Neuroblastoma Patients’ Outcome and Chromosomal Instability
Abstract
:1. Introduction
2. Results
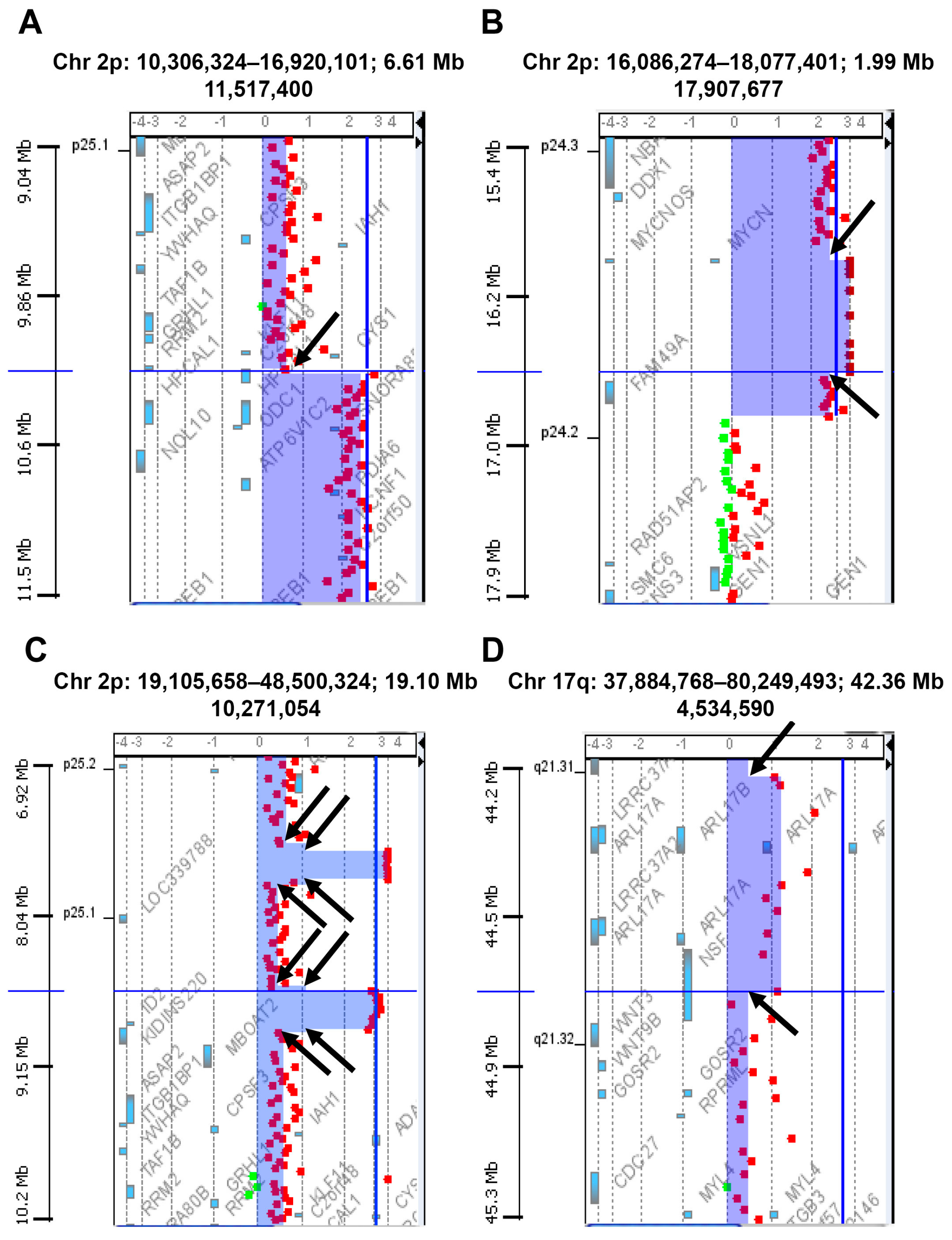
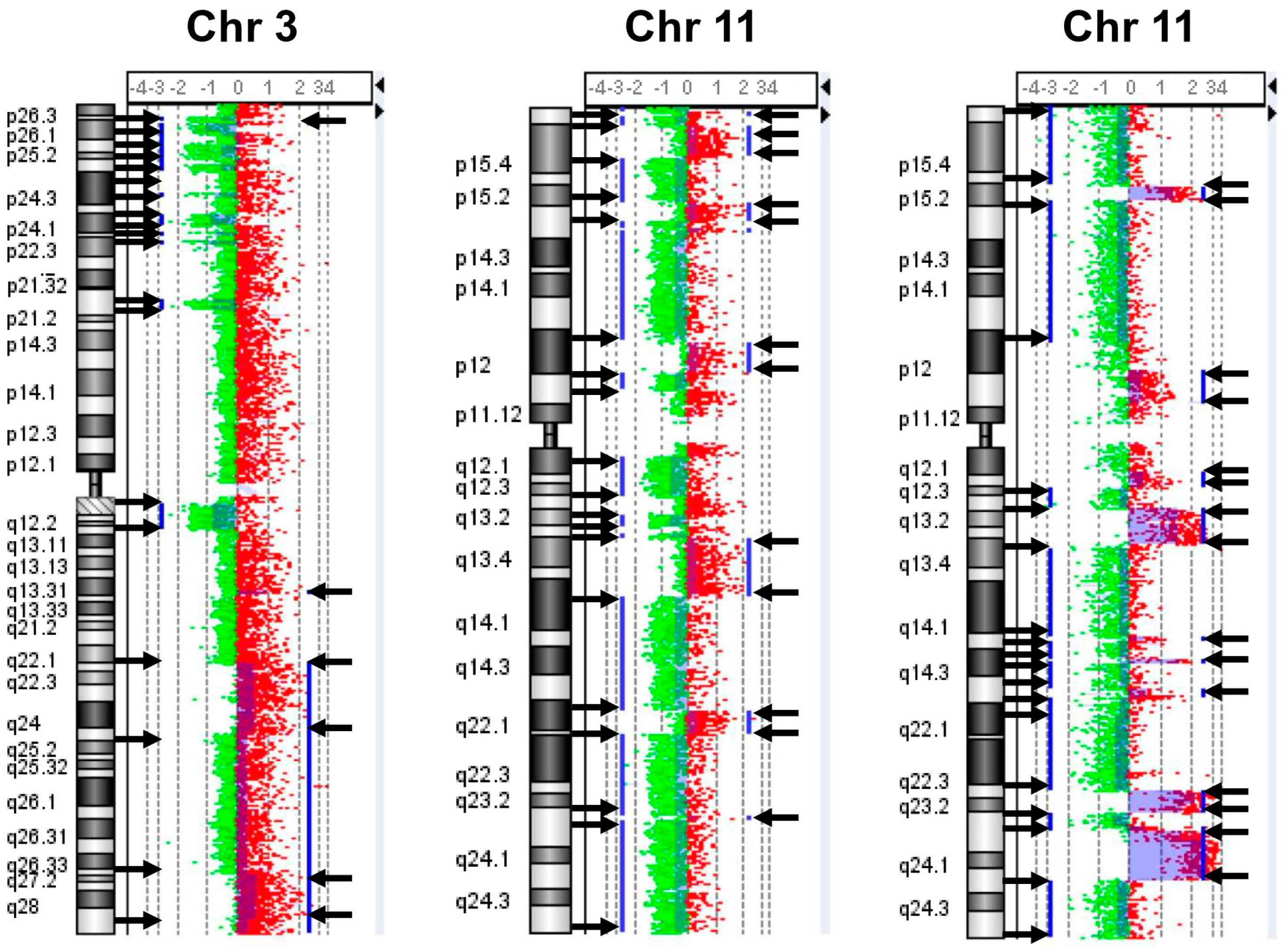
2.1. Chromosomal Instability (CIN) in Neuroblastoma Samples
2.2. Association between BPI Index and Main Prognostic Factors
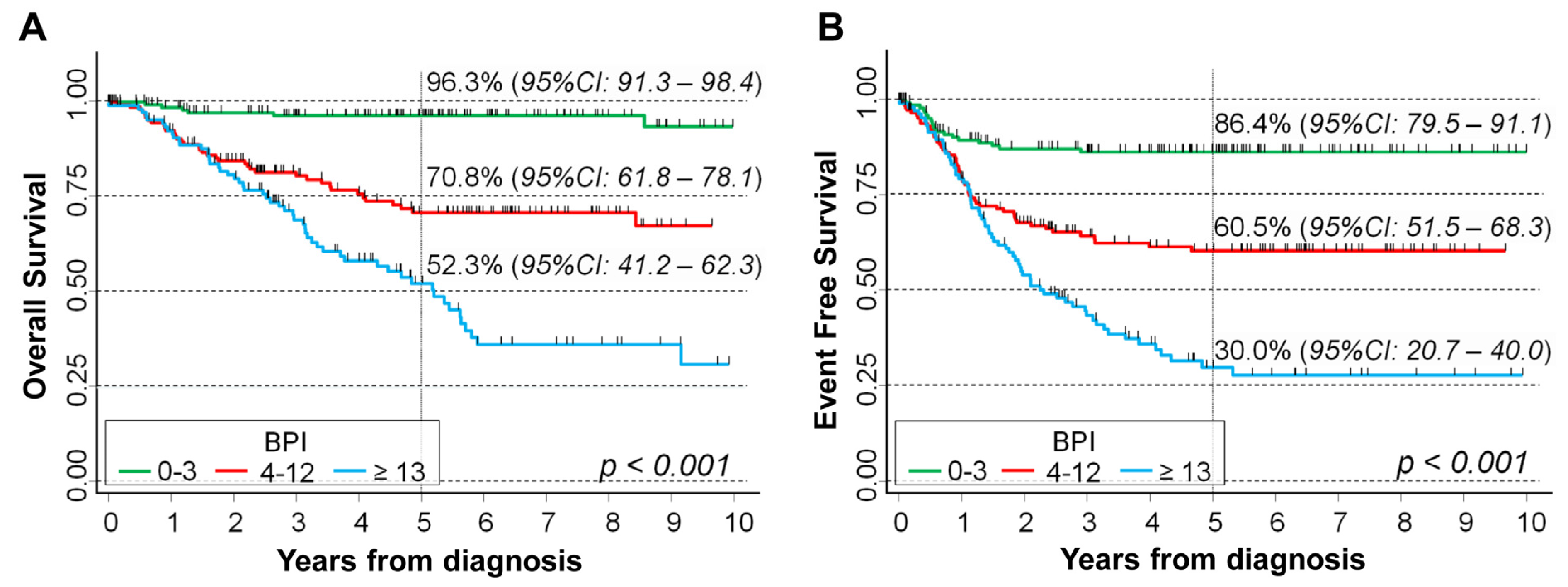
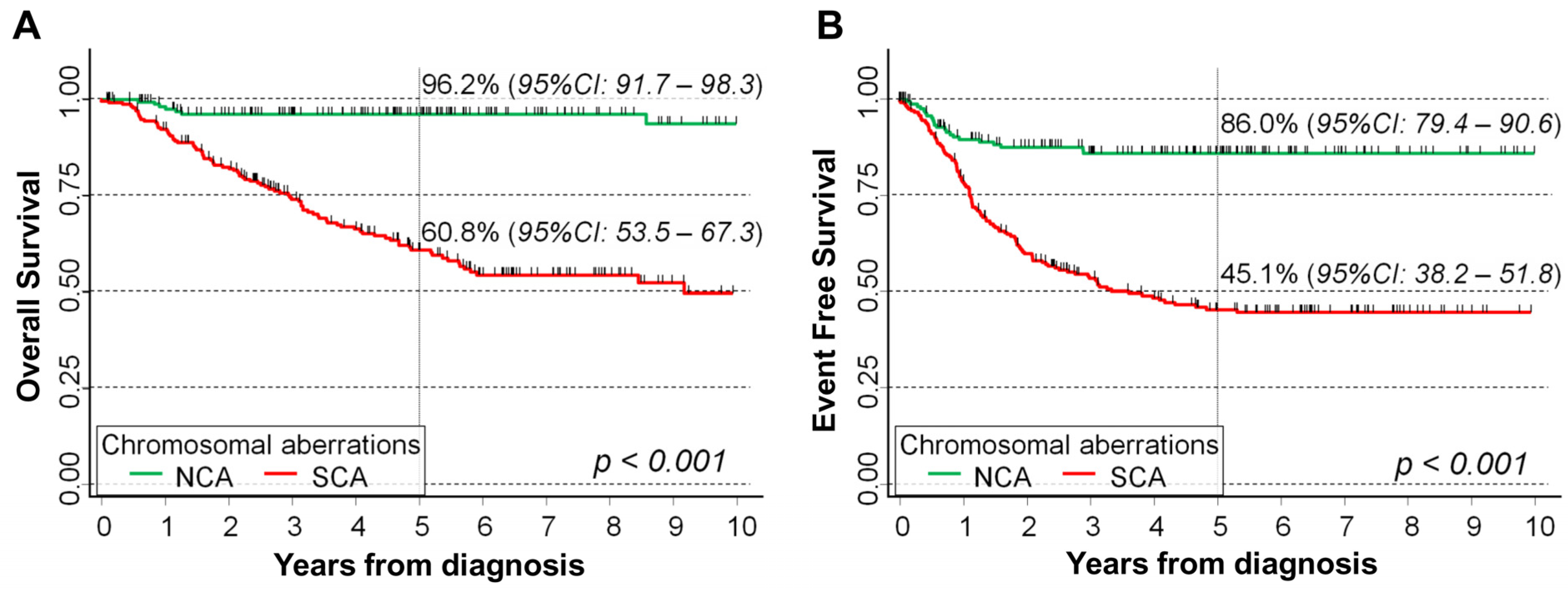
2.3. BPI Values and Survival of NB Patients
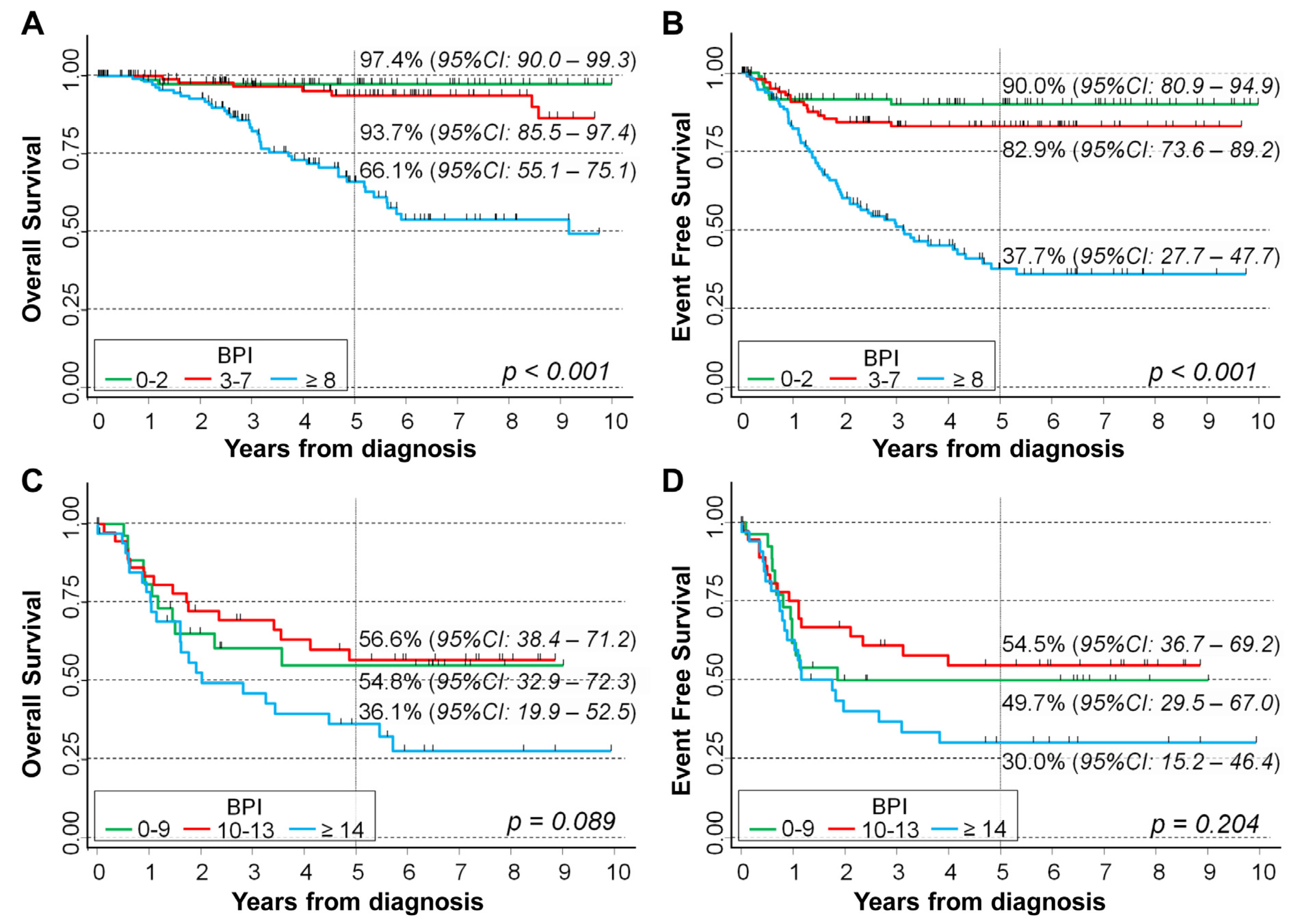
2.4. Assessing the Independent Potential Prognostic Role of BPI Index in Patients with Cromosome Abnormalities
3. Discussion
4. Materials and Methods
4.1. Neuroblastoma Samples
4.2. Genomic Profile Analysis
4.3. Patients’ Characteristics
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular pathogenesis and therapy. Ann. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma-A Neural Crest Derived Embryonal Malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, Z.; Wang, H.; Wang, L.; Li, T.; Xiao, R.; Xie, T.; Han, R.; Dong, R.; Liu, H.; et al. The super elongation complex drives transcriptional addiction in MYCN-amplified neuroblastoma. Sci. Adv. 2023, 9, eadf0005. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M. MYCN in neuronal tumours. Cancer Lett. 2004, 204, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Janoueix-Lerosey, I.; Schleiermacher, G.; Michels, E.; Mosseri, V.; Ribeiro, A.; Lequin, D.; Vermeulen, J.; Couturier, J.; Peuchmaur, M.; Valent, A.; et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J. Clin. Oncol. 2009, 27, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Pezzolo, A.; Rossi, E.; Gimelli, S.; Parodi, F.; Negri, F.; Conte, M.; Pistorio, A.; Sementa, A.; Pistoia, V.; Zuffardi, O.; et al. Presence of 1q gain and absence of 7p gain are new predictors of local or metastatic relapse in localized resectable neuroblastoma. Neuro. Oncol. 2009, 11, 192–200. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Janoueix-Lerosey, I.; Ribeiro, A.; Klijanienko, J.; Couturier, J.; Pierron, G.; Mosseri, V.; Valent, A.; Auger, N.; Plantaz, D.; et al. Accumulation of segmental alterations determines progression in neuroblastoma. J. Clin. Oncol. 2010, 28, 3122–3130. [Google Scholar] [CrossRef]
- Pezzolo, A.; Sementa, A.R.; Lerone, M.; Morini, M.; Ognibene, M.; Defferrari, R.; Mazzocco, K.; Conte, M.; Gigliotti, A.R.; Garaventa, A.; et al. Constitutional 3p26.3 terminal microdeletion in an adolescent with neuroblastoma. Cancer Biol. Ther. 2017, 18, 285–289. [Google Scholar] [CrossRef]
- Depuydt, P.; Boeva, V.; Hocking, T.D.; Cannoodt, R.; Ambros, I.M.; Ambros, P.F.; Asgharzadeh, S.; Attiyeh, E.F.; Combaret, V.; Defferrari, R.; et al. Genomic Amplifications and Distal 6q Loss: Novel Markers for Poor Survival in High-risk Neuroblastoma Patients. J. Natl. Cancer Inst. 2018, 110, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, L.; Ognibene, M.; Morini, M.; Conte, M.; Di Cataldo, A.; Tondo, A.; D’Angelo, P.; Castellano, A.; Garaventa, A.; Lasorsa, V.A.; et al. Genomic co-amplification of CDK4/MDM2/FRS2 is associated with very poor prognosis and atypical clinical features in neuroblastoma patients. Genes Chromosomes Cancer 2020, 59, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, V.A.; Cimmino, F.; Ognibene, M.; Mazzocco, K.; Erminio, G.; Morini, M.; Conte, M.; Iolascon, A.; Pezzolo, A.; Capasso, M. 19p loss is significantly enriched in older age neuroblastoma patients and correlates with poor prognosis. NPJ Genom. Med. 2020, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, M.; Morini, M.; Garaventa, A.; Podestà, M.; Pezzolo, A. Identification of a minimal region of loss on chromosome 6q27 associated with poor survival of high-risk neuroblastoma patients. Cancer Biol. Ther. 2020, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.H.; Eggert, A. Neuroblastoma. Crit. Rev. Oncog. 2015, 20, 245–270. [Google Scholar] [CrossRef]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef]
- Naranjo, A.; Irwin, M.S.; Hogarty, M.D.; Cohn, S.L.; Park, J.R.; London, W.B. Statistical Framework in Support of a Revised Children’s Oncology Group Neuroblastoma Risk Classification System. JCO Clin. Cancer Inform. 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef]
- Ackermann, S.; Cartolano, M.; Hero, B.; Welte, A.; Kahlert, Y.; Roderwieser, A.; Bartenhagen, C.; Walter, E.; Gecht, J.; Kerschke, L.; et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 2018, 362, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Koneru, B.; Lopez, G.; Farooqi, A.; Conkrite, K.L.; Nguyen, T.H.; Macha, S.J.; Modi, A.; Rokita, J.L.; Urias, E.; Hindle, A.; et al. Telomere Maintenance Mechanisms Define Clinical Outcome in High-Risk Neuroblastoma. Cancer Res. 2020, 80, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Burrell, R.A.; Endesfelder, D.; Novelli, M.R.; Swanton, C. Cancer chromosomal instability: Therapeutic and diagnostic challenges. EMBO Rep. 2012, 13, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Compton, D.A. Chromosomal instability and cancer: A complex relationship with therapeutic potential. J. Clin. Invest. 2012, 122, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instability in colorectal cancers. Nature 1997, 386, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instabilities in human cancers. Nature 1998, 396, 643–649. [Google Scholar] [CrossRef]
- Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim. Biophys. Acta 2008, 1786, 32–40. [Google Scholar] [CrossRef]
- Thompson, S.L.; Compton, D.A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008, 180, 665–672. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Cantley, L.C. The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell 2018, 174, 1347–1360. [Google Scholar] [CrossRef]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139–150. [Google Scholar] [CrossRef]
- Gronroos, E.; López-García, C. Tolerance of Chromosomal Instability in Cancer: Mechanisms and Therapeutic Opportunities. Cancer Res. 2018, 78, 6529–6535. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McClelland, S.E.; Endesfelder, D.; Groth, P.; Weller, M.C.; Shaikh, N.; Domingo, E.; Kanu, N.; Dewhurst, S.M.; Gronroos, E.; et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 2013, 494, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.S.; Singh, A.T.K. Cell-cycle Checkpoints and Aneuploidy on the Path to Cancer. In Vivo 2018, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.; Said, M.; Naim, V. DNA Replication Stress and Chromosomal Instability: Dangerous Liaisons. Genes 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Amon, A. Context is everything: Aneuploidy in cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, B.; Berbegall, A.P.; Martin-Vañó, S.; Castel, V.; Navarro, S.; Noguera, R. Imbalance between genomic gain and loss identifies high-risk neuroblastoma patients with worse outcomes. Neoplasia 2021, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tonini, G.P.; Capasso, M. Genetic predisposition and chromosome instability in neuroblastoma. Cancer Metastasis Rev. 2020, 39, 275–285. [Google Scholar] [CrossRef]
- Cortés-Ciriano, I.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.Z.; Pellman, D.S.; et al. Comprehensive analysis of chromothripsis in 2658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef]
- Sansregret, L.; Swanton, C. The Role of Aneuploidy in Cancer Evolution. Cold Spring Harb Perspect. Med. 2017, 7, a028373. [Google Scholar] [CrossRef]
- Chunduri, N.K.; Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019, 21, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Thompson, S.L.; Manning, A.L.; Compton, D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hieronymus, H.; Murali, R.; Tin, A.; Yadav, K.; Abida, W.; Moller, H.; Berney, D.; Scher, H.; Carver, B.; Scardino, P.; et al. Tumor copy number alteration burden is a pan-cancer prognostic factor associated with recurrence and death. Elife 2018, 7, e37294. [Google Scholar] [CrossRef] [PubMed]
- Fusco, P.; Esposito, M.R.; Tonini, G.P. Chromosome instability in neuroblastoma. Oncol. Lett. 2018, 16, 6887–6894. [Google Scholar] [CrossRef] [PubMed]
- Bedekovics, T.; Hussain, S.; Zhang, Y.; Ali, A.; Jeon, Y.J.; Galardy, P.J. USP24 Is a Cancer-Associated Ubiquitin Hydrolase, Novel Tumor Suppressor, and Chromosome Instability Gene Deleted in Neuroblastoma. Cancer Res. 2021, 81, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Chromosomes and cancer cells. Chromosome Res. 2011, 19, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Guedj, M.; Jones, N.; Sfar, S.; Brouste, V.; Elarouci, N.; Banneau, G.; Orsetti, B.; Primois, C.; de Lara, C.T.; et al. An array CGH based genomic instability index (G2I) is predictive of clinical outcome in breast cancer and reveals a subset of tumors without lymph node involvement but with poor prognosis. BMC Med. Genom. 2012, 5, 54. [Google Scholar] [CrossRef]
- Przybytkowski, E.; Lenkiewicz, E.; Barrett, M.T.; Klein, K.; Nabavi, S.; Greenwood, C.M.; Basik, M. Chromosome-breakage genomic instability and chromothripsis in breast cancer. BMC Genom. 2014, 15, 579. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Koster, J.; Zwijnenburg, D.A.; van Sluis, P.; Valentijn, L.J.; van der Ploeg, I.; Hamdi, M.; van Nes, J.; Westerman, B.A.; van Arkel, J.; et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012, 483, 589–593. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Tubio, J.M.; Estivill, X. Cancer: When catastrophe strikes a cell. Nature 2011, 470, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.L.; Zhang, C.Z.; Pellman, D. Chromothripsis: A New Mechanism for Rapid Karyotype Evolution. Annu. Rev. Genet. 2015, 49, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Paolini, L.; Hussain, S.; Galardy, P.J. Chromosome instability in neuroblastoma: A pathway to aggressive disease. Front. Oncol. 2022, 12, 988972. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, M.; De Marco, P.; Parodi, S.; Meli, M.; Di Cataldo, A.; Zara, F.; Pezzolo, A. Genomic Analysis Made It Possible to Identify Gene-Driver Alterations Covering the Time Window between Diagnosis of Neuroblastoma 4S and the Progression to Stage 4. Int. J. Mol. Sci. 2022, 23, 6513. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; van der Burg, M.; Szuhai, K.; Kops, G.J.; Medema, R.H. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 2011, 333, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Haupt, R.; Garaventa, A.; Gambini, C.; Parodi, S.; Cangemi, G.; Casale, F.; Viscardi, E.; Bianchi, M.; Prete, A.; Jenkner, A.; et al. Improved survival of children with neuroblastoma between 1979 and 2005: A report of the Italian Neuroblastoma Registry. J. Clin. Oncol. 2010, 28, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Fu, J.; Vegesna, R.; Mao, Y.; Heathcock, L.E.; Torres-Garcia, W.; Ezhilarasan, R.; Wang, S.; McKenna, A.; Chin, L.; et al. A survey of intragenic breakpoints in glioblastoma identifies a distinct subset associated with poor survival. Genes Dev. 2013, 27, 1462–1472. [Google Scholar] [CrossRef]
- Smida, J.; Xu, H.; Zhang, Y.; Baumhoer, D.; Ribi, S.; Kovac, M.; von Luettichau, I.; Bielack, S.; O’Leary, V.B.; Leib-Mösch, C.; et al. Genome-wide analysis of somatic copy number alterations and chromosomal breakages in osteosarcoma. Int. J. Cancer. 2017, 141, 816–828. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Survival Analysis—Regression Modelling of Time to Event Data, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]

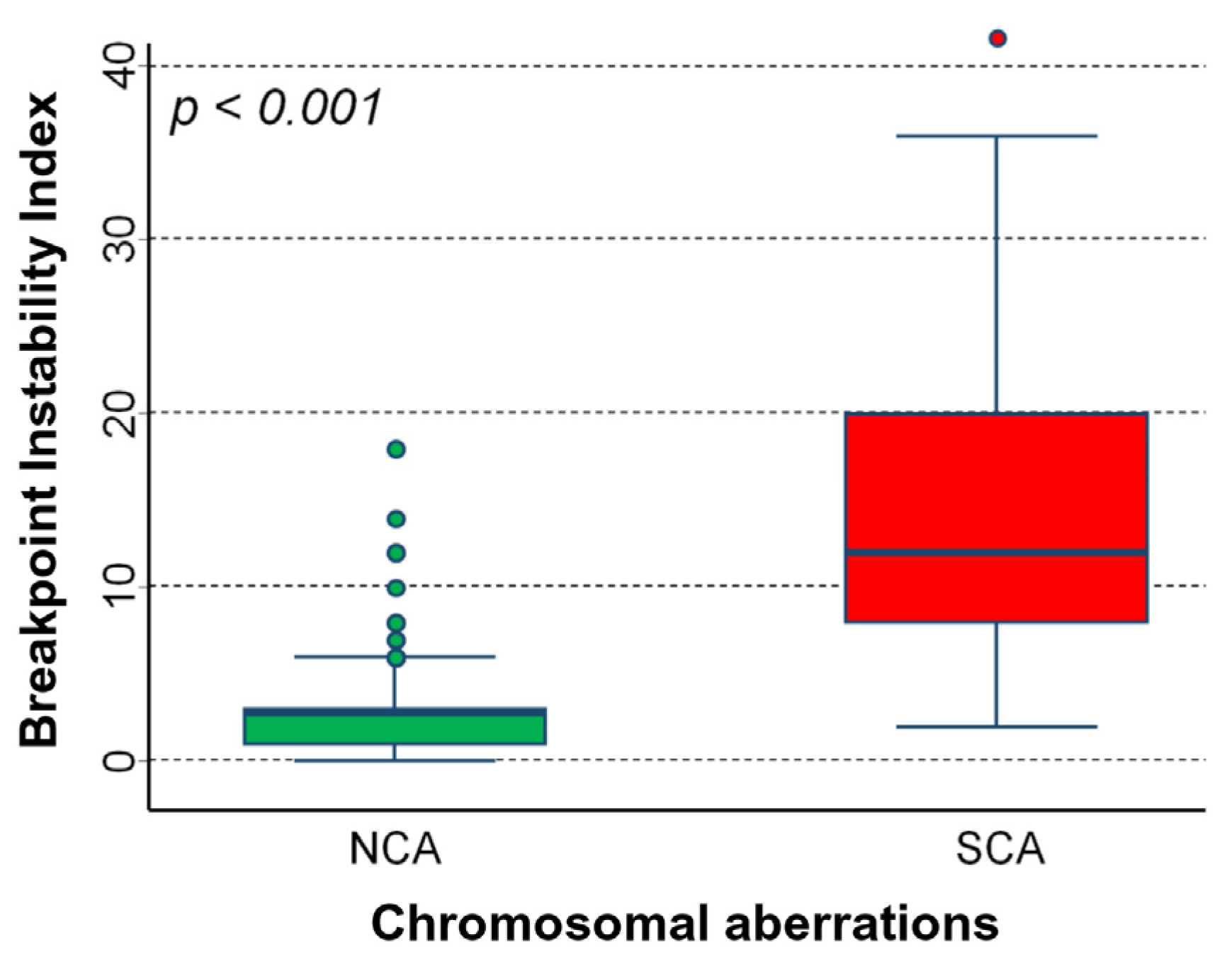

| Prognostic Factors | N | Median | IQR | p |
|---|---|---|---|---|
| Age at diagnosis | <0.001 | |||
| <18 months | 232 | 3 | 2–8 | |
| 18–59 months | 169 | 12 | 8–18 | |
| ≥60 months | 50 | 18 | 8–24 | |
| INRG Stage | <0.001 | |||
| L1 | 166 | 3 | 2–6 | |
| L2 | 81 | 4 | 3–12 | |
| M | 166 | 12 | 8–20 | |
| MS | 20 | 3.5 | 1–5.5 | |
| MYCN status | <0.001 | |||
| Non amplified | 328 | 4 | 2–12 | |
| Amplified | 100 | 12 | 8–16 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | <0.001 # | <0.001 # | |||||
| 0–3 | 167/6 | 1 (ref.) | - | 1 (ref.) | - | ||
| 4–12 | 160/39 | 7.5 | 3.2–17.8 | 2.6 | 0.99–6.7 | ||
| ≥13 | 124/55 | 16.5 | 7.1–38.4 | 5.0 | 2.0–13.0 | ||
| Original variable | 451/100 | 1.08 | 1.06–1.09 | <0.001 | 1.04 | 1.02–1.07 | 0.001 |
| Event Free Survival | <0.001 # | <0.001 # | |||||
| 0–3 | 167/19 | 1 (ref.) | - | 1 (ref.) | - | ||
| 4–12 | 160/55 | 3.4 | 2.0–5.7 | 2.3 | 1.2–4.3 | ||
| ≥13 | 124/69 | 6.4 | 3.8–10.6 | 3.8 | 1.9–7.3 | ||
| Original variable | 451/143 | 1.06 | 1.04–1.08 | <0.001 | 1.03 | 1.01–1.05 | 0.003 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | <0.001 # | 0.002 # | |||||
| 0–2 | 92/2 | 1 (ref.) | - | 1 (ref.) | - | ||
| 3–7 | 111/7 | 2.7 | 0.55–12.8 | 2.0 | 0.39–10.0 | ||
| ≥8 | 125/41 | 17.3 | 4.2–71.7 | 5.3 | 1.2–24.2 | ||
| Original variable | 328/50 | 1.10 | 1.07–1.12 | <0.001 | 1.05 | 1.02–1.08 | 0.002 |
| Event Free Survival | <0.001 # | <0.001 # | |||||
| 0–2 | 92/8 | 1 (ref.) | - | 1 (ref.) | - | ||
| 3–7 | 111/16 | 1.6 | 0.70–3.8 | 1.6 | 0.67–4.0 | ||
| ≥8 | 125/65 | 7.3 | 3.5–15.3 | 5.5 | 2.3–13.4 | ||
| Original variable | 328/89 | 1.07 | 1.05–1.09 | <0.001 | 1.04 | 1.02–1.07 | 0.001 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | 0.087 # | 0.122 # | |||||
| 0–9 | 27/11 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–13 | 37/15 | 0.87 | 0.40–1.9 | 0.85 | 0.38–1.9 | ||
| ≥14 | 36/22 | 1.7 | 0.84–3.6 | 1.7 | 0.81–3.6 | ||
| Original variable | 100/48 | 1.03 | 0.99–1.07 | 0.168 | 1.02 | 0.98–1.06 | 0.313 |
| Event Free Survival | 0.202 # | 0.264 # | |||||
| 0–9 | 27/13 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–13 | 37/16 | 0.79 | 0.38–1.7 | 0.75 | 0.35–1.6 | ||
| ≥14 | 36/22 | 1.5 | 0.74–2.9 | 0.329 | 1.4 | 0.70–2.9 | |
| Original variable | 100/51 | 1.02 | 0.98–1.05 | 0.329 | 1.01 | 0.97–1.05 | 0.558 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | <0.001 # | 0.002 # | |||||
| 0–2 | 84/2 | 1 (ref.) | - | 1 (ref.) | - | ||
| 3–5 | 80/2 | 1.0 | 0.14–7.1 | 1.1 | 0.16–7.9 | ||
| ≥6 | 83/24 | 11.9 | 2.8–50.4 | 6.3 | 1.4–28.6 | ||
| Original variable | 247/28 | 1.12 | 1.08–1.16 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
| Event Free Survival | <0.001 # | <0.001 # | |||||
| 0–2 | 84/7 | 1 (ref.) | - | 1 (ref.) | - | ||
| 3–5 | 80/8 | 1.2 | 0.43–3.3 | 1.2 | 0.44–3.4 | ||
| ≥6 | 83/35 | 6.0 | 2.7–13.6 | 5.5 | 2.3–13.3 | ||
| Original variable | 247/50 | 1.10 | 1.07–1.13 | <0.001 | 1.10 | 1.05–1.14 | <0.001 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | n.e. | n.e. | |||||
| 0–9 | 42/13 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–16 | 66/28 | 1.7 | 0.88–3.3 | 1.5 | 0.75–2.8 | ||
| ≥17 | 58/31 | 2.2 | 1.1–4.2 | 2.3 | 1.1–4.5 | ||
| Original variable | 166/72 | 1.02 | 1.00–1.05 | 0.077 | 1.02 | 1.00–1.05 | 0.090 |
| Event Free Survival | n.e. | n.e. | |||||
| 0–9 | 42/18 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–16 | 66/34 | 1.4 | 0.77–2.4 | 1.2 | 0.67–2.1 | ||
| ≥17 | 58/38 | 1.6 | 0.91–2.8 | 1.5 | 0.81–2.7 | ||
| Original variable | 166/90 | 1.01 | 0.99–1.04 | 0.257 | 1.00 | 0.98–1.03 | 0.464 |
| Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| BPI | N/O | HR | 95%CI | p | HR | 95%CI | p |
| Overall Survival | <0.001 # | 0.006 # | |||||
| 0–9 | 71/15 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–15 | 88/29 | 1.7 | 0.91–3.2 | 1.2 | 0.61–2.2 | ||
| ≥16 | 102/49 | 2.9 | 1.6–5.2 | 2.2 | 1.2–4.0 | ||
| Original variable | 261/93 | 1.04 | 1.02–1.06 | 0.001 | 1.03 | 1.00–1.06 | 0.049 |
| Event Free Survival | <0.001 # | 0.008 # | |||||
| 0–9 | 71/23 | 1 (ref.) | - | 1 (ref.) | - | ||
| 10–15 | 88/37 | 1.4 | 0.81–2.3 | 1.2 | 0.68–2.1 | ||
| ≥16 | 102/61 | 2.3 | 1.4–3.7 | 1.9 | 1.1–3.3 | ||
| Original variable | 261/121 | 1.03 | 1.01–1.05 | 0.003 | 1.02 | 1.00–1.05 | 0.074 |
| Patients’ Characteristics | N | % ‡ |
|---|---|---|
| Gender | ||
| Males | 248 | 55.0 |
| Females | 203 | 45.0 |
| Age at diagnosis | ||
| <18 months | 232 | 51.4 |
| 18–59 months | 169 | 37.5 |
| ≥60 months | 50 | 11.1 |
| INRG Stage | ||
| L1 | 166 | 38.3 |
| L2 | 81 | 18.7 |
| M | 166 | 38.3 |
| MS | 20 | 4.6 |
| Missing | 18 | 4.0 |
| MYCN status | ||
| Non amplified | 328 | 76.6 |
| Amplified | 100 | 23.4 |
| Missing | 23 | 5.1 |
| Events | 143 | 31.7 |
| Deaths | 100 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognibene, M.; De Marco, P.; Amoroso, L.; Fragola, M.; Zara, F.; Parodi, S.; Pezzolo, A. Neuroblastoma Patients’ Outcome and Chromosomal Instability. Int. J. Mol. Sci. 2023, 24, 15514. https://doi.org/10.3390/ijms242115514
Ognibene M, De Marco P, Amoroso L, Fragola M, Zara F, Parodi S, Pezzolo A. Neuroblastoma Patients’ Outcome and Chromosomal Instability. International Journal of Molecular Sciences. 2023; 24(21):15514. https://doi.org/10.3390/ijms242115514
Chicago/Turabian StyleOgnibene, Marzia, Patrizia De Marco, Loredana Amoroso, Martina Fragola, Federico Zara, Stefano Parodi, and Annalisa Pezzolo. 2023. "Neuroblastoma Patients’ Outcome and Chromosomal Instability" International Journal of Molecular Sciences 24, no. 21: 15514. https://doi.org/10.3390/ijms242115514
APA StyleOgnibene, M., De Marco, P., Amoroso, L., Fragola, M., Zara, F., Parodi, S., & Pezzolo, A. (2023). Neuroblastoma Patients’ Outcome and Chromosomal Instability. International Journal of Molecular Sciences, 24(21), 15514. https://doi.org/10.3390/ijms242115514






