Circular RNA circIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts
Abstract
:1. Introduction
2. Results
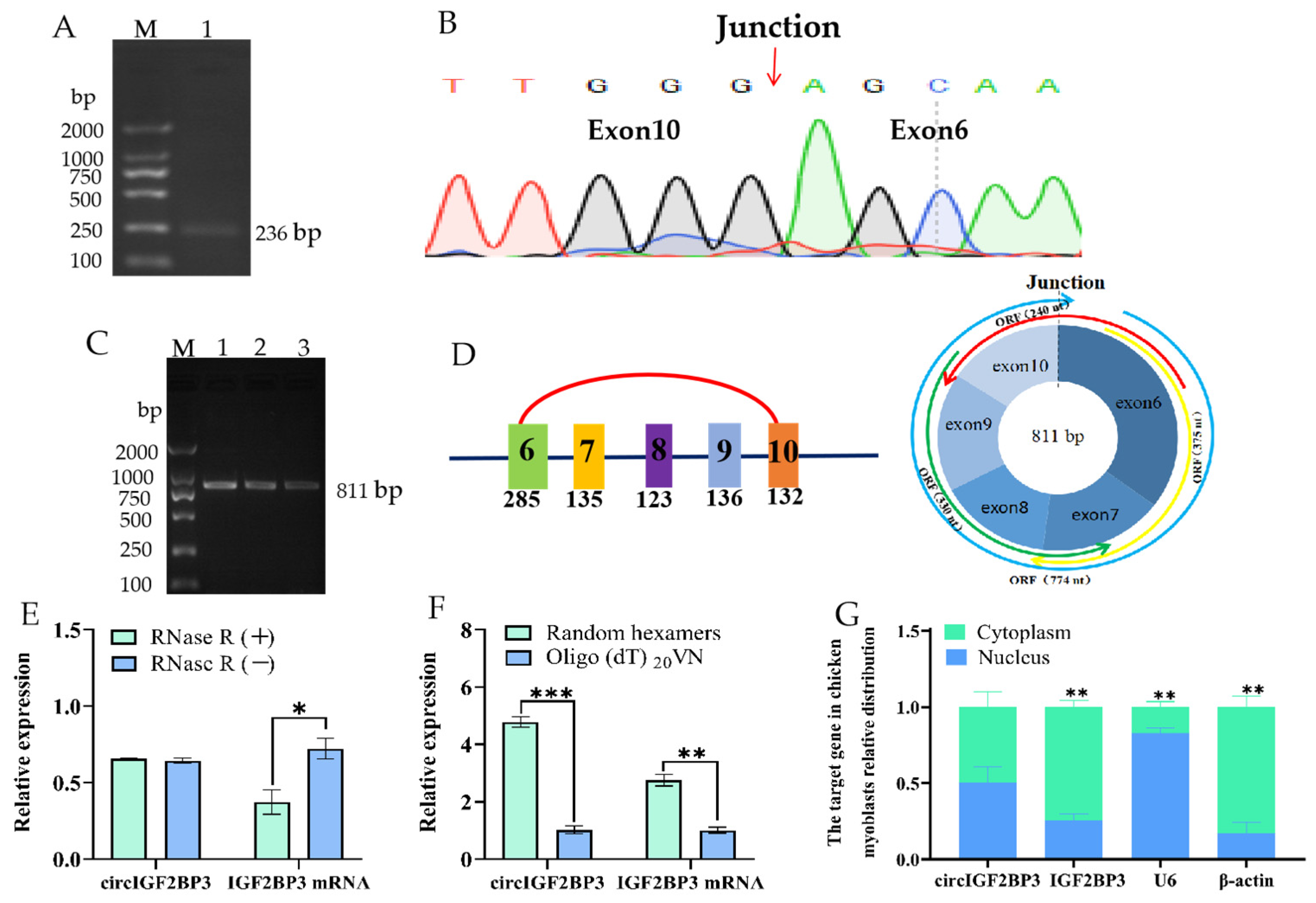
2.1. Circular Features of the Chicken CircIGF2BP3
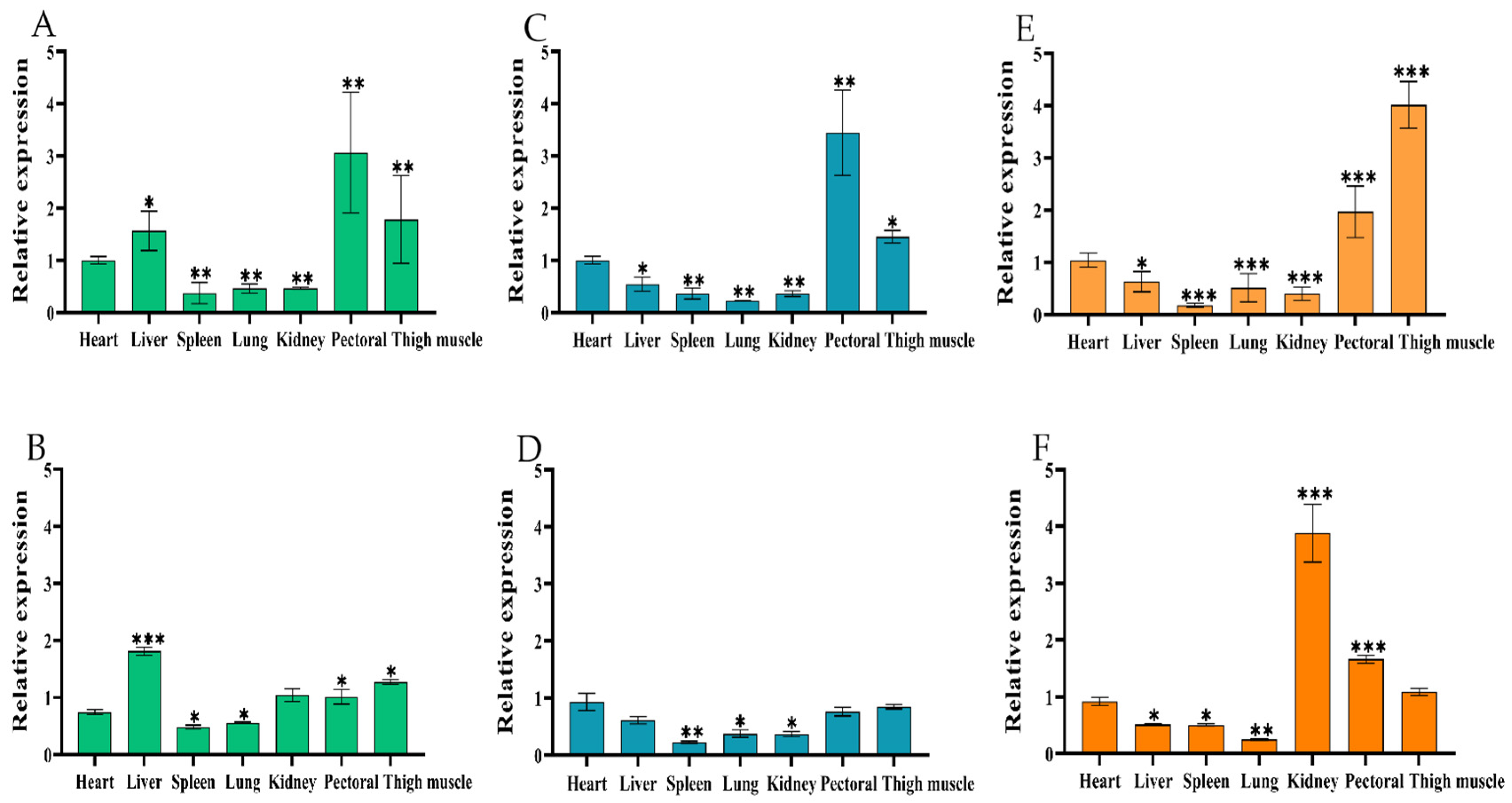
2.2. Spatiotemporal Expression Pattern of Chicken CircIGF2BP3
2.2.1. Tissue Expression Profile of CircIGF2BP3
2.2.2. Temporal Expression Pattern of CircIGF2BP3
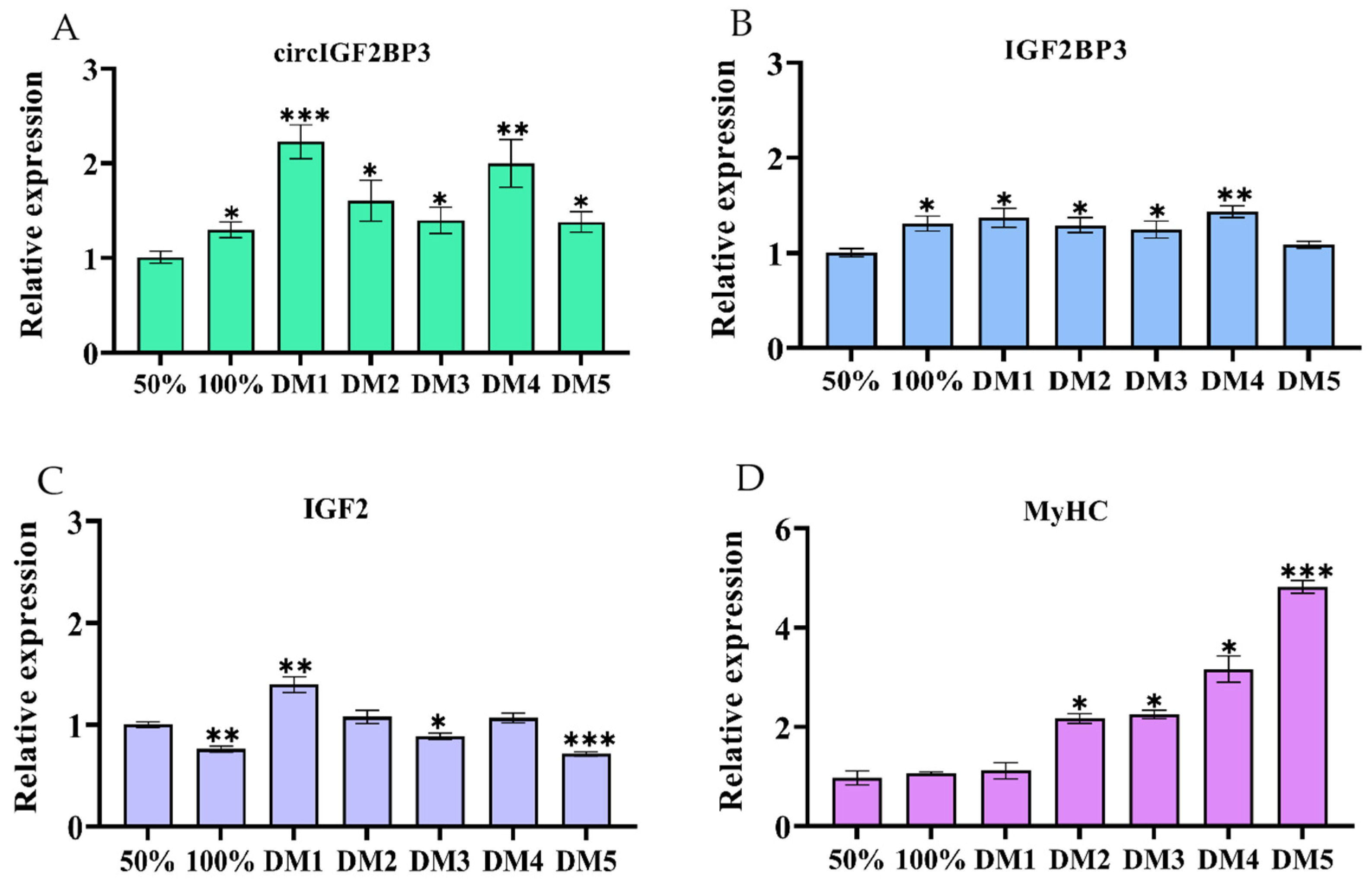
2.3. CircIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts
2.3.1. CircIGF2BP3 Expression in the Proliferation and Differentiation of Myoblasts
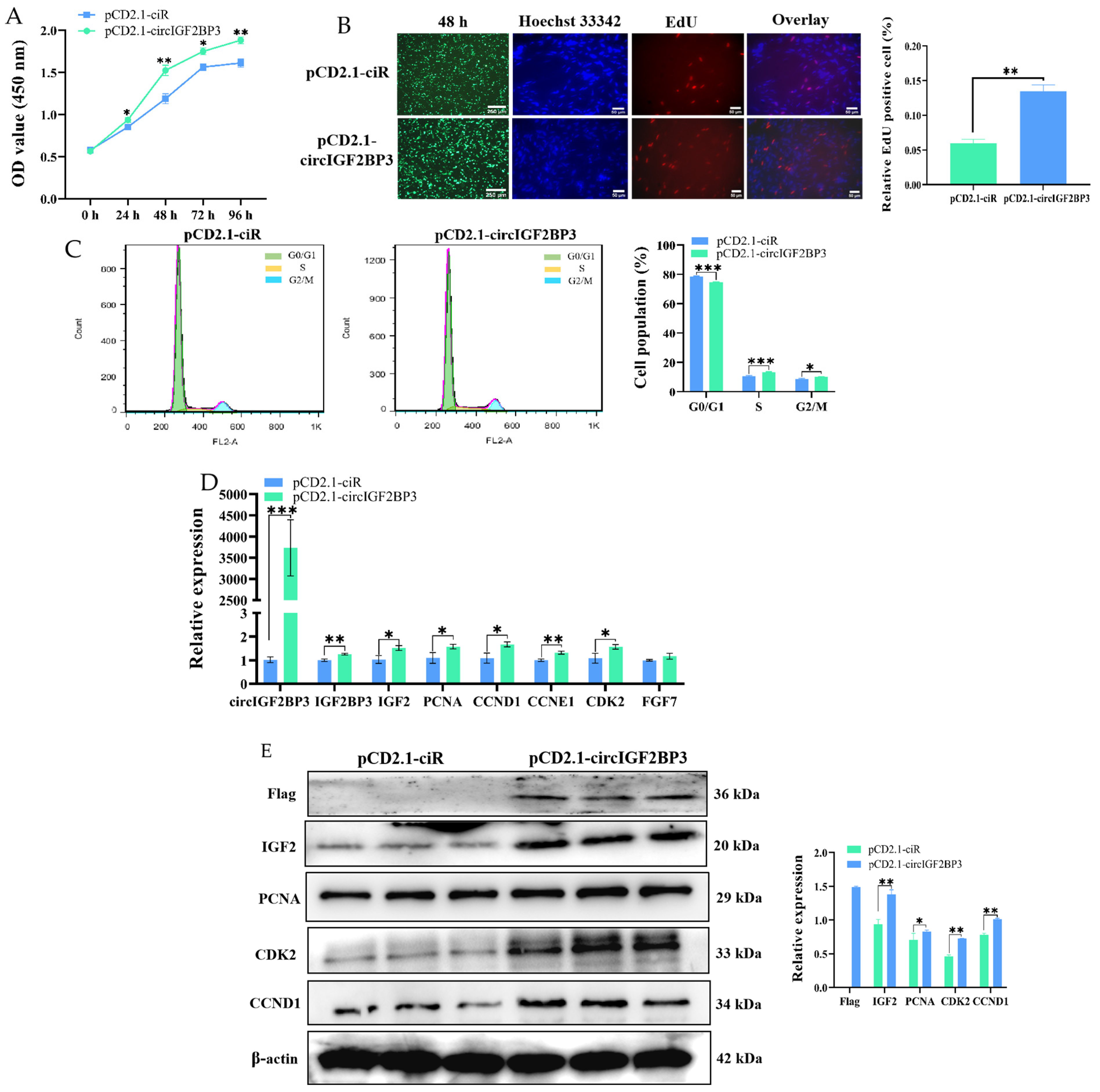
2.3.2. CircIGF2BP3 Promotes the Proliferation of Myoblasts
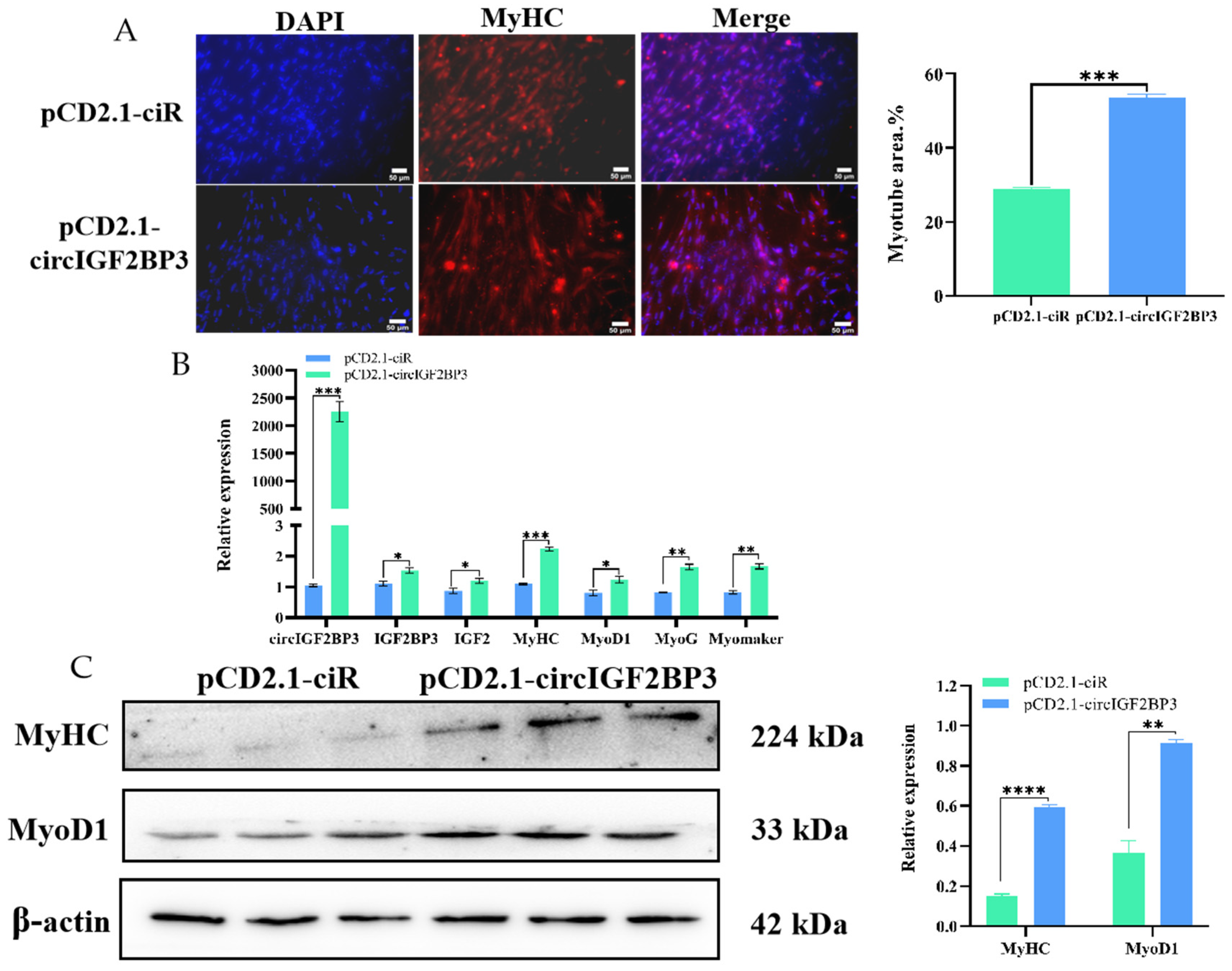
2.3.3. CircIGF2BP3 Promotes Myoblast Differentiation
3. Discussion
4. Materials and Methods
4.1. Ethics Standards
4.2. Animal
4.3. Full-Length Cloning and Validation of Loop Formation in CircIGF2BP3
4.4. Construction of Chicken CircIGF2BP3 Overexpression Vector
4.5. Cell Culture and Transfection
4.6. RNA Extraction and Quantitative Real-Time PCR (RT-PCR)
4.7. Nuclear-Cytoplasm Separation
4.8. CCK-8 Assay
4.9. EdU Assay
4.10. Flow Cytometry
4.11. Immunofluorescence
4.12. Western Blot
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Wang, J.; Zheng, Y.; Zhang, J.; Chen, S.; Zhao, F. Comprehensive identification of internal structure and alternative splicing events in circular RNAs. Nat. Commun. 2016, 7, 12060. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tsukahara, T. A view of pre-mRNA splicing from RNase R resistant RNAs. Mol. Sci. 2014, 15, 9331–9342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Nie, L.; Ding, X.; Zhang, X.; Zhao, W.; Xu, X.; Kyei, B.; Dai, D.; Zhan, S.; et al. MyoD-induced circular RNA CDR1as promotes myogenic differentiation of skeletal muscle satellite cells. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 807–821. [Google Scholar] [CrossRef]
- Wang, X.; Cao, X.; Dong, D.; Shen, X.; Cheng, J.; Jiang, R.; Yang, Z.; Peng, S.; Huang, Y.; Lan, X.; et al. Circular RNA TTN Acts As a miR-432 Sponge to Facilitate Proliferation and Differentiation of Myoblasts via the IGF2/PI3K/AKT Signaling Pathway. Mol. Ther. Nucleic Acids 2019, 18, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.M.; Hugouvieux, V.; Nayak, A.; Conos, S.A.; Capovilla, G.; Cildir, G.; Jourdain, A.; Tergaonkar, V.; Schmid, M.; Zubieta, C.; et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 2017, 3, 17053. [Google Scholar] [CrossRef]
- Legnini, I.; Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. CircZNF609 is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Lin, C.; Zhang, J.; Shen, Z. Ranslation role of circRNAs in cancers. J. Clin. Lab. Anal. 2021, 35, 23866. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Zhou, Y.; Wang, Q.; Zheng, Z.; Xu, B.; Wu, C.; Zhou, Q.; Hu, W.; Wu, C.; et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol. Cancer 2019, 18, 47. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Nielsen, F.C.; Nielsen, J.; Christiansen, J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand. J. Clin. Lab. Investig. Suppl. 2001, 234, 93–99. [Google Scholar] [CrossRef]
- Suvasini, R.; Shruti, B.; Thota, B.; Shinde, S.V.; Friedmann-Morvinski, D.; Nawaz, Z.; Prasanna, K.V.; Thennarasu, K.; Hegde, A.S.; Arivazhagan, A.; et al. Insulin growth factor-2 binding protein 3 (IGF2BP3) is a glioblastoma-specific marker that activates phosphatidylinositol 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) pathways by modulating IGF-2. J. Biol. Chem. 2011, 286, 25882–25890. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Yan, J.; Chen, M.; Zhu, M.; Tang, Y.; Liu, S.; Tang, Z. A comprehensive epigenome atlas reveals DNA methylation regulating skeletal muscle development. Nucleic Acids Res. 2021, 49, 1313–1329. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, S.; Mu, H.; Tang, X.; Ou, Y.; Chen, J.; Ma, Y.; Li, Y. Analysis of differentially expressed genes and signaling pathways related to intramuscular fat deposition in skeletal muscle of sex-linked dwarf chickens. Biomed Res. Int. 2014, 2014, 724274. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Shen, X.; Zhao, J.; Cao, X.; Han, S.; Cui, C.; Chen, Y.; Wei, Y.; Xia, L.; et al. miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1655. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Das, D.; Abdelmohsen, K.; Panda, A.C. Circular RNAs in myogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, T.; She, Y.; Wu, K.; Gu, S.; Li, L.; Dong, C.; Chen, C.; Zhou, Y. N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer 2021, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, W.; Li, X.; Zhang, J.; Chen, S.; Zhang, J.L.; Yang, L.; Chen, L.L. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016, 15, 611–624. [Google Scholar] [CrossRef]
- Dong, R.; Ma, X.K.; Chen, L.L.; Yang, L. Increased complexity of circRNA expression during species evolution. RNA Biol. 2017, 14, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, Y.; Fan, X.; Liang, G.; Wang, Z.; Li, J.; Wang, L.; Chen, Y.; Adetula, A.A.; Tang, Y.; et al. circRNAome profiling reveals circFgfr2 regulates myogenesis and muscle regeneration via a feedback loop. J. Cachexia Sarcopenia Muscle 2022, 13, 696–712. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, W.; Hu, X.; Mao, H.; Liu, S.; Chen, B. Transcriptome RNA Sequencing Reveals That Circular RNAs Are Abundantly Expressed in Embryonic Breast Muscle of Duck. Vet. Sci. 2023, 10, 75. [Google Scholar] [CrossRef]
- Muroya, S.; Tanabe, R.; Nakajima, I.; Chikuni, K. Molecular characteristics and site specific distribution of the pigment of the silky fowl. J. Vet. Med. Sci. 2000, 62, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, A.; Wang, Q.; Wang, H.; Dong, D.; Liu, L. Transcriptome analysis of embryonic muscle development in Chengkou Mountain Chicken. BMC Genom. 2021, 22, 431. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, K.; Zhang, J.; Ling, X.; Zhang, X.; Zhang, L.; Li, P.; Wei, Q.; Zhang, T.; Wang, X.; et al. Identification of crucial circRNAs in skeletal muscle during chicken embryonic development. BMC Genom. 2022, 23, 330. [Google Scholar] [CrossRef]
- Ouyang, H.; Chen, X.; Li, W.; Li, Z.; Nie, Q.; Zhang, X. Circular RNA circSVIL Promotes Myoblast Proliferation and Differentiation by Sponging miR-203 in Chicken. Front. Genet. 2018, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ouyang, H.; Wang, Z.; Chen, B.; Nie, Q. A Novel Circular RNA Generated by FGFR2 Gene Promotes Myoblast Proliferation and Differentiation by Sponging miR-133a-5p and miR-29b-1-5p. Cells 2018, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wei, Y.; Liu, W.; You, G.; Tang, S.; Su, Z.; Du, M.; He, J.; Zhao, J.; Tian, Y.; et al. A Novel Circular RNA circITSN2 Targets the miR-218-5p/LMO7 Axis to Promote Chicken Embryonic Myoblast Proliferation and Differentiation. Front. Cell Dev. Biol. 2021, 9, 748844. [Google Scholar] [CrossRef]
- Shen, X.; Wei, Y.; You, G.; Liu, W.; Amevor, F.K.; Zhang, Y.; He, H.; Ma, M.; Zhang, Y.; Li, D.; et al. Circular PPP1R13B RNA Promotes Chicken Skeletal Muscle Satellite Cell Proliferation and Differentiation via Targeting miR-9-5p. Animals 2021, 11, 2396. [Google Scholar] [CrossRef]
- Yin, H.; Shen, X.; Zhao, J.; Cao, X.; He, H.; Han, S.; Chen, Y.; Cui, C.; Wei, Y.; Wang, Y.; et al. Circular RNA CircFAM188B Encodes a Protein That Regulates Proliferation and Differentiation of Chicken Skeletal Muscle Satellite Cells. Front. Cell Dev. Biol. 2020, 8, 522588. [Google Scholar] [CrossRef]
- Cai, C.K.; Zhao, G.Y.; Tian, L.Y.; Liu, L.; Yan, K.; Ma, Y.L.; Ji, Z.W.; Li, X.X.; Han, K.; Gao, J.; et al. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol. Rep. 2012, 28, 1764–1770. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, Y.; Yin, X.; Li, T.; Chen, F.; Wu, P.; Zhang, S.; Wang, J.; Zhang, G. MicroRNA-214 Inhibits Chicken Myoblasts Proliferation, Promotes Their Differentiation, and Targets the TRMT61A Gene. Genes 2020, 11, 1400. [Google Scholar] [CrossRef]

| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Annealing Temperature (°C) | Product Length (bp) |
|---|---|---|---|---|
| circIGF2BP3-C | CTGAATGCCTTGGGTCTGTTCC | GGCCCCTCTGTCCAAATCCA | 64.5 | 811 |
| circIGF2BP3-full | CCCAAATGGTGGATATGAAG | AGCAATAGAAAAACTGAACG | 58 | 236 |
| β-actin | ACTGACCGCGTTACTCCC | GCAACCATCACACCCTGATGTC | 60 | 165 |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Annealing Temperature (°C) | Product Length (bp) |
|---|---|---|---|---|
| circIGF2BP3 | TCTGAATGCCTTGGGTCTGTT | GTCCAAATCCACGTCGTCCC | 64.5 | 228 |
| IGF2BP3-201 | CTGCTGCTGCTTCATATCCACCATTT | CTCAGCTTGGCATCTGGTCCTTC | 60 | 184 |
| IGF2-202 | CTGGCCTATGCGTTGGATTCAG | GTTGATCCTCCTGTTATTTCGTCCC | 60 | 147 |
| PCNA | CTCTGAGGGCTTCGACACCT | ATCCGCATTGTCTTCTGCTCT | 60 | 133 |
| CCND1 | AACCCACCTTCCATGATCGC | CTGTTCTTGGCAGGCTCGTA | 60 | 168 |
| CCNE1 | TTACGCTGTCCCCTGTTGAC | CCCAATTCCCACGCTACACT | 60 | 265 |
| CDK2 | GTACAAGGCCCGGAACAAGG | TTCTCCGTGTGGATCACGTC | 60 | 159 |
| FGF7 | AAAGACAGAAGGCAGGTCGG | TGCAAGCTAAAGATATAGTGCCCA | 60 | 193 |
| MyoD1 | GCTACTACACGGAATCACCAAAT | CTGGGCTCCACTGTCACTCA | 58 | 200 |
| MyoG | CGGAGGCTGAAGAAGGTGAA | CGGTCCTCTGCCTGGTCAT | 60 | 320 |
| MyHC | CTCCTCACGCTTTGGTAA | TGATAGTCGTATGGGTTGGT | 54 | 213 |
| Myomaker | TGGGTGTCCCTGATGGC | CCCGATGGGTCCTGAGTAG | 60 | 135 |
| Sno-U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT | 60 | 222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lin, J.; Jiao, Z.; Zhang, L.; Guo, D.; An, L.; Xie, T.; Lin, S. Circular RNA circIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts. Int. J. Mol. Sci. 2023, 24, 15545. https://doi.org/10.3390/ijms242115545
Wang X, Lin J, Jiao Z, Zhang L, Guo D, An L, Xie T, Lin S. Circular RNA circIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts. International Journal of Molecular Sciences. 2023; 24(21):15545. https://doi.org/10.3390/ijms242115545
Chicago/Turabian StyleWang, Xiaotong, Junyuan Lin, Zhenhai Jiao, Li Zhang, Dongxue Guo, Lilong An, Tingting Xie, and Shudai Lin. 2023. "Circular RNA circIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts" International Journal of Molecular Sciences 24, no. 21: 15545. https://doi.org/10.3390/ijms242115545
APA StyleWang, X., Lin, J., Jiao, Z., Zhang, L., Guo, D., An, L., Xie, T., & Lin, S. (2023). Circular RNA circIGF2BP3 Promotes the Proliferation and Differentiation of Chicken Primary Myoblasts. International Journal of Molecular Sciences, 24(21), 15545. https://doi.org/10.3390/ijms242115545





