Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of Mesenchymal Stromal Cells from Bone Marrow and Perinatal Tissues
2.2. Definition of Culture Conditions for Hematopoietic Stem and Progenitor Cell Expansion
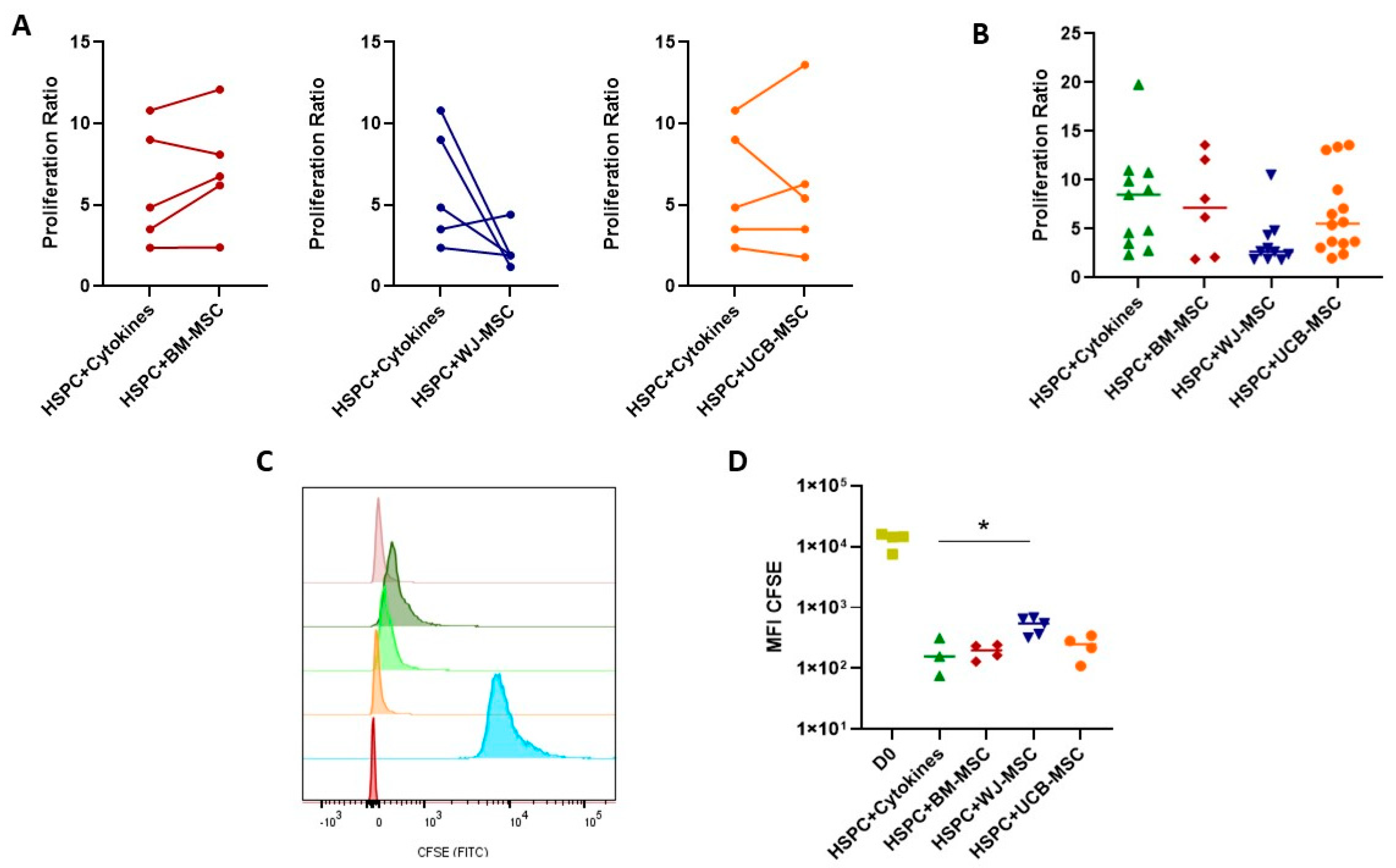
2.3. Evaluation of the Supportive Capacity of Perinatal Mesenchymal Stromal Cells in Hematopoietic Stem- and Progenitor-Cell Proliferation
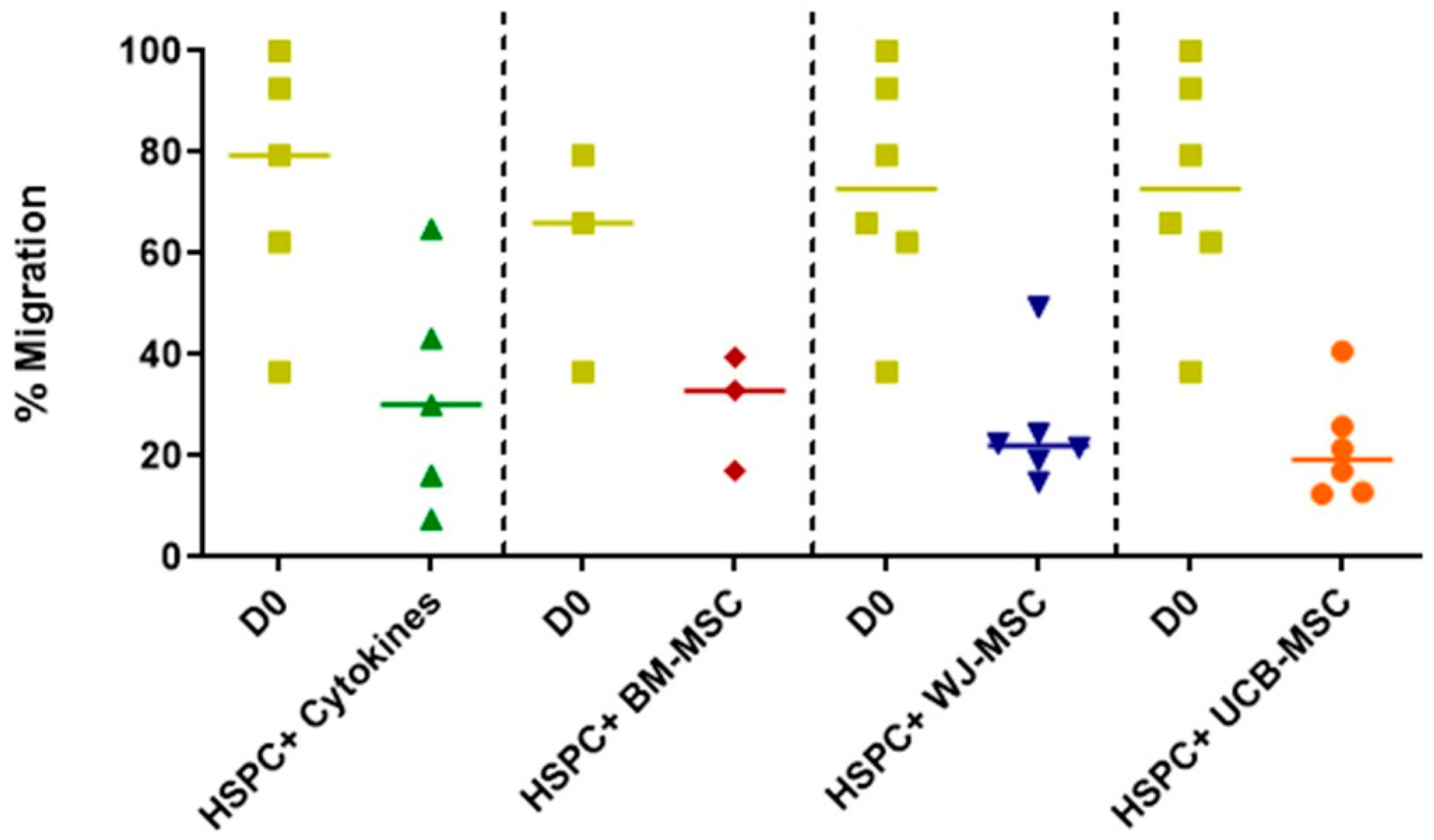
2.4. Evaluation of the Migratory Profile of Hematopoietic Stem and Progenitor Cells in Co-Culture with Mesenchymal Stromal Cells from Perinatal Tissues
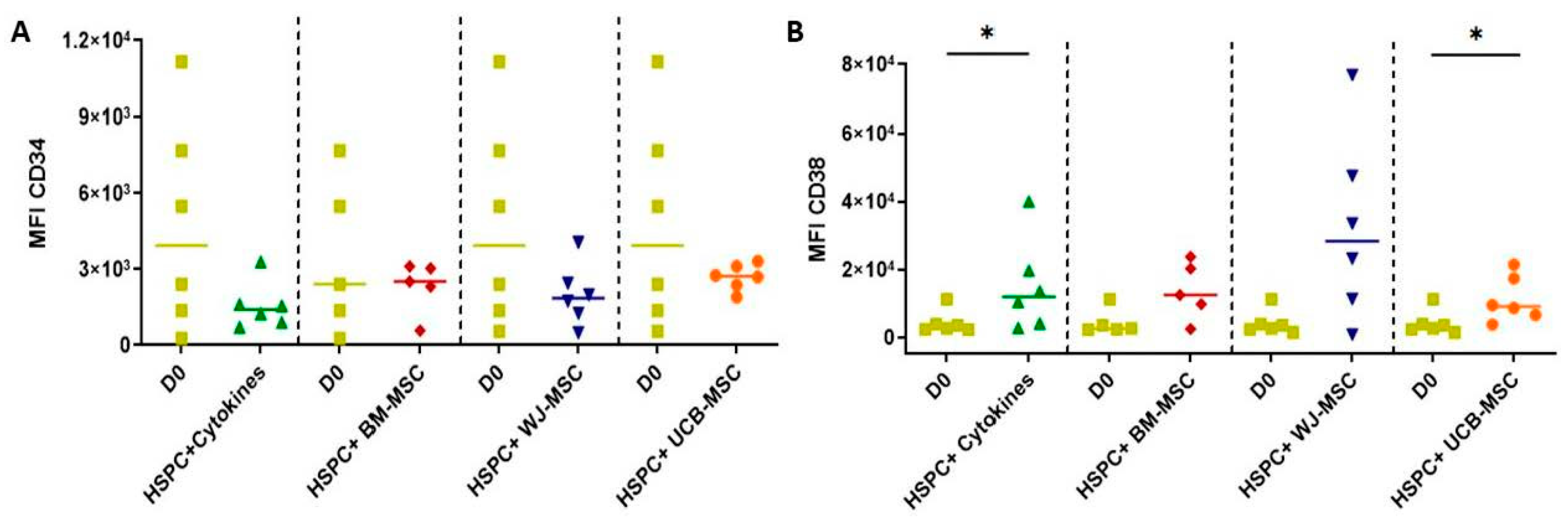
2.5. Determination of the Expression of Primitive and Lineage-Committed Markers in Hematopoietic Stem Progenitor Cells Cultured with Mesenchymal Stromal Cells from Perinatal Tissues
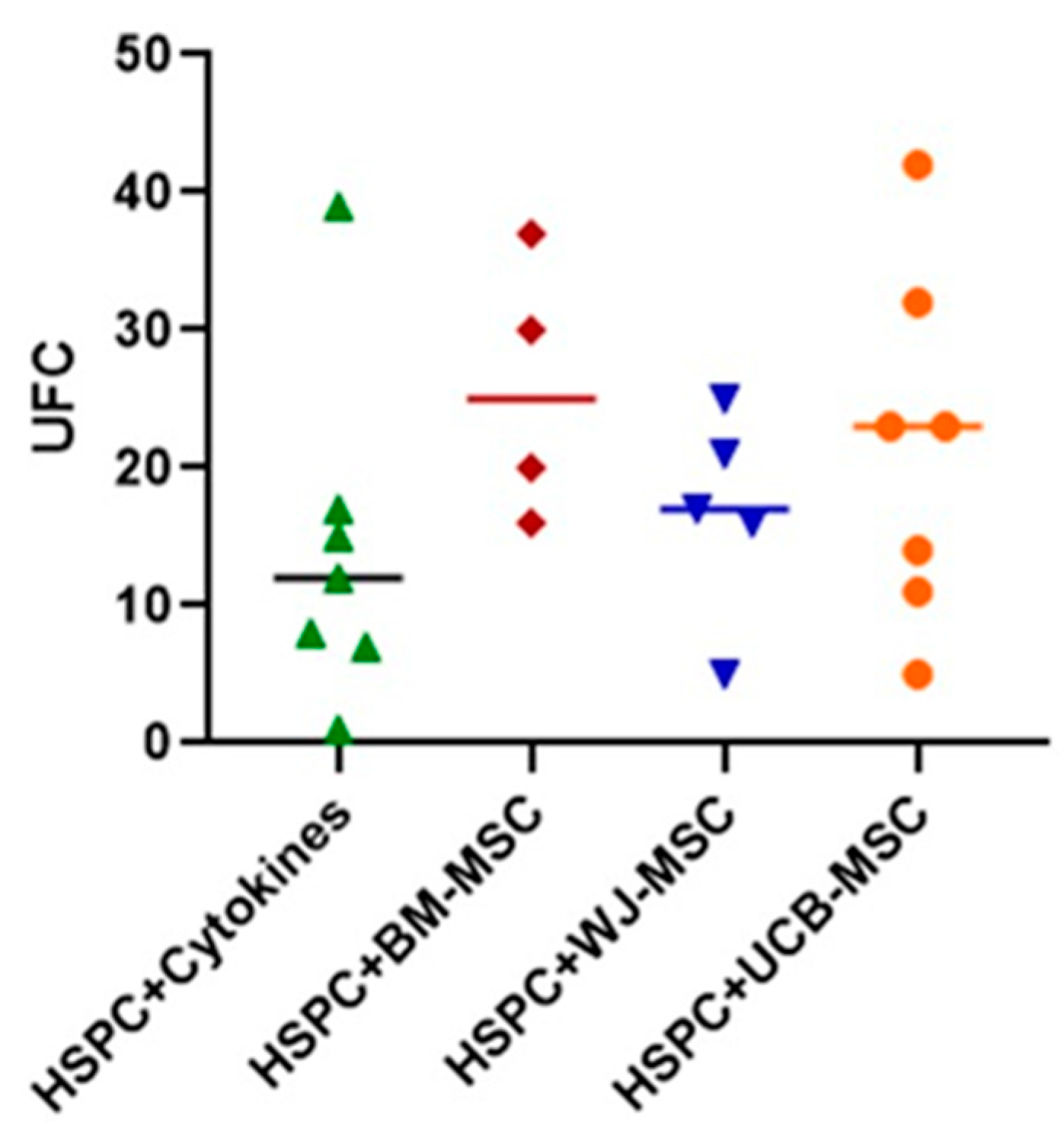
2.6. Evaluation of the Clonogenic Potential of Hematopoietic Stem Progenitor Cells in Co-Culture with Mesenchymal Stromal Cells from Perinatal Tissues
2.7. Evaluation of the Secretory Profile of Hematopoietic Stem Cells in Co-Culture with Mesenchymal Stromal Cells from Perinatal Tissues
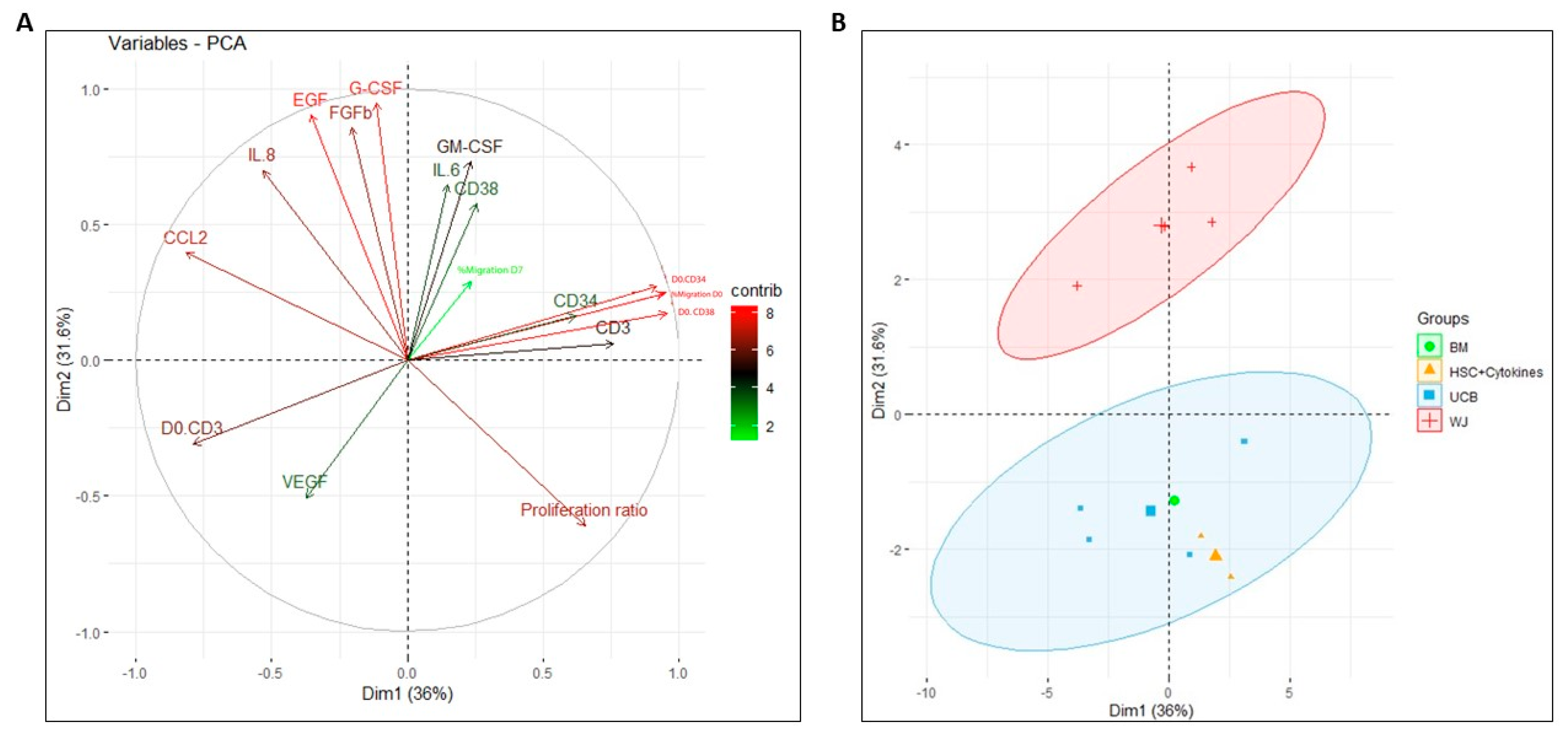
2.8. Principal Component Analysis for Assessing the Impact on Functionality Variables of HSPCs during Co-Culture with Perinatal-Tissue-Derived MSCs
3. Discussion
4. Materials and Methods
4.1. Donor Selection
4.2. Isolation of Cell Lines from BM MSCs
4.3. Isolation of Cell Lines from WJ MSCs
4.4. Isolation of Cell Lines from UCB MSCs
4.5. Immunophenotypic Characterization of MSCs
4.6. CD34+ HSPCs Immunomagnetic Isolation
4.7. Irradiation of MSCs
4.8. Co-Culture of MSCs with HSPCs
4.9. Assessment of Proliferation Capacity
4.10. Evaluation of Migration Capacity
4.11. Determination of Differentiation Capacity
4.12. Determination of Clonogenic Potential
4.13. Identification and Quantification of Cytokines in MSC Monoculture and Co-Culture Conditioned Media
4.14. Variable Correlation Analysis
4.15. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emiloju, O.E.; Potdar, R.; Jorge, V.; Gupta, S.; Varadi, G. Clinical Advancement and Challenges of Ex Vivo Expansion of Human Cord Blood Cells. Clin. Hematol. Int. 2019, 2, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.C.; Boitano, A.E.; Delaney, C.S.; Shpall, E.J.; Wagner, J.E. Advances in Umbilical Cord Blood Manipulation—From Niche to Bedside. Nat. Rev. Clin. Oncol. 2015, 12, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic Stem Cell Activity and Interactions with the Niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E. Current Status of Umbilical Cord Blood Hematopoietic Stem Cell Transplantation. Exp. Hematol. 2000, 28, 1197–1205. [Google Scholar] [CrossRef]
- MacMillan, M.L.; Robin, M.; Harris, A.C.; DeFor, T.E.; Martin, P.J.; Alousi, A.; Ho, V.T.; Bolaños-Meade, J.; Ferrara, J.L.M.; Jones, R.; et al. A Refined Risk Score for Acute Graft-versus-Host Disease That Predicts Response to Initial Therapy, Survival, and Transplant-Related Mortality. Biol. Blood Marrow Transpl. 2015, 21, 761–767. [Google Scholar] [CrossRef]
- Gounari, E.; Daniilidis, A.; Tsagias, N.; Michopoulou, A.; Kouzi, K.; Koliakos, G. Isolation of a Novel Embryonic Stem Cell Cord Blood–Derived Population with in Vitro Hematopoietic Capacity in the Presence of Wharton’s Jelly–Derived Mesenchymal Stromal Cells. Cytotherapy 2019, 21, 246–259. [Google Scholar] [CrossRef]
- de Lima, M.; McNiece, I.; Robinson, S.N.; Munsell, M.; Eapen, M.; Horowitz, M.; Alousi, A.; Saliba, R.; McMannis, J.D.; Kaur, I.; et al. Cord-Blood Engraftment with Ex Vivo Mesenchymal-Cell Coculture. N. Engl. J. Med. 2012, 367, 2305–2315. [Google Scholar] [CrossRef]
- Metheny, L.; Caimi, P.; de Lima, M. Cord Blood Transplantation: Can We Make It Better? Front. Oncol. 2013, 3, 238. [Google Scholar] [CrossRef]
- Eapen, M. A Resurgence of Cord Blood Transplantation? Lancet Haematol. 2020, 7, e89–e90. [Google Scholar] [CrossRef]
- Hofmeister, C.C.; Zhang, J.; Knight, K.L.; Le, P.; Stiff, P.J. Ex Vivo Expansion of Umbilical Cord Blood Stem Cells for Transplantation: Growing Knowledge from the Hematopoietic Niche. Bone Marrow Transpl. 2007, 39, 11–23. [Google Scholar] [CrossRef]
- Laughlin, M.J.; Eapen, M.; Rubinstein, P.; Wagner, J.E.; Zhang, M.-J.; Champlin, R.E.; Stevens, C.; Barker, J.N.; Gale, R.P.; Lazarus, H.M.; et al. Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia. N. Engl. J. Med. 2004, 351, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Choi, M.; Wnendt, S.; Kraus, M.; Teng, E.; Leong, H.F.; Merchav, S. Enhanced In Vivo Homing of Uncultured and Selectively Amplified Cord Blood CD34+ Cells by Cotransplantation with Cord Blood-Derived Unrestricted Somatic Stem Cells. Stem Cells 2007, 25, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Orticelli, V.; Papait, A.; Vertua, E.; Bonassi Signoroni, P.; Romele, P.; Di Pietro, L.; Magatti, M.; Teofili, L.; Silini, A.R.; Parolini, O. Human Amniotic Mesenchymal Stromal Cells Support the Ex Vivo Expansion of Cord Blood Hematopoietic Stem Cells. Stem Cells Transl. Med. 2021, 10, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.N.; Ng, J.; Niu, T.; Yang, H.; McMannis, J.D.; Karandish, S.; Kaur, I.; Fu, P.; Del Angel, M.; Messinger, R.; et al. Superior Ex Vivo Cord Blood Expansion Following Co-Culture with Bone Marrow-Derived Mesenchymal Stem Cells. Bone Marrow Transpl. 2006, 37, 359–366. [Google Scholar] [CrossRef]
- Mendelson, A.; Frenette, P.S. Hematopoietic Stem Cell Niche Maintenance during Homeostasis and Regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef]
- Schofield, R. The Relationship between the Spleen Colony-Forming Cell and the Haemopoietic Stem Cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Zimran, E.; Papa, L.; Hoffman, R. Ex Vivo Expansion of Hematopoietic Stem Cells: Finally Transitioning from the Lab to the Clinic. Blood Rev. 2021, 50, 100853. [Google Scholar] [CrossRef]
- Boulais, P.E.; Frenette, P.S. Making Sense of Hematopoietic Stem Cell Niches. Blood 2015, 125, 2621–2629. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Frausin, S.; Viventi, S.; Verga Falzacappa, L.; Quattromani, M.J.; Leanza, G.; Tommasini, A.; Valencic, E. Wharton’s Jelly Derived Mesenchymal Stromal Cells: Biological Properties, Induction of Neuronal Phenotype and Current Applications in Neurodegeneration Research. Acta Histochem. 2015, 117, 329–338. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological Characterization of Multipotent Mesenchymal Stromal Cells—The International Society for Cellular Therapy (ISCT) Working Proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Lo Iacono, M.; Baiamonte, E.; Di Stefano, R.; Spina, B.; Sacco, M.; Di Maggio, R.; Vitrano, A.; Maggio, A.; Acuto, S. Wharton’s Jelly—MSC As Effective Tool for Expansion of Purified Human Cord Blood CD34+ Hematopoietic Stem Cells. Blood 2015, 126, 4770. [Google Scholar] [CrossRef]
- Bakhshi, T.; Zabriskie, R.C.; Bodie, S.; Kidd, S.; Ramin, S.; Paganessi, L.A.; Gregory, S.A.; Fung, H.C.; Christopherson, K.W. Mesenchymal Stem Cells from the Wharton’s Jelly of Umbilical Cord Segments Provide Stromal Support for the Maintenance of Cord Blood Hematopoietic Stem Cells during Long-Term Ex Vivo Culture. Transfusion 2008, 48, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Gauthaman, K.; Cheyyatraivendran, S.; Lin, H.D.; Biswas, A.; Bongso, A. Human Umbilical Cord Wharton’s Jelly Stem Cells and Its Conditioned Medium Support Hematopoietic Stem Cell Expansion Ex Vivo. J. Cell. Biochem. 2012, 113, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Grau-Vorster, M.; Laitinen, A.; Nystedt, J.; Vives, J. HLA-DR Expression in Clinical-Grade Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells: A Two-Site Study. Stem Cell Res. Ther. 2019, 10, 164. [Google Scholar] [CrossRef]
- Nold, P.; Hackstein, H.; Riedlinger, T.; Kasper, C.; Neumann, A.; Mernberger, M.; Fölsch, C.; Schmitt, J.; Fuchs-Winkelmann, S.; Barckhausen, C.; et al. Immunosuppressive Capabilities of Mesenchymal Stromal Cells Are Maintained under Hypoxic Growth Conditions and after Gamma Irradiation. Cytotherapy 2015, 17, 152–162. [Google Scholar] [CrossRef]
- Preciado, S.; Muntión, S.; Rico, A.; Pérez-Romasanta, L.A.; Ramos, T.L.; Ortega, R.; Borrajo, J.; Corchete, L.A.; Rodríguez, C.; Díez-Campelo, M.; et al. Mesenchymal Stromal Cell Irradiation Interferes with the Adipogenic/Osteogenic Differentiation Balance and Improves Their Hematopoietic-Supporting Ability. Biol. Blood Marrow Transpl. 2018, 24, 443–451. [Google Scholar] [CrossRef]
- Breems, D.A.; Blokland, E.A.W.; Siebel, K.E.; Mayen, A.E.M.; Engels, L.J.A.; Ploemacher, R.E. Stroma-Contact Prevents Loss of Hematopoietic Stem Cell Quality During Ex Vivo Expansion of CD34+ Mobilized Peripheral Blood Stem Cells. Blood 1998, 91, 111–117. [Google Scholar] [CrossRef]
- Pineault, N.; Abu-Khader, A. Advances in Umbilical Cord Blood Stem Cell Expansion and Clinical Translation. Exp. Hematol. 2015, 43, 498–513. [Google Scholar] [CrossRef]
- Mobaraki, S.N.; Abroun, S.; Atashi, A.; Kaviani, S. Evaluation of Expansion and Maintenance of Umbilical Cord Blood CD34+ Cells in the Co-Culture with Umbilical Cord Blood-Derived Mesenchymal Stem Cells in The Presence of Microcarrier Beads. Cell J. 2023, 25, 184–193. [Google Scholar] [CrossRef]
- Hoggatt, J.; Pelus, L.M. Mobilization of Hematopoietic Stem Cells from the Bone Marrow Niche to the Blood Compartment. Stem Cell Res. Ther. 2011, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Shirvaikar, N.; Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem. Res. Int. 2012, 2012, 685267. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Ghanavat, M.; Shahrabi, S.; Gatavizadeh, Z.; Saki, N. The Effect of Inflammatory Factors and Their Inhibitors on the Hematopoietic Stem Cells Fate. Cell Biol. Int. 2021, 45, 900–912. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The Physical Microenvironment of Hematopoietic Stem Cells and Its Emerging Roles in Engineering Applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Djedaini, M.; Hoffman, R. Ex Vivo HSC Expansion Challenges the Paradigm of Unidirectional Human Hematopoiesis. Ann. N. Y. Acad. Sci. 2020, 1466, 39–50. [Google Scholar] [CrossRef]
- Crippa, S.; Bernardo, M.E. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere 2018, 2, e151. [Google Scholar] [CrossRef]
- Maeda, K.; Malykhin, A.; Teague-Weber, B.N.; Sun, X.-H.; Farris, A.D.; Coggeshall, K.M. Interleukin-6 Aborts Lymphopoiesis and Elevates Production of Myeloid Cells in Systemic Lupus Erythematosus-Prone B6.Sle1.Yaa Animals. Blood 2009, 113, 4534–4540. [Google Scholar] [CrossRef]
- Tie, R.; Li, H.; Cai, S.; Liang, Z.; Shan, W.; Wang, B.; Tan, Y.; Zheng, W.; Huang, H. Interleukin-6 Signaling Regulates Hematopoietic Stem Cell Emergence. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Su, Y.; Xu, C.; Cheng, W.; Zhao, Y.; Sui, L.; Zhao, Y. Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 1277. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic Stem Cell Self-Renewal in Vivo and Ex Vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Santi, L.; Bosotti, R.; Porro, G.; Bernardo, M.E. Bone Marrow-Derived Mesenchymal Stromal Cells: A Novel Target to Optimize Hematopoietic Stem Cell Transplantation Protocols in Hematological Malignancies and Rare Genetic Disorders. J. Clin. Med. 2019, 9, 2. [Google Scholar] [CrossRef]
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016, 1314709. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Kuchroo, P.; Dave, V.; Vijayan, A.; Viswanathan, C.; Ghosh, D. Paracrine Factors Secreted by Umbilical Cord-Derived Mesenchymal Stem Cells Induce Angiogenesis in Vitro by a VEGF-Independent Pathway. Stem Cells Dev. 2015, 24, 437–450. [Google Scholar] [CrossRef]
- Dooley, D.C.; Oppenlander, B.K.; Spurgin, P.; Mead, J.H.; Novak, F.P.; Plunkett, M.; Beckstead, J.; Heinrich, M.C. Basic Fibroblast Growth Factor and Epidermal Growth Factor Downmodulate the Growth of Hematopoietic Cells in Long-Term Stromal Cultures. J. Cell. Physiol. 1995, 165, 386–397. [Google Scholar] [CrossRef]
- Aqmasheh, S.; Shamsasanjan, K.; Akbarzadehlaleh, P.; Pashoutan Sarvar, D.; Timari, H. Effects of Mesenchymal Stem Cell Derivatives on Hematopoiesis and Hematopoietic Stem Cells. Adv. Pharm. Bull. 2017, 7, 165–177. [Google Scholar] [CrossRef]
- Crippa, S.; Santi, L.; Berti, M.; De Ponti, G.; Bernardo, M.E. Role of Ex Vivo Expanded Mesenchymal Stromal Cells in Determining Hematopoietic Stem Cell Transplantation Outcome. Front. Cell Dev. Biol. 2021, 9, 663316. [Google Scholar] [CrossRef]
- Kang, K.-W.; Lee, S.-J.; Kim, J.H.; Lee, B.-H.; Kim, S.J.; Park, Y.; Kim, B.S. Etoposide-Mediated Interleukin-8 Secretion from Bone Marrow Stromal Cells Induces Hematopoietic Stem Cell Mobilization. BMC Cancer 2020, 20, 619. [Google Scholar] [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, as Well as Their Bone Marrow Microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Panahi, M.; Saraygord-Afshari, N.; Taheri, N.; Bilici, M.; Jafari, D.; Alizadeh, E. The Secretome of Mesenchymal Stem Cells and Oxidative Stress: Challenges and Opportunities in Cell-Free Regenerative Medicine. Mol. Biol. Rep. 2021, 48, 5607–5619. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Agrawal, D.K. Hematopoietic Stem and Progenitor Cells in Inflammation and Allergy. Front. Immunol. 2013, 4, 428. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pretty Heatmaps CRAN—Package Pheatmap. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 28 June 2023).
- Kassambara, A. Package “factoextra” Type Package Title Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://github.com/kassambara/factoextra/issues (accessed on 28 June 2023).
- R Development Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 June 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, X.; Lara, A.M.; Llano-León, M.; López-González, D.A.; Hernández-Mejía, D.G.; Bustos, R.H.; Camacho-Rodríguez, B.; Perdomo-Arciniegas, A.-M. Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood. Int. J. Mol. Sci. 2023, 24, 15544. https://doi.org/10.3390/ijms242115544
Bonilla X, Lara AM, Llano-León M, López-González DA, Hernández-Mejía DG, Bustos RH, Camacho-Rodríguez B, Perdomo-Arciniegas A-M. Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood. International Journal of Molecular Sciences. 2023; 24(21):15544. https://doi.org/10.3390/ijms242115544
Chicago/Turabian StyleBonilla, Ximena, Ana Milena Lara, Manuela Llano-León, David A. López-González, David G. Hernández-Mejía, Rosa Helena Bustos, Bernardo Camacho-Rodríguez, and Ana-María Perdomo-Arciniegas. 2023. "Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood" International Journal of Molecular Sciences 24, no. 21: 15544. https://doi.org/10.3390/ijms242115544
APA StyleBonilla, X., Lara, A. M., Llano-León, M., López-González, D. A., Hernández-Mejía, D. G., Bustos, R. H., Camacho-Rodríguez, B., & Perdomo-Arciniegas, A.-M. (2023). Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood. International Journal of Molecular Sciences, 24(21), 15544. https://doi.org/10.3390/ijms242115544




