACCATCAGC-3′ Reverse primer 5′-GAGGCCACCCTTGAGGTC-3′ |
| MATE2 | Forward primer 5′-TGCTTCCCAGTTCCTCTCAG-3′ Reverse primer 5′-GAAGATGTCATTGCCCTGGT-3′ |
| MDR1 | Forward primer 5′-CCTAGGAGTACTCACTTCAGGA-3′ Reverse primer 5′-AAGATCCATTCCGACCTCGC-3′ |
| megalin | Forward primer 5′-TGGATGTGAAAGCGGTCCTC-3′ Reverse primer 5′-ACTCAACACAGGTACGGCTG-3′ |
| MRP2 | Forward primer 5′-ACGCAGTCCAGGAATCATGC-3′ Reverse primer 5′-AAAACCAGGAGCCATGTGCC-3′ |
| MRP4 | Forward primer 5′-GTGGCCGTGATTCCTTGGAT-3′ Reverse primer 5′-GGCATCCAGAGTTTTTGCCAG-3′ |
| MRP5 | Forward primer 5′-CTGAAGCCCATCCGGACTAC-3′ Reverse primer 5′-CACCATGAAGGCTGGTCCAC-3′ |
| OAT1 | Forward primer 5′-TGTCATCAACTCCCTGGGTCG-3′ Reverse primer 5′-CCCCAGCACAGCAAGAGAGGT-3′ |
| OAT2 | Forward primer 5′-AGCCTACGTGAGTACCCTGG-3′ Reverse primer 5′-CACTCCAGCTCCAGTGGC-3′ |
| OAT3 | Forward primer 5′-CACGAGCCCTCCAATCAGTA-3′ Reverse primer 5′-CTGGGTCTACAACAGCACCA-3′ |
| OAT4 | Hs00945829_m1 |
| OCT1 | Forward primer 5′-CCCCTCATTTTGTTTGCGGT-3′ Reverse primer 5′-TTTCTCCCAAGGTTCTCGGC-3′ |
| OCT2 | Forward primer 5′-GATAGTCTGCCTGGTCAATGCTG-3′ Reverse primer 5′-GAGCCGGTAGACCAGGAATG-3′ |
| SGLT1 | Forward primer 5′-TGGCCACTTCCAATGTTACT-3′ Reverse primer 5′-GGGACTGTTGGAGGCTTCTT-3′ |
| SGLT2 | Forward primer 5′-GTCATCGCGCTTCTGGGCAT-3′ Reverse primer 5′-GGAAGGCGTAACCCATGAGGAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, H.-Y.; Yen, T.-H.; Wu, F.-Y.; Cheng, C.-M.; Liu, J.-W.; Fan, Y.-T.; Huang, J.-J.; Nien, C.-Y. Delivery and Transcriptome Assessment of an In Vitro Three-Dimensional Proximal Tubule Model Established by Human Kidney 2 Cells in Clinical Gelatin Sponges. Int. J. Mol. Sci. 2023, 24, 15547. https://doi.org/10.3390/ijms242115547
Hsiao H-Y, Yen T-H, Wu F-Y, Cheng C-M, Liu J-W, Fan Y-T, Huang J-J, Nien C-Y. Delivery and Transcriptome Assessment of an In Vitro Three-Dimensional Proximal Tubule Model Established by Human Kidney 2 Cells in Clinical Gelatin Sponges. International Journal of Molecular Sciences. 2023; 24(21):15547. https://doi.org/10.3390/ijms242115547
Chicago/Turabian StyleHsiao, Hui-Yi, Tzung-Hai Yen, Fang-Yu Wu, Chao-Min Cheng, Jia-Wei Liu, Yu-Ting Fan, Jung-Ju Huang, and Chung-Yi Nien. 2023. "Delivery and Transcriptome Assessment of an In Vitro Three-Dimensional Proximal Tubule Model Established by Human Kidney 2 Cells in Clinical Gelatin Sponges" International Journal of Molecular Sciences 24, no. 21: 15547. https://doi.org/10.3390/ijms242115547
APA StyleHsiao, H.-Y., Yen, T.-H., Wu, F.-Y., Cheng, C.-M., Liu, J.-W., Fan, Y.-T., Huang, J.-J., & Nien, C.-Y. (2023). Delivery and Transcriptome Assessment of an In Vitro Three-Dimensional Proximal Tubule Model Established by Human Kidney 2 Cells in Clinical Gelatin Sponges. International Journal of Molecular Sciences, 24(21), 15547. https://doi.org/10.3390/ijms242115547







