Abstract
Edible berries such as the fruits of black chokeberry (Aronia melanocarpa (Michx.) Elliott) and bilberry (Vaccinium myrtillus L.) are considered to be rich in phenolic compounds, which are nowadays attracting great interest due to their promising health benefits. The main objective of our study was to investigate, for the first time, their inhibitory properties on Src tyrosine kinase activity, as this enzyme plays an important role in multiple cellular processes and is activated in both cancer and inflammatory cells. In hydroethanolic fruit extracts, 5.0–5.9% of total polyphenols were determined spectrophotometrically, including high amounts of hydroxycinnamic acid derivatives. HPLC analysis revealed that the black chokeberry and bilberry extracts contained 2.05 mg/g and 2.54 mg/g of chlorogenic acid, respectively. Using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay, the extracts studied were found to have comparable inhibitory effects on Src tyrosine kinase, with IC50 values of 366 µg/mL and 369 µg/mL, respectively. The results also indicated that chlorogenic acid contributes significantly to the observed effect. In addition, both fruit extracts exhibited antioxidant activity by scavenging DPPH and NO radicals with SC50 values of 153–352 µg/mL. Our study suggested that black chokeberry and bilberry fruits may be beneficial in cancer and other inflammation-related diseases.
1. Introduction
Berry fruits, their processed foods, and dietary supplements have become popular as a result of their promising health benefits. In addition to valuable nutrients, dark-colored berries are rich in bioactive compounds, of which the structurally diverse phenolic compounds are the most abundant. Numerous studies have shown that polyphenol-rich berries possess a broad spectrum of biological and pharmacological activities that have a positive effect on aging and age-related chronic diseases [1,2].
Black chokeberry (Aronia melanocarpa (Michx.) Elliott) of the family Rosaceae is a perennial shrub native to eastern North America. It is now widely cultivated in many European countries, but its fruit is rarely eaten fresh and unprocessed because of its tart and sour-bitter taste. So, the fruits are often used to make various food products including syrups, juices, jellies, alcoholic and energy drinks, teas, and dietary supplements. Native Americans used to treat colds with the fruit of black chokeberry [3]. Today, the fruit and flowers are known as traditional remedies for flu and to strengthen the immune system [4]. Black chokeberry is also used for hypertension and atherosclerosis [5]. It has one of the most polyphenol-rich berry fruits, with a high content of proanthocyanidins, anthocyanins, and phenolic acids, while flavonoids form the smallest class of phenolic compounds [3,4]. It is estimated that fresh fruits contain up to 3 g of polyphenols per 100 g [6]. High polyphenol content has been associated with a variety of health-promoting effects, including anti-inflammatory, antioxidant, immunomodulatory, anticarcinogenic, antimicrobial, antidiabetic, cardioprotective, liver-protective, and neuroprotective effects [7,8,9,10,11,12,13].
Bilberry (Vaccinium myrtillus L.) is a wild-growing shrub that belongs to the Erica-ceae family. It is native to Eurasia and, unlike black chokeberry, is not cultivated. The pleasant, sweet, and slightly astringent taste of the fruit makes it very popular as a fresh fruit and processed food or as a dietary supplement. Based on long-standing traditional use, the dried ripe fruit of bilberry (Myrtilli fructus siccus) is considered a medicinal product for the treatment of mild diarrhea and inflammation of the oral mucosa, while the fresh ripe fruit (Myrtilli fructus recens) is recommended for the treatment of capillary fragility and symptoms of venous insufficiency [14,15]. Bilberry fruit is a rich source of biologically active compounds, especially phenolic compounds such as anthocyanins, hydroxycinnamic acid derivatives, flavonoids, and proanthocyanidins [16,17,18]. They exhibit anti-inflammatory activity, which could translate into potential preventive and therapeutic effects in metabolic syndrome, cancer, diabetes, and cardiovascular, ophthalmic, and neurological diseases [19,20].
Both black chokeberry and bilberry fruits contain high levels of hydroxycinnamic acids, of which chlorogenic acid is the most abundant. This is an ester of caffeic acid and quinic acid, that is widely distributed in plants. We consume the majority of chlorogenic acid from coffee, but it is also found in various herbs, fruits, and vegetables that we have in our diet [21,22]. Previous studies have shown its multiple biological and pharmacological effects, including antioxidant, anti-inflammatory, anticancer, antihyperlipidemic, antidiabetic, antihypertensive, and antineurodegenerative effects [23]. To exert their functions, chlorogenic acid and other phenolic compounds target various molecular mechanisms and signaling pathways. If we consider ROS-induced oxidative damage and various pathologies in different organ systems, it is clear that oxidative stress leads to the development of various disorders and aging processes [24]. By interacting with nucleic acids (DNA damage and strand breaks), lipids (lipid peroxidation), and proteins, especially cysteine residues, ROS can cause cell toxicity. ROS also has effects on several signaling pathways, such as modulation of NF-κB/Nrf2 activation, which plays an important role in regulating cellular responses to oxidative stress and inflammation [25]. Numerous studies have demonstrated that bioactive phenolic compounds in berries may act as ROS radical scavengers by donating electrons to free radicals, thus protecting DNA, proteins, and lipids from oxidative damage. Some systematic reviews and meta-analyses of clinical studies have described the health benefits of certain berry species and phenolic compounds on cardiovascular diseases, metabolic disorders, and cancer. However, future research is needed to clarify the mechanisms of action of the bioactive berry constituents and their health benefits [26].
Most signal transduction pathways in humans are regulated by protein kinases through the phosphorylation of their protein substrates. Protein phosphorylation is an important cellular regulatory mechanism as many enzymes and receptors are activated/deactivated by it [27,28]. The Src family of non-receptor tyrosine kinases in humans comprises eleven members, of which Src, Yes, and Fyn are present in almost all cells, whereas the other members are restricted to specific tissues and organs [29]. Src tyrosine kinases phosphorylate tyrosine residues on target proteins and are thus involved in the regulation of cellular signaling pathways that control numerous processes important for the maintenance of cellular homeostasis and survival [30]. To activate a tyrosine kinase receptor, key amino acids must be in the correct positions to facilitate phosphate group transfer, and the peptide-substrate binding site must be accessible [31]. Src tyrosine kinases play an important role in carcinogenesis, as their excessive activation and expression lead to cell proliferation disorders associated with tumor invasion, metastasis, and angiogenesis [30,32]. Recent scientific evidence also points to the role of the Src kinase family in the development of inflammation-related diseases, as they also control the transmission of signals associated with the inflammatory response [33,34,35].
Because of the integral role of protein kinases in the regulation of intracellular homeostasis and the transduction of extracellular signals, dysregulation of their activity is directly related to numerous progressive diseases. Therefore, small molecules that inhibit protein kinases are now among the most-studied drug classes for the treatment of various diseases such as cancer and inflammatory and autoimmune diseases [36,37]. Given their enormous therapeutic potential and drug-like properties, kinase inhibitors are a rapidly growing and important category of target therapeutics. Most protein kinase inhibitors already approved or about to be approved for clinical use, as well as most kinase inhibitors currently in clinical trials, target tyrosine kinases. Among these kinase inhibitors, those derived from various plant sources are also described in the literature. They belong to different classes of compounds, including polyphenols [38].
With this background, we aimed to evaluate the antioxidant properties of black chokeberry and bilberry fruit extracts and, for the first time, to investigate their ability to inhibit Src tyrosine kinase activity. Since chlorogenic acid is widely distributed in plants and is one of the most important phenolic acids in the human diet, it is intensively studied for its health-promoting activities [23]. However, the role of chlorogenic acid as an inhibitor of Src kinases or as a scavenger of NO has not yet been investigated. Therefore, our further objective was to evaluate the role of chlorogenic acid, one of the most abundant phenolic constituents of black chokeberry and bilberry fruits, in the biological effects of interest.
2. Results and Discussion
2.1. Content of Polyphenols in Black Chokeberry and Bilberry Fruit Extracts
The contents of total polyphenols, and the individual proportions of the various polyphenol groups in the hydroethanolic fruit extracts, were determined spectrophotometrically. Table 1 shows that both the black chokeberry and bilberry extracts were rich in phenolic constituents (5.90% and 4.96%, respectively). The most abundant compounds in both extracts studied were phenolic acids. The black chokeberry extract contained a high 4.09% of hydroxycinnamic acids, while the percentage in the blueberry extract was 1.51%. Anthocyanins were also present in considerable amounts in the fruits of chokeberry (0.41%) and blueberry (1.06%), while the percentage of flavonoids was much lower (0.17% and 0.12%, respectively).

Table 1.
Content of total polyphenols, hydroxycinnamic acids, anthocyanins, and flavonoids (%, g/100 g of extract) in black chokeberry and bilberry fruit extracts.
Our study confirms previous findings on the high content of polyphenols in the fruit, juice, and extracts of black chokeberry [5,39,40]. A review of literature data shows that phenolic compounds in black chokeberry have been extensively studied. Among anthocyanins, cyanidin glycosides were identified as particularly representative, with cyanidin-3-galactoside predominating. Fruits were found to contain large amounts of procyanidins composed of flavan-3-ol monomers, mainly epicatechin. The glycosides of quercetin were identified to be the most abundant flavonoids. In addition, previous studies have shown that the fruit of black chokeberry is characterized by a high content of phenolic acids, mainly chlorogenic acid and neochlorogenic acid [22,41,42].
The results of spectrophotometric determination of total polyphenols and individual polyphenol groups in the bilberry extract are in agreement with previous studies on bilberry fruit [43,44,45]. The bilberry extract had a slightly lower total polyphenol content than black chokeberry. However, the bilberry-fruit extract contained about 2.5 times more anthocyanins but less phenolic acids. Literature studies have shown that chlorogenic acid and glycosides of cyanidin, delphinidin, and quercetin are the predominant single phenolic constituents of bilberry fruit [45,46].
2.2. Content of Phenolic Acids and Flavonoids in Black Chokeberry and Bilberry Fruit Extracts
TLC analysis revealed that chlorogenic acid was the main phenolic compound in both the chokeberry and bilberry extracts. In addition to chlorogenic acid, black chokeberry also contained a significant amount of neochlorogenic acid, which was not the case for the bilberry. As can be seen in the HPLC chromatograms shown in Figure 1, the chlorogenic acid peak in both samples stands out, with a retention time of 18.44 min. The extract from black chokeberry fruit contained 2.05 mg/g of chlorogenic acid, while 2.54 mg/g of chlorogenic acid was determined in bilberry-fruit extract. Several previous studies have also demonstrated high levels of chlorogenic acid in black chokeberry fruit. For example, about 1 mg/g of chlorogenic acid was determined in a German sample [47]. In fresh fruits from Russia and Belarus, it was identified as the main hydroxycinnamic acid in different varieties (0.2–0.8 mg/g), accounting for more than 50% of the total content [41]. Research on samples from Poland showed that, as the fruit ripens, the proportion of chlorogenic acid decreases in favor of anthocyanins [22]. In light of the results obtained for bilberry, similar levels of chlorogenic acid were found in the extracts of bilberry fruit from Romania and Montenegro [17,45]. Our results confirmed that the hydroethanolic extracts of black chokeberry and bilberry are rich natural sources of chlorogenic acid, which is considered an essential component of their health benefits, including antioxidant, anti-inflammatory, anticarcinogenic, antidiabetic, and cardioprotective properties [22].
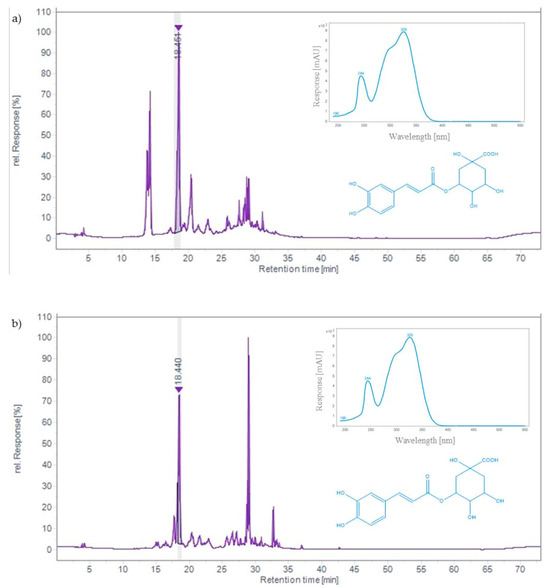
Figure 1.
HPLC chromatograms of hydroethanolic extracts of black chokeberry fruits (a) and bilberry fruits (b) with UV-spectrum of chlorogenic acid recorded at 320 nm.
In addition to chlorogenic acid, neochlorogenic acid was also detected in black chokeberry fruit, while bilberry fruit did not contain it. This finding is reflected in the HPLC chromatograms (Figure 1), where the peak of neochlorogenic acid (Rt = 14.12) is seen only in the extract of black chokeberry. Our results showed that the extract contained a high amount of neochlorogenic acid (2.07 mg/g), which was comparable to chlorogenic acid (Table 2).

Table 2.
Content of phenolic acids and flavonoids (mg/g of extract) of black chokeberry and bilberry extracts determined by the HPLC-DAD method.
Previous studies also support the presence of neochlorogenic acid in black chokeberry fruit. Kaloudi et al. [48] detected neochlorogenic acid and chlorogenic acid in fresh fruits from Greece in a ratio of 1:1.8. Neochlorogenic acid was also determined in the fresh fruits of five chokeberry cultivars from Belarus, Russia, and Denmark. Its content varied between 0.15 and 0.46 mg/g, being 1.3–1.8 times lower than that of chlorogenic acid [41]. Quercetin glycosides were found to be the major flavonoid compounds in the fruits of black chokeberry and bilberry. The results summarized in Table 2 show that bilberry contains the highest amount of isoquercitrin (1.36 mg/g). This 3-O-glucoside of quercetin was also present in the extract of black chokeberry but in a lower amount (0.67 mg/g), along with a significant amount of 3-O-galactoside (0.50 mg/g) and 3-O-rutinoside (0.34 mg/g). Our results are consistent with previous reports of the highest content of quercetin compounds among flavonoids in the fruits of black chokeberry [49] and blueberry [49,50].
2.3. Src Tyrosine Kinase Inhibitory Activity of Black Chokeberry and Bilberry Fruit Extracts
Src tyrosine kinase (Src) is the first proto-oncogene tyrosine kinase ever described. Both expression and activation of Src are enhanced in various human cancers and correlate with malignancy progression and the development of metastasis and invasion. The role of Src in oncogenesis has led to the discovery of other members of the Src protein kinase family and the search for cancer therapies. Because of increasing evidence of its crucial role in tumor progression, Src has emerged as a promising target for anticancer therapy [51,52]. Src is primarily involved in numerous signaling pathways that regulate and maintain cell metabolism, cell contact, and cell migration. It also controls signal transduction associated with inflammatory responses [53]. A previous study reported that Src triggers macrophage-mediated inflammatory responses. Various inflammatory diseases, such as rheumatoid arthritis, atherosclerosis, cancer, obesity, and diabetes, are closely related to macrophage activation [54]. In recent years, numerous natural products have been described as starting points for new chemical compounds that act as protein kinase inhibitors and are potential drug candidates [55].
The present study evaluated black chokeberry and bilberry fruit hydroethanolic extracts for their inhibitory effect on Src activity using a time-resolved fluorescence resonance energy transfer assay (TR-FRET), with staurosporine serving as a positive control (IC50 = 0.70 ± 0.05 ng/mL). The studied extracts inhibited Src activity in a dose-dependent manner (Figure 2) and showed a comparatively significant inhibitory effect, with IC50 values of 366 ± 17.02 µg/mL and 369 ± 21.64 µg/mL, respectively. Inhibition of Src activity by chlorogenic acid, one of the most abundant constituents of the extracts studied, was also tested. The results showed that chlorogenic acid significantly contributed to the inhibition of Src activity, with an IC50 value of 122 ± 9.13 µg/mL, which was a three-times-stronger activity than that of the extracts studied. The primary responsibility of chlorogenic acid for the inhibitory effects of the extracts is also reflected in the result that black chokeberry and bilberry extracts with approximately equal amounts of chlorogenic acid showed very similar inhibitory effects on Src activity.
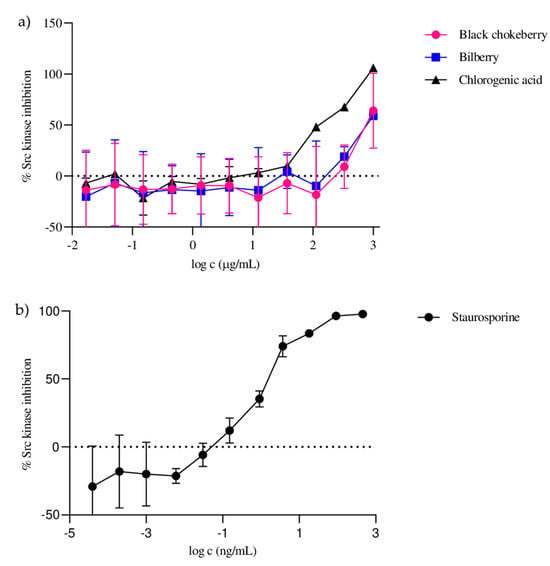
Figure 2.
Dose–response curves of Src kinase inhibition for black chokeberry fruits, bilberry fruits, chlorogenic acid (a) and staurosporine (b).
These are the first data on the inhibitory effects of chokeberry and bilberry on Src activity, helping to clarify the mechanisms of action by which they may exert anticancer and anti-inflammatory effects. Previously, black chokeberry extracts and their isolated bioactive components have shown growth-inhibitory effects on various human cancer cells, including HT-29 and Caco-2 colon cancer, MCF-7 and MDA-MB-231 breast cancer, A549 and H1299 non-small-cell lung cancer, and BGC-803 gastric cancer [9,56]. They have been found to inhibit the cell proliferation of cancer cells and arrest the cell cycle through various mechanisms of action, such as reducing the expression of cyclin A and B genes [57], upregulating the tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 [58], inducing c-Myc protein degradation [59], decreasing the stability of β-catenin, and thus inhibiting the expression of related proteins in the Wnt/β-catenin signaling pathway [60]. The anticancer activity of bilberry has also been the subject of previous studies demonstrating its antiproliferative and proapoptotic properties [61]. Bilberry-fruit extract and its polyphenol fraction have shown an anticancer effect on hormone-dependent (LNCaP) and hormone-independent (PC3 and DU-145) prostate cancer cell lines [62], and HSC-3 oral carcinoma cells [63]. The growth and invasive potential of human H1229 non-small-cell lung cancer cells were suppressed by bilberry anthocyanins [64], which were found to induce redox-sensitive caspase 3-related apoptosis in B-cell chronic lymphocytic leukemia through dysregulation of the Bcl-2/Bad pathway [65]. Bilberry was also able to inhibit proliferation and induce apoptosis in MCF7 breast cancer cells via a mechanism that has no effect on microtubules or mitosis at the lowest effective concentrations, while microtubule organization was affected by the higher concentrations [66]. Studies on colorectal cancer indicated that bilberry inhibited intestinal tumor formation and cancer cell growth, which was confirmed by an uncontrolled pilot human study [67].
Many studies have shown a close relationship between cancer and chronic inflammation [68]. Src is overactive in both cancer cells and immune cells that infiltrate tumors. It is also involved in cytokine-mediated communication between cancer and inflammatory cells [69]. Therefore, inhibition of Src activity is certainly an important mechanism to prevent chronic inflammation and thus the occurrence of cancer and other diseases associated with inflammation. Accordingly, our results demonstrated the inhibitory potential of black chokeberry and bilberry. In terms of their anti-inflammatory properties, previous studies have shown that black chokeberry inhibits the release of inflammatory markers such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-8, as well as the activation of nuclear factor-kappa B (NF-κB) [6,70]. Bilberry has also been found to exert anti-inflammatory effects via multiple mechanisms of action, including reducing the expression of TNF-α, IL-6, and IL-1β, inducing nitric oxide synthases and cyclooxygenases, and altering the NF-κB and Janus kinase signal transducer and activator of transcription signaling pathways [71].
Our findings extend the knowledge of the potent biological activity of chlorogenic acid and are consistent with previous results indicating its great potential in the prevention and treatment of various types of cancer and inflammatory diseases. For example, a recent review indicated that chlorogenic acid has excellent protective effects against various liver diseases associated with different signaling pathways, including AMP-activated protein kinase (AMPK) and extracellular signal-regulated kinases 1 and 2 (ERK1/2) [72]. Zhou et al. [73] demonstrated that chlorogenic acid has an anti-glioma effect on U373 cells by downregulating the SRC/MAPKs signaling pathway. Chlorogenic acid was also found to inhibit Bcr-Abl tyrosine kinase and trigger p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemia cells [74]. An isomer of chlorogenic acid, 3-O-caffeoylquinic acid has been shown to regulate lipopolysaccharide-induced TNF-α production in microglia, deactivate c-Src and abrogate c-Src activation during proinflammatory microglia stimulation, which prevents ROS generation in these cells [75]. In addition to in vitro evaluation and animal models, chlorogenic acid has also been tested in a clinical setting. A phase I trial of chlorogenic acid injection demonstrated clinical benefits for patients with high-grade glioma, who had relapsed after previous standard therapies, and a favorable safety profile up to a dose of 5.5 mg/kg [76].
2.4. Antioxidant Activity of Black Chokeberry and Bilberry Fruit Extracts
The antioxidant activity of the fruit extracts at concentrations of 6.25–800 µg/mL was evaluated in comparison with chlorogenic acid using two different assays. The DPPH assay is commonly used to evaluate the ability of natural products to scavenge free radicals. Unlike the DPPH radical, a stable synthetic free radical, NO is an important signaling molecule involved in the regulation of various physiological processes such as neurotransmission, smooth muscle relaxation, blood pressure regulation, vasodilation, defensive mechanisms, cell function, and control of inflammatory and immunological responses. However, when NO is formed in excessive amounts, it can react with superoxide anions to form peroxynitrite anion, a very damaging species for proteins, lipids, and DNA [77,78] Therefore, the ability of plant extracts and their pure constituents to directly scavenge NO may be useful in preventing pathological conditions associated with oxidative stress. Both studied fruit extracts showed a concentration-dependent ability to scavenge DPPH and NO radicals (Table 3 and Table 4). In both cases, black chokeberry was a more effective radical scavenger than bilberry, whose percent inhibition values were consistently lower. At the lowest concentrations tested, chlorogenic acid had an effect similar to that of the extracts at concentrations of 50–100 µg/mL. The ability of chlorogenic acid to inhibit 90% of DPPH radicals was already achieved at a concentration of 100 µg/mL, but at the highest concentration tested, there was no significant difference compared to black chokeberry, while the effect was slightly higher compared to bilberry.

Table 3.
DPPH radical-scavenging activity (%) of black chokeberry and bilberry fruit extracts in comparison with chlorogenic acid.

Table 4.
NO radical-scavenging activity (%) of black chokeberry and bilberry fruit extracts in comparison with chlorogenic acid.
Black chokeberry also lagged behind chlorogenic acid when tested for its ability to scavenge NO radicals at lower concentrations. However, at the highest concentration tested, black chokeberry showed better efficacy. It scavenged more than 90% of the NO radicals generated, while chlorogenic acid scavenged less than 80% of the radicals. The bilberry extract at the highest concentration tested achieved an effect of slightly more than 60%, again indicating a weaker antioxidant effect compared to the black chokeberry extract.
The DPPH and NO radical scavenging activities of the tested extracts and chlorogenic acid were evaluated using SC50 values, which represent the concentrations of the tested sample required to scavenge the original radical concentration by 50%. The results are presented in Table 5. The lowest SC50 value of 18.39 µg/mL and 20.41 µg/mL obtained for chlorogenic acid showed the highest ability to scavenge DPPH and NO radicals, respectively. Black chokeberry extract has stronger antioxidant properties than bilberry extract, as the SC50 values are twice as low. Regardless of the fact that two completely different radicals are used, it is interesting to note that chlorogenic acid has the same antioxidant activity, as evidenced by very similar SC50 values. Moreover, the same is true for the fruit extracts tested.

Table 5.
Comparative SC50 values (µg/mL) of black chokeberry and bilberry fruit extracts and chlorogenic acids obtained for DPPH and NO radical-scavenging activity.
The presented results showing the strong antioxidant activity of the hydroethanolic extracts of the two tested fruits are in agreement with the data in the literature [42,61]. Compared to other small dark fruits, black chokeberry is generally considered to have a stronger radical-scavenging activity due to its higher content of phenolic compounds [3]. The antioxidant activity of black chokeberry and bilberry has been confirmed in various tests such as the DPPH radical-scavenging assay [3,42,61], which is the most commonly used. Data on their ability to scavenge NO radicals, however, are scarce. Denev et al. [47] demonstrated the antioxidant activity of anthocyanin-rich extracts of bilberry and black chokeberry via the electrochemical measurement of NO radicals. Kolarov et al. [79] showed that bilberry fruit extract has a high ability to scavenge NO radicals, which correlates with the content of anthocyanins and flavan-3-ols, but they did not investigate phenolic acids and their influence on the antioxidant activity of bilberry fruits. Bilberry was also found to suppress nitric oxide (NO) and pro-inflammatory cytokine formation in LPS-induced RAW 264.7 cells and to exhibit antioxidant and anti-inflammatory properties in vitro [80]. Accordingly, our study confirmed the high capability of the tested fruit extracts to scavenge free radicals and thus prevent oxidative stress, which contributes significantly to the etiology of various chronic diseases.
3. Materials and Methods
3.1. Chemicals
Adenosine 5′-triphosphate disodium salt dehydrate (ATP), anhydrous sodium sulfate, aluminium chloride, 2-aminoethyl diphenylborinate, acetonitrile (HPLC grade), chlorogenic acid (95%), 2,2-diphenyl-1-picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), ethyl acetate, ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), gallic acid (95%), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), hyperoside, isoquercitrin, magnesium chloride (MgCl2), neochlorogenic acid, phosphate buffer saline (PBS), polyethylene glycol 4000 (PEG 4000), rutin, sulphanilamide, staurosporine and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Src (08-173) was obtained from Carna Bioscience (Natic, MA, USA). LANCE Ultra ULight-TK peptide (TRF0127-M), LANCE detection buffer 10× (CR97-100), LANCE europium-labeled anti-phosphotyrosine (PT66) antibody were purchased from Perkin Elmer (Waltham, MA, USA). Dithiothreitol (DTT) was purchased from Bio-Rad (Hercules, CA, USA). N-(1-naphthyl) ethylenediamine dihydrochloride (NED), sodium molybdate and sodium nitrite were provided by Fluka (Buchs, Switzerland). Acetic, formic, hydrochloric and phosphoric acids were obtained from Kemika (Zagreb, Croatia). Aceton, ethanol, methanol and sodium carbonate decahydrate were purchased from Gram-Mol (Zagreb, Croatia). Folin-Ciocalteau’s phenol reagent, and sodium nitroprusside were purchased from Merck (Darmstadt, Germany).
3.2. Plant Material and Extract Preparation
Ripe fruits of black chokeberry (Aronia melanocarpa (Michx.)) and bilberry (Vaccinium myrtillus L.) originating from Zagreb’s surrounding area were purchased at the local market. The plant samples were authenticated by the Department of Pharmacognosy, Faculty of Pharmacy and Biochemistry (University of Zagreb, Zagreb, Croatia). Fresh berries (10.00 g) were macerated with 50% ethanol (100 mL) for 24 h. After maceration, samples were extracted for an additional 15 min in an ultrasonic bath and filtered through Whatman paper No. 1, using a Büchner funnel. The ethanol was removed in a rotary evaporator. The residues were freeze-dried and used for further studies. The extraction yields for black chokeberry and bilberry were 29.56% and 26.32%, respectively.
3.3. TLC Analysis
Phenolic compounds were detected by thin-layer chromatography. Aliquots (10 μL) of the freeze-dried hydroethanolic extracts and standards were applied to a precoated silica gel 60 F254 TLC plate and developed in glass chambers previously saturated with the mobile phases: ethyl acetate—formic acid—acetic acid—water (100:11:11:27, v/v/v/v). The chromatograms obtained were dried in a stream of air for a few minutes. The flavonoids and phenolic acids were analyzed under UV light at 365 nm after spraying with a natural products-polyethylene glycol reagent (1% methanolic solution of 2-aminoethyl diphenylborinate and 5% ethanolic solution of PEG 4000 [81].
3.4. Total Polyphenols Determination
Total polyphenol content was determined according to the procedure described by Feng et al. [82], with slight modifications. Briefly, 0.5 mL of the freeze-dried hydroethanolic extracts or standard of the appropriate concentration was added to the volumetric flask and mixed with 1 mL of the Folin-Ciocalteau’s phenol reagent, 10 mL of distilled water, and made up to a volume of 25 mL with 10% sodium carbonate. Samples were shaken well and stored in the dark at room temperature for 30 min before absorbance was measured at 760 nm. Gallic acid was used to generate a calibration curve between 50 and 600 µg/mL at 8 concentration levels. Results were expressed as gallic acid equivalents (GAE) per 100 g of sample.
3.5. Determination of Hydroxycinnamic Acid Derivatives
Hydroxycinnamic acid derivatives were determined according to the European Pharmacopoeia [83] with slight modifications. Freeze-dried hydroethanolic extracts (0.20 g) were dissolved in 50% ethanol (50 mL). An aliquot of the extract (1.0 mL) was mixed with 0.5 M hydrochloric acid (2 mL), Arnow’s reagent (10% aqueous solution of sodium nitrite and sodium molybdate, 2 mL), and 8.5% sodium hydroxide (2 mL) and diluted with water to 10.0 mL. The absorbance was measured at 525 nm against the blank sample. The percentage of total hydroxycinnamic acid content was calculated and expressed as chlorogenic acid according to the equation (%) = A × 2.65/m. A represents the absorbance of the test solution while m represents the mass of the extract in grams.
3.6. Total Anthocyanins Determination
The anthocyanin content was determined according to the European Pharmacopoeia [83] with slight modifications. Briefly, 0.05 g of the freeze-dried hydroethanolic extracts were dissolved in water (1 mL). The solution was then diluted 50-fold with 0.1% of hydrochloric acid. The absorbance of the solution was measured at 528 nm against 0.1% hydrochloric acid as a blank. The content of anthocyanins was expressed as cyanidin 3-O-glucoside chloride according to the equation (%) = A ×50/718 × m. A represents the absorbance of the test solution, m represents the mass of the extract in grams, and 718 represents the specific absorbance of cyanidin 3-O-glucoside chloride at 528 nm.
3.7. Total Flavonoids Determination
The flavonoid content was determined spectrophotometrically according to the method described in the European Pharmacopoeia [83]. Freeze-dried hydroethanolic extracts (0.600 g) were mixed with 1.0 mL of hexamethylenetetramine (5 g/L), 20 mL of acetone, and 2.0 mL of hydrochloric acid (250 g/L) and heated in a water bath under reflux for 30 min. After cooling, the extracts were filtered through cotton, and the residue was extracted twice with 20 mL of acetone for 10 min and diluted to 100 mL with acetone. The obtained extract was mixed with 20 mL of distilled water and extracted with ethyl acetate. The ethyl acetate fractions were washed with distilled water, filtered over anhydrous sodium sulphate, and diluted with ethyl acetate to 50.0 mL. An aliquot (10.0 mL) was mixed with 1.0 mL of an aluminum chloride solution and diluted to 25.0 mL with a 5% methanolic solution of acetic acid. The absorbance of the test solution was measured after 30 min at 425 nm. Total flavonoids were expressed as isoquercitrin and calculated according to the following equation: (%) = A × 1.25/m. A represents the absorbance of the test solution, while m represents the mass of the extract in grams.
3.8. Determination of Phenolic Acids and Flavonoids by High-Performance Liquid Chromatography
Identification and quantification of phenolic acids and flavonoids in freeze-dried ethanolic extracts were performed using an Agilent 1260 Infinity II liquid chromatograph equipped with an autosampler, quaternary pump, column oven, and DAD detector (Agilent Technologies, Santa Clara, CA, USA) according to the method described previously [84]. The analysis was performed using Zorbax Eclipse XDB-C18 column (4.6 × 250 mm, particle size 5 µm, Agilent Technologies). The mobile phase consisted of 2% (v/v, A) formic acid and acetonitrile (B). Elution was performed with the following gradient: 100–91% A from 0 to 12 min, 91–87% A from 12 to 20 min, 87–67% A from 20 to 30 min, 67% A from 30 to 32 min, 67–57% A from 32 to 42 min, 57% A from 42 to 60 min, 57–100% A from 60 to 60.50 min and 100% A from 60.50 to 73 min at a flow rate of 0.8 mL/min and a column temperature of 40 °C. Samples were prepared by dissolving in 50% EtOH to give a concentration of 30 mg/mL, while the concentration of standards was 2 mg/mL. Samples were filtered through a 0.45 µm syringe filter. Peaks were identified by comparing retention times and UV/VIS spectra with those of the standard solution. Chlorogenic and neochlorogenic acid were quantified at 330 nm using calibration curves obtained by linear regression analysis of six calibration points, y = 3120x + 376.9, R2 = 0.9992 for chlorogenic acid and y = 3433.3x + 326.85, R2 = 0.9977. Rutin, hyperoside and isoquercitrine were quantified at 240 and 360 nm using calibration curves obtained by linear regression analysis of six calibration points. The calibration curves for rutin, hyperoside and isoquercitrine were y = 947.11 − 28.683, R2 = 0.9949; y = 2115x − 4.5056, R2 = 0.99998; y = 1419.4x + 15.089, R2 = 1. Results were expressed as mg of phenolic acid or flavonoid per g of extract.
3.9. Src Tyrosine Kinase Inhibition Assay
To evaluate the inhibition of Src tyrosine kinase (Src), a time-resolved fluorescence resonance energy transfer (TR-FRET) tyrosine kinase assay was used according to the procedure described by Jelić et al. [85]. The assay was performed using a low-volume 384-well plate (Storplate-384-deep-well-V plate, Perkin Elmer, Waltham, MA, USA). The mother plate contained samples dissolved in DMSO, which were serially diluted 1:3 using an automated Janus pipetting workstation (Janus Integrator Platform, 8 tips, AJ8001, Perkin Elmer). Samples (100 nL) were then transferred to the test plate using a nanovolume dispenser (Mosquito 3019-002, TTP Labtech, Melbourn, UK). In the first step, the kinase reaction was started by mixing 0.5 nM Src (5 µL) with a combination of 50 nM peptide substrate (2.5 µL) and 200 µM ATP (2.5 µL). After three hours of incubation at 20 ± 2 °C, the mixture was diluted in kinase buffer containing 50 mM HEPES, 1 mM EGTA, 10 mM MgCl2, 2 mM DTT, and 0.01% Tween 20. Samples were analyzed at a concentration range of 0.02–1000 µg/mL. In the second step, 10 mM EDTA (5 µL) and 1 nM of Europium-labeled anti-phospho antibodies (5 µL) prepared in LANCE detection buffer were added and then incubated at 20 ± 2 °C for 3 h. TR-FRET signal was recorded at Ex340/Em615-665 using an EnVision plate reader (XciteMultilabel Reader, 2104-0020, Perkin Elmer). The final concentration of DMSO was 1%. Staurosporine was used as a reference control substance.
3.10. DPPH Radical-Scavenging Assay
The DPPH radical-scavenging assay was performed according to the method described by Harput et al. [86] with minor modifications. Appropriate serial dilutions of the freeze-dried hydroethanolic extracts and chlorogenic acid (800–6.25 µg/mL) in 50% EtOH were prepared in 96-well plates. Freshly dissolved 0.36 mM DPPH• (70 µL) was added to the 130 µL of a sample, and the reaction mixture was shaken vigorously and incubated in the dark for 30 min. The absorbance was measured at 492 nm using a microplate reader (Chromate, Palm City, FL, USA). The radical-scavenging activity was determined by comparing the absorbance with that of the blank (100%) containing only DPPH• and solvent.
3.11. NO Radical-Scavenging Assay
The radical-scavenging activity of nitric oxide was investigated according to the method described by Jing et al. [87] with slight modifications. To determine the NO radical-scavenging activity of the freeze-dried hydroethanolic extracts and chlorogenic acid, 80 µL of the serially diluted samples (800–6.25 µg/mL) were added to the 96-well plate and 80 µL of 2 mM sodium nitroprusside, dissolved in PBS (0.01 mM, pH 7.4), was added to each well. The plate was incubated under light at room temperature for 120 min. After incubation, 40 µL of 1% sulphanilamide in phosphoric acid was added and held for 5 min. Finally, 40 µL of 0.1% NED was added and the absorbance was immediately measured at 545 nm on the microplate reader (Chromate, Palm City, FL, USA). The NO radical-scavenging activity was calculated by comparing the absorbance with that of the blank (100%) containing all reagents except the tested sample.
3.12. Statistical Analysis
All experiments were performed in triplicate and results are expressed as mean ± standard deviation. The SC50 and IC50 values were calculated by linear regression extrapolation. Differences between the obtained results were determined using the ordinary one-way ANOVA and post hoc Tukey’s multiple comparisons tests. Pearson’s correlation coefficient was calculated to determine the degree of association between the two variables. All statistical analyses were performed using GraphPad Prism software (version 8.4.3) and Microsoft Excel (Microsoft office 365). All values with p < 0.05 were considered statistically significant.
4. Conclusions
As far as we know, this study is the first to demonstrate the inhibitory properties of black chokeberry and bilberry fruits on Src tyrosine kinase activity. The hydroethanolic fruit extracts studied significantly suppressed Src activity. The inhibitory effect was comparable in both and was mainly due to the presence of chlorogenic acid. These extracts also showed a strong ability to scavenge NO radicals, with black chokeberry being a more potent antioxidant. Our results contribute to the clarification of the complex mechanisms of the anticancer and anti-inflammatory effects of black chokeberry and bilberry, in which phenolic components play an important role. In particular, the current study highlights the importance of hydroxycinnamic acids, especially chlorogenic acid, which has great potential to become a leading component in the discovery of new anticancer and anti-inflammatory drugs. In addition, further research is needed to adequately support the use of black chokeberry and blueberry fruits in the prevention and treatment of diseases associated with oxidative stress and chronic inflammation.
Author Contributions
Conceptualization, S.V.-K.; methodology, D.J., M.B.Š. and B.B.; formal analysis, M.B.Š., D.J. and T.P.; investigation, B.B., M.B.Š., D.J., T.P., M.M. and E.Š.; writing—original draft preparation, S.V.-K., M.B.Š., B.B. and S.L.; writing—review and editing, S.V.-K.; visualization, M.B.Š.; supervision, S.V.-K.; project administration, E.Š.; funding acquisition, S.V.-K. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Development of functional drink in sustainable packaging JamINNO+” (KK.01.2.1.02.0305), co-financed by the European Union through the European Regional Development Fund—Operational Program Competitiveness and Cohesion.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Author Dubravko Jelić was employed by the company Selvita Ltd.; Ekaterina Šprajc and Sandy Lovković were employed by the company Jamnica plus Ltd. and had no financial or non-financial interests from this research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative Phytochemical Analysis of Aronia melanocarpa L. Fruit Juices on Bulgarian Market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-W.; Kim, J.-E.; Nam, Y.-E.; Kim, W.-S.; Lee, I.; Yim, S.-V.; Kwon, O. Eight-Week Supplementation of Aronia Berry Extract Promoted the Glutathione Defence System against Acute Aerobic Exercise-Induced Oxidative Load Immediately and 30 Min Post-Exercise in Healthy Adults: A Double-Blind, Randomised Controlled Trial. J. Hum. Nutr. Diet. 2023, 36, 1589–1599. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Parfenov, A.; Sharonova, N.; Nikitin, E.; Zobov, V. Radical Scavenging Actions and Immunomodulatory Activity of Aronia melanocarpa Propylene Glycol Extracts. Plants 2021, 10, 2458. [Google Scholar] [CrossRef]
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer Effects of Extracts from Three Different Chokeberry Species. Nutr. Cancer 2021, 73, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of Blood Glucose Levels and Obesity in Mice given Aronia Juice by Inhibition of Dipeptidyl Peptidase IV and α-Glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Qin, B.; Anderson, R.A. An Extract of Chokeberry Attenuates Weight Gain and Modulates Insulin, Adipogenic and Inflammatory Signaling Pathways in Epididymal Adipose Tissue of Rats Fed a Fructose-Rich Diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, X.; Liu, S.; Liu, Y.; Wang, D. Aronia melanocarpa Prevents Alcohol-Induced Chronic Liver Injury via Regulation of Nrf2 Signaling in C57BL/6 Mice. Oxid. Med. Cell Longev. 2020, 2020, e4054520. [Google Scholar] [CrossRef]
- Lee, H.Y.; Weon, J.B.; Jung, Y.S.; Kim, N.Y.; Kim, M.K.; Ma, C.J. Cognitive-Enhancing Effect of Aronia melanocarpa Extract against Memory Impairment Induced by Scopolamine in Mice. Evid. Based Complement. Alternat. Med. 2016, 2016, e6145926. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Herbal Medicinal Products Committee HMPC. European Union Herbal Monograph on Vaccinium myrtillus L., Fructus Siccus. EMA/HMPC/678995/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-siccus_en.pdf (accessed on 7 July 2023).
- European Medicines Agency. Herbal Medicinal Products Committee HMPC. European Union Herbal Monograph on Vaccinium myrtillus L., Fructus Recens. EMA/HMPC/375808/2014. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-recens_en.pdf (accessed on 7 July 2023).
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillus L.) Growing at Different Locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Dare, A.P.; Günther, C.S.; Grey, A.C.; Guo, G.; Demarais, N.J.; Cordiner, S.; McGhie, T.K.; Boldingh, H.; Hunt, M.; Deng, C.; et al. Resolving the developmental distribution patterns of polyphenols and related primary metabolites in bilberry (Vaccinium myrtillus) fruit. Food Chem. 2022, 374, 131703. [Google Scholar] [CrossRef]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Restivo, I.; Basilicata, M.G.; Giardina, I.C.; Massaro, A.; Pepe, G.; Salviati, E.; Pecoraro, C.; Carbone, D.; Cascioferro, S.; Parrino, B.; et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants 2023, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Burns, G.; Mears, K.; Sweeney, M.; Blanton, C. The Effect of Berry Consumption on Oxidative Stress Biomarkers: A Systematic Review of Randomized Controlled Trials in Humans. Antioxidants 2023, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Burton, R.A.; Wu, H.; Lipchik, A.M.; Craddock, B.P.; Mo, H.; Parker, L.L.; Miller, W.T.; Post, C.B. Substrate Binding to Src: A New Perspective on Tyrosine Kinase Substrate Recognition from NMR and Molecular Dynamics. Protein Sci. 2020, 29, 350–359. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The Crucial Role of Protein Phosphorylation in Cell Signaling and Its Use as Targeted Therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Rivera-Torres, J.; San José, E. Src Tyrosine Kinase Inhibitors: New Perspectives on Their Immune, Antiviral, and Senotherapeutic Potential. Front. Pharmacol. 2019, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned So Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, N.; Giovannetti, E.; Carbone, D.; Leonetti, A.; Rolfo, C.D.; Peters, G.J. Resistance to epidermal growth factor receptor inhibition in non-small cell lung cancer. Cancer Drug Resist. 2018, 1, 230–249. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Brian, B.F.; Freedman, T.S. The Src-family Kinase Lyn in Immunoreceptor Signaling. Endocrinology 2021, 162, bqab152. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, D.; Zhang, L.; Wei, W. Src Family Protein Kinase Controls the Fate of B Cells in Autoimmune Diseases. Inflammation 2021, 44, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Szilveszter, K.P.; Németh, T.; Mócsai, A. Tyrosine Kinases in Autoimmune and Inflammatory Skin Diseases. Front. Immunol. 2019, 10, 1862. [Google Scholar] [CrossRef]
- Ayala-Aguilera, C.C.; Valero, T.; Lorente-Macías, A.; Baillache, D.J.; Croke, S.; Unciti-Broceta, A. Small Molecule Kinase Inhibitor Drugs (1995–2021): Medical Indication, Pharmacology, and Synthesis. J. Med. Chem. 2022, 65, 1047–1131. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Cascioferro, S.M.; Parrino, B.; Diana, P. Multi or Single-Kinase Inhibitors to Counteract Drug Resistance in Cancer: What is New? Curr. Med. Chem. 2023, 30, 776–782. [Google Scholar] [CrossRef]
- Nishal, S.; Jhawat, V.; Gupta, S.; Phaugat, P. Utilization of kinase inhibitors as novel therapeutic drug targets: A review. Oncol. Res. 2022, 30, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Vignolini, P.; Ieri, F.; Heimler, D. Polyphenols and Volatile Compounds in Commercial Chokeberry (Aronia melanocarpa) Products. Nat. Prod. Commun. 2016, 11, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Landeka Jurčević, I.; Panjkota Krbavčić, I.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, M.A.; Perova, I.B.; Eller, K.I.; Akimov, M.Y.; Sukhanova, A.M.; Rodionova, G.M.; Ramenskaya, G.V. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules 2023, 28, 4101. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Georgescu, C.; Frum, A.; Virchea, L.-I.; Sumacheva, A.; Shamtsyan, M.; Gligor, F.-G.; Olah, N.K.; Mathe, E.; Mironescu, M. Geographicf Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules 2022, 27, 4986. [Google Scholar] [CrossRef] [PubMed]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [PubMed]
- Veljković, M.; Pavlović, D.R.; Stojiljković, N.; Ilić, S.; Jovanović, I.; Poklar Ulrih, N.; Rakić, V.; Veličković, L.; Sokolović, D. Bilberry: Chemical Profiling, In Vitro and In Vivo Antioxidant Activity and Nephroprotective Effect against Gentamicin Toxicity in Rats. Phytother. Res. 2017, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, M. Black Chokeberry (Aronia melanocarpa) Polyphenols Reveal Different Antioxidant, Antimicrobial and Neutrophil-Modulating Activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. White versus blue: Does the wild ‘albino’ bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem. 2016, 211, 876–882. [Google Scholar] [CrossRef]
- Simatou, A.; Simatos, S.; Goulielmaki, M.; Spandidos, D.A.; Baliou, S.; Zoumpourlis, V. Historical Retrospective of the SRC Oncogene and New Perspectives (Review). Mol. Clin. Oncol. 2020, 13, 21. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating Metabolism in Cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, J.; Cheng, Q. Proto-Oncogene Tyrosine-Protein Kinase SRC (Src) Inhibition in Microglia Relieves Neuroinflammation in Neuropathic Pain Mouse Models. Bioengineered 2021, 12, 11390–11398. [Google Scholar] [CrossRef]
- Byeon, S.E.; Yi, Y.-S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The Role of Src Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2012, 2012, 512926. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-Rich Extract from Aronia meloncarpa E. Induces a Cell Cycle Block in Colon Cancer but not Normal Colonic Cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Bermùdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Upregulation of Tumor Suppressor Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 in Human Colon Cancer Caco-2 cells Following Repetitive Exposure to Dietary Levels of a Polyphenol-Rich Chokeberry Juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Deng, H.-J.; Yun, B.-S.; Lee, D.-S. Triterpene Acid ( 3-O-p-Coumaroyltormentic Acid) Isolated from Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa Induce Apoptosis in Caco-2 Cells through Wnt/β-Catenin Signaling Pathway. Chem. Biodivers. 2020, 17, e2000654. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Di Serio, C.; Renai, L.; Scordo, C.V.A.; Checchini, L.; Ungar, A.; Tarantini, F.; Bartoletti, R. Vaccinium myrtillus L. Extract and its Native Polyphenol-Recombined Mixture Have Anti-Proliferative and Pro-Apoptotic Effects on Human Prostate Cancer Cell Lines. Phytother. Res. 2021, 35, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Mauramo, M.; Onali, T.; Wahbi, W.; Vasara, J.; Lampinen, A.; Mauramo, E.; Kivimäki, A.; Martens, S.; Häggman, H.; Sutinen, M.; et al. Bilberry (Vaccinium myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.-M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry Extract (Antho 50) Selectively Induces Redox-Sensitive Caspase 3-related Apoptosis in Chronic Lymphocytic Leukemia Cells by Targeting the Bcl-2/Bad Pathway. Sci. Rep. 2015, 11, 5:8996. [Google Scholar] [CrossRef]
- Nguyen, V.; Tang, J.; Oroudjev, E.; Lee, C.J.; Marasigan, C.; Wilson, L.; Ayoub, G. Cytotoxic Effects of Bilberry Extract on MCF7-GFP-Tubulin Breast Cancer Cells. J. Med. Food 2010, 13, 278–285. [Google Scholar] [CrossRef]
- Onali, T.; Kivimäki, A.; Mauramo, M.; Salo, T.; Korpela, R. Anticancer Effects of Lingonberry and Bilberry on Digestive Tract Cancers. Antioxidants 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic Inflammation: Key Player and Biomarker-Set to Predict and Prevent Cancer Development and Progression Based on Individualized Patient Profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Pham, H.; Pandol, S.J.; Ptasznik, A. Src as the Link between Inflammation and Cancer. Front. Physiol. 2014, 4, 416. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) Concentrate Inhibits NF-κB and Synergizes with Selenium to Inhibit the Release of Pro-Inflammatory Mediators in Macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic Acids: A Pharmacological Systematic Review on Their Hepatoprotective Effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, F.; Chen, J.; Zhang, S.; Wang, H. Chlorogenic Acid Inhibits Human Glioma U373 Cell Progression via Regulating the SRC/MAPKs Signal Pathway: Based on Network Pharmacology Analysis. Drug Des. Devel Ther. 2021, 15, 1369–1383. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.; Biswas, T.; Roy, K.C.; Mandal, S.; Mandal, C.; Pal, B.C.; Bhattacharya, S.; Rakshit, S.; Bhattacharya, D.K.; Chaudhuri, U.; et al. Chlorogenic Acid Inhibits Bcr-Abl Tyrosine Kinase and Triggers p38 Mitogen-Activated Protein Kinase-Dependent Apoptosis in Chronic Myelogenous Leukemic Cells. Blood 2004, 104, 2514–2522. [Google Scholar] [CrossRef]
- Socodato, R.; Portugal, C.C.; Canedo, T.; Domith, I.; Oliveira, N.A.; Paes-de-Carvalho, R.; Relvas, J.B.; Cossenza, M. c-Src Deactivation by the Polyphenol 3-O-Caffeoylquinic Acid Abrogates Reactive Oxygen Species-Mediated Glutamate Release from Microglia and Neuronal Excitotoxicity. Free Radic. Biol. Med. 2015, 79, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. 1 Phase I Study of Chlorogenic Acid Injection for Recurrent High-Grade Glioma with Long-Term Follow-up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef]
- Kindl, M.; Blažeković, B.; Bucar, F.; Vladimir-Knežević, S. Antioxidant and Anticholinesterase Potential of Six Thymus Species. Evid. Based Complement. Alternat Med. 2015, 2015, 403950. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Kolarov, R.; Peić Tukuljac, M.; Kolbas, A.; Kolbas, N.; Barać, G.; Ognjanov, V.; Ljubojević, M.; Prvulović, D. Antioxidant Capacity of Wild-Growing Bilberry, Elderberry, and Strawberry Fruits. Acta Hort. Regiotec. 2021, 24, 119–126. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Chun, E.M.; Mijan, M.A.; Park, S.H.; Moon, S.-K.; Lim, B.O. Anthocyanins Profiling of Bilberry (Vaccinium myrtillus L.) Extract that Elucidates Antioxidant and Anti-Inflammatory Effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S.; Zgainski, E.M. Drogenanalyse, 2nd ed.; Springer: Berlin, Germany, 2009; pp. 195–197. [Google Scholar]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of Phenolic Compounds in Olive Leaves by Different Drying and Storage Methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 2 May 2023).
- Ye, L.; Zhou, S.; Liu, L.; Liu, L.; Waters, D.L.E.; Zhong, K.; Zhou, X.; Ma, X.; Liu, X. Phenolic Compounds and Antioxidant Capacity of Brown Rice in China. Int. J. Food Eng. 2016, 12, 537–546. [Google Scholar] [CrossRef]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef]
- Harput, U.S.; Genç, Y.; Khan, N.; Saracoglu, İ. Radical Scavenging Effects of Different Veronica Species. Rec. Nat. Prod. 2011, 5, 100–107. [Google Scholar]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant Potential, Total Phenolic and Total Flavonoid Contents of Rhododendron anthopogonoides and its Protective Effect on Hypoxia-induced Injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).