Innate Immunity in Autoimmune Thyroid Disease during Pregnancy
Abstract
1. Introduction
2. The Delicate Thyroid Equilibrium in Pregnancy
3. Autoimmune Hyperthyroidism in Pregnancy
4. Autoimmune Hypothyroidism in Pregnancy
5. The Immunological Background of AITD
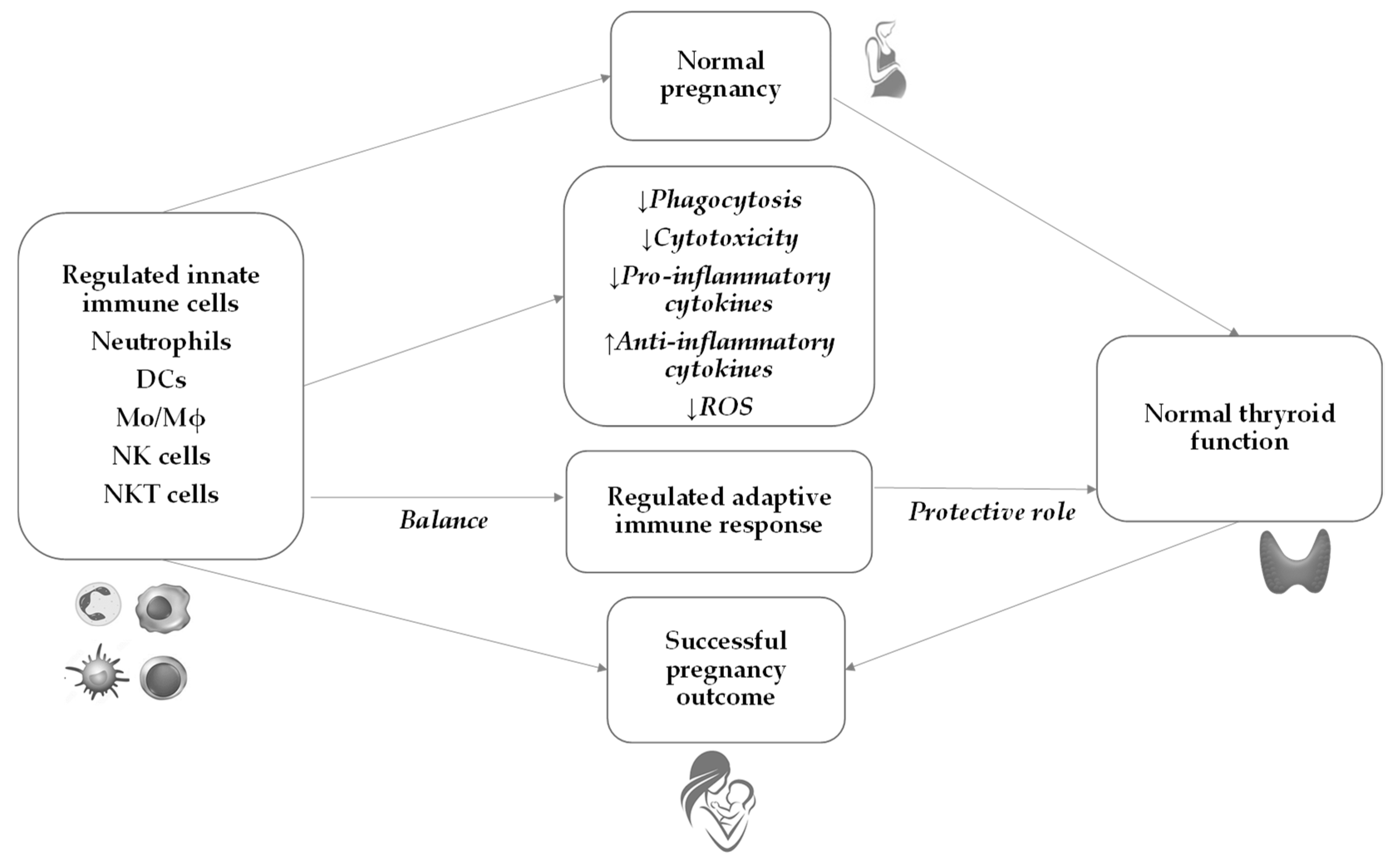
6. Innate Immunity during Normal Pregnancy
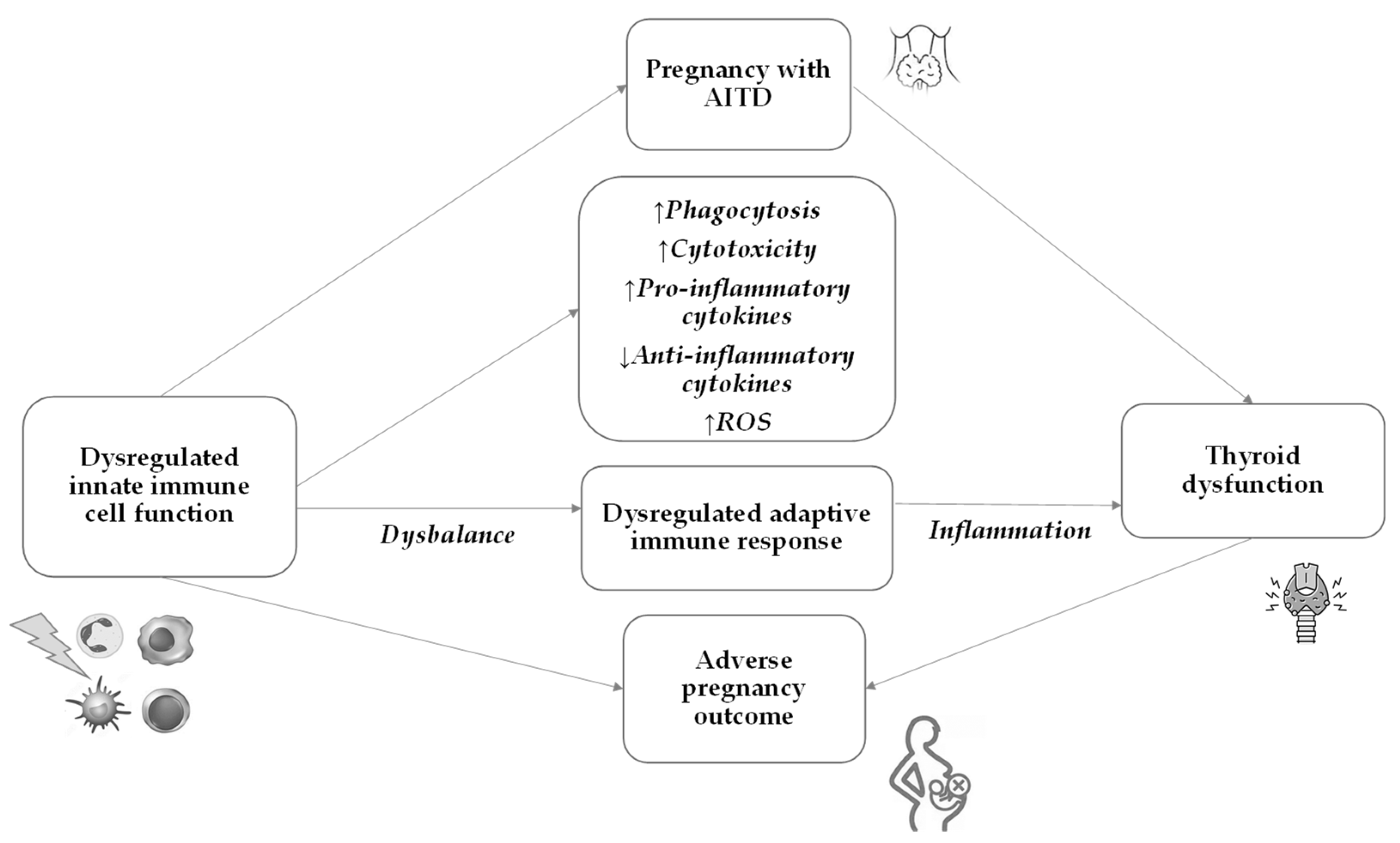
7. Innate Immunity in AITD and Pregnancy
7.1. Dendritic Cells
7.2. Macrophages
7.3. Neutrophils
7.4. NK Cells
7.5. NKT Cells
NKT Cells in Pregnant Women with AITD
| Cells of Innate Immunity | AITD | Normal Pregnancy | Pregnancy with AITD | ||
|---|---|---|---|---|---|
| HT | GD | HT | GD | ||
| Neutrophils |
| N/A | |||
| Monocytes/Macrophages | N/A | ||||
| Dendritic cells | N/A |
| N/A | ||
| NK cells | N/A |
| |||
| NKT cells |
|
| N/A | ||
8. Emerging Causal Connection: Microbiota and the Thyroid (Dis)balance
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, J.S.; Amaya-Amaya, J.; Anaya, J.M. Thyroid disease and autoimmune diseases. In Autoimmunity: From Bench to Bedside [Internet]; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013; Chapter 30. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459466/ (accessed on 8 July 2023).
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, O. Prevalence and Trends of Thyroid Disease among Adults, 1999–2018. Endocr. Pract. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef]
- Wang, B.; Shao, X.; Song, R.; Xu, D.; Zhang, J. The Emerging Role of Epigenetics in Autoimmune Thyroid Diseases. Front. Immunol. 2017, 8, 396. [Google Scholar] [CrossRef]
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent advances in understanding autoimmune thyroid disease: The tallest tree in the forest of polyautoimmunity. F1000Resarch 2017, 6, 1776. [Google Scholar] [CrossRef] [PubMed]
- Brix, T.H.; Hegedüs, L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin. Endocrinol. 2012, 76, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Kalarani, I.B.; Veerabathiran, R. Impact of iodine intake on the pathogenesis of autoimmune thyroid disease in children and adults. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 256–264. [Google Scholar] [CrossRef]
- Strikić Đula, I.; Pleić, N.; Babić Leko, M.; Gunjača, I.; Torlak, V.; Brdar, D.; Punda, A.; Polašek, O.; Hayward, C.; Zemunik, T. Epidemiology of Hypothyroidism, Hyperthyroidism and Positive Thyroid Antibodies in the Croatian Population. Biology 2022, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Tomer, Y.; Huber, A. The etiology of autoimmune thyroid disease: A story of genes and environment. J. Autoimmun. 2009, 32, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Barad, D.H. Gender as risk factor for autoimmune diseases. J. Autoimmun. 2007, 28, 1–6. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical hypothyroidism: A review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Rodondi, N.; Den Elzen, W.P.; Bauer, D.C.; Cappola, A.R.; Razvi, S.; Walsh, J.P.; Asvold, B.O.; Iervasi, G.; Imaizumi, M.; Collet, T.H.; et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010, 304, 1365–1374. [Google Scholar] [CrossRef]
- Chaker, L.; Baumgartner, C.; Den Elzen, W.P.; Ikram, M.A.; Blum, M.R.; Collet, T.H.; Bakker, S.J.; Dehghan, A.; Drechsler, C.; Luben, R.N.; et al. Subclinical hypothyroid¬ism and the risk of stroke events and fatal stroke: An individual participant data analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2181–2191. [Google Scholar] [CrossRef]
- Khosrotehrani, K.; Johnson, K.L.; Cha, D.H.; Salomon, R.N.; Bianchi, D.W. Transfer of fetal cells with multilineage potential to maternal tissue. J. Am. Med. Assoc. 2004, 292, 75–80. [Google Scholar] [CrossRef]
- Yap, Y.W.; Onyekwelu, E.; Alam, U. Thyroid disease in pregnancy. Clin. Med. 2023, 23, 125–128. [Google Scholar] [CrossRef]
- Ain, K.B.; Mori, Y.; Refetoff, S. Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: A mechanism for estrogen-induced elevation of serum TBG concentration. J. Clin. Endocrinol. Metab. 1987, 65, 689–696. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Tan, A.; Knox, E.; Kilby, M.D.; Franklyn, J.; Coomarasamy, A. Association between thyroid autoantibodies and miscarriage and preterm birth: Meta-analysis of evidence. BMJ 2011, 342, d2616. [Google Scholar] [CrossRef] [PubMed]
- Tańska, K.; Gietka-Czernel, M.; Glinicki, P.; Kozakowski, J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front. Endocrinol. 2023, 13, 1049665. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Lashley, E.E.L.O.; Vermeulen, N.; van der Hoorn, M.L.P. Identifying discrepancies between clinical practice and evidence-based guideline in recurrent pregnancy loss care, a tool for clinical guideline implementation. BMC Pregnancy Childbirth 2023, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Q.; Zhao, H.; Xiong, Y.; Li, X. Association between levothyroxine treatment for maternal subclinical hypothyroidism with negative TPOAb and early child neurodevelopment: A prospective real-world clinical trial. Acta Obstet. Gynecol. Scand. 2023, 102, 1183–1192. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, H.; Ren, M.; Gao, Y.; Sun, K.; Wu, H.; Ding, R.; Wang, J.; Li, Z.; Liu, D.; et al. Thyroid autoimmunity and adverse pregnancy outcomes: A multiple center retrospective study. Front. Endocrinol. 2023, 14, 1081851. [Google Scholar] [CrossRef]
- Li, M.; He, Y.; Mao, Y.; Yang, L.; Chen, L.; Du, J.; Chen, Q.; Zhu, Q.; Liu, J.; Zhou, W. Preconception thyroid-stimulating hormone levels and adverse pregnancy outcomes. Clin. Endocrinol. 2022, 97, 339–346. [Google Scholar] [CrossRef]
- Knøsgaard, L.; Andersen, S.; Hansen, A.B.; Vestergaard, P.; Andersen, S.L. Maternal hypothyroidism and adverse outcomes of pregnancy. Clin. Endocrinol. 2023, 98, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Alibrandi, A.; Di Mauro, M.; Paola, G.; Perdichizzi, L.G.; Granese, R.; Giacobbe, A.; Scilipoti, A.; Ragonese, M.; Ercoli, A.; et al. Preconception Thyrotropin Levels and Thyroid Function at Early Gestation in Women with Hashimoto Thyroiditis. J. Clin. Endocrinol. Metab. 2023, 108, e464–e473. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Perez, D.B.; Jani, P.; Maheshwari, R.; Shah, D. Thyroid function in infants born to women with hypothyroidism: An observational study at an Australian tertiary perinatal centre. Clin. Endocrinol. 2023, 98, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Maganha, C.A.; Mattar, R.; Mesa Júnior, C.O.; Marui, S.; Solha, S.T.G.; Teixeira, P.F.D.S.; Zaconeta, A.C.M.; Souza, R.T. Screening, diagnosis and management of hyperthyroidism in pregnancy. Rev. Bras. Ginecol. Obstet. 2022, 44, 806–818. [Google Scholar] [CrossRef]
- Cooper, D.S.; Laurberg, P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 238–249. [Google Scholar] [CrossRef]
- Samuels, S.L.; Namoc, S.M.; Bauer, A.J. Neonatal Thyrotoxicosis. Clin. Perinatol. 2018, 45, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiu, M.L.; Bors, R.G.; Gheorghisan-Galateanu, A.A.; Pop, A.L.; Cretoiu, D.; Varlas, V.N. Hyperthyroidism in Pregnancy: The Delicate Balance between Too Much or Too Little Antithyroid Drug. J. Clin. Med. 2021, 10, 3742. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Huang, Z.; Pan, X.; Leung, W.; Chen, L.; Zhang, Y.; Wang, L.; Sima, Y.; Gober, H.J.; et al. The Clinical Value and Variation of Antithyroid Antibodies during Pregnancy. Dis. Markers 2020, 2020, 8871951. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Pearce, E.N. An update on thyroid disorders in the postpartum period. J. Endocrinol. Investig. 2022, 45, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Thyroid Disease in Pregnancy. ACOG Practice Bulletin, Number 223. Obstet. Gynecol. 2020, 135, e261–e274. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef]
- Caron, P. Management of thyrotoxicosis and pregnancy: Review of the current literature and an update of the care pathway. Ann. Endocrinol. 2022, 83, 226–231. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Sasso, E.B.; Barton, L.; Mestman, J.H. Graves’ hyperthyroidism in pregnancy: A clinical review. Clin. Diabetes Endocrinol. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S. Hypothyroidism in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 308–319. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Herman Mahečić, D.; Marinković Radošević, J.; Strinović Morić, M.; Bilić-Ćurčić, I. Hypothyroidism and pregnancy: Still a controversial issue. Gynecol. Endocrinol. 2020, 36, 776–780. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Pearce, E.N. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018, 6, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Dhillon-Smith, R.K.; Tobias, A.; Smith, P.P.; Middleton, L.J.; Sunner, K.K.; Baker, K.; Farrell-Carver, S.; Bender-Atik, R.; Agrawal, R.; Bhatia, K.; et al. The prevalence of thyroid dysfunction and autoimmunity in women with history of miscarriage or subfertility. J. Clin. Endocrinol. Metab. 2020, 105, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef]
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Ye, X.P.; Zhou, Z.; Zhu, C.F.; Li, R.; Fang, Y.; Zhang, R.J.; Li, L.; Liu, W.; Wang, Z.; et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat. Commun. 2022, 13, 775. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Morshed, S.A.; Latif, R.; Davies, T.F. Delineating the autoimmune mechanisms in Graves’ disease. Immunol. Res. 2012, 54, 191–203. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Pathogenesis of thyroid auto-immune disease: The role of cellular mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- Diefenbach, A.; Colonna, M.; Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41, 354–365. [Google Scholar] [CrossRef]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. Innate immune recognition of cancer. Annu. Rev. Immunol. 2015, 33, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vely, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef]
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and molecular basis of thyroid autoimmunity. Eur. Thyroid. J. 2022, 11, e210024. [Google Scholar] [CrossRef]
- Domínguez-Andrés, J.; Novakovic, B.; Li, Y.; Scicluna, B.P.; Gresnigt, M.S.; Arts, R.J.W.; Oosting, M.; Moorlag, S.J.C.F.M.; Groh, L.A.; Zwaag, J.; et al. The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab. 2019, 29, 211–220.e5. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Mancilla-Herrera, I.; Vega-Sánchez, R.; Díaz, L.; Zaga-Clavellina, V. Innate Immune Cells and Toll-like Receptor-Dependent Responses at the Maternal-Fetal Interface. Int. J. Mol. Sci. 2019, 20, 3654. [Google Scholar] [CrossRef]
- Cristiani, C.M.; Palella, E.; Sottile, R.; Tallerico, R.; Garofalo, C.; Carbone, E. Human NK Cell Subsets in Pregnancy and Disease: Toward a New Biological Complexity. Front. Immunol. 2016, 7, 656. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef]
- Lurie, S.; Rahamim, E.; Piper, I.; Golan, A.; Sadan, O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Hasler, P.; Vokalova, L.; van Breda, S.V.; Lapaire, O.; Than, N.G.; Hoesli, I.; Rossi, S.W. The role of neutrophil activation in determining the outcome of pregnancy and modulation by hormones and/or cytokines. Clin. Exp. Immunol. 2019, 198, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Feng, C.; Zhang, X.; Lu, J.; Zhao, Y. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation 2016, 40, 311. [Google Scholar] [CrossRef]
- True, H.; Blanton, M.; Sureshchandra, S.; Messaoudi, I. Monocytes and macrophages in pregnancy: The good, the bad, and the ugly. Immunol. Rev. 2022, 308, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Akira, S. Toll-like receptors and innate immunity. J. Mol. Med. 2006, 84, 712–725. [Google Scholar] [CrossRef]
- Lampé, R.; Kövér, Á.; Szűcs, S.; Pál, L.; Árnyas, E.; Ádány, R.; Póka, R. Phagocytic index of neutrophil granulocytes and monocytes in healthy and preeclamptic pregnancy. J. Reprod. Immunol. 2015, 107, 26–30. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Göhner, C.; Plösch, T.; Faas, M.M. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta 2017, 60 (Suppl. 1), S41–S51. [Google Scholar] [CrossRef]
- Ramhorst, R.; Grasso, E.; Paparini, D.; Hauk, V.; Gallino, L.; Calo, G.; Vota, D.; Perez Leiros, C. Decoding the chemokine network that links leukocytes with decidual cells and the trophoblast during early implantation. Cell Adhes. Migr. 2016, 10, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Negishi, Y.; Takahashi, H.; Kuwabara, Y.; Takeshita, T. Innate immune cells in reproduction. J. Obstet. Gynaecol. Res. 2018, 44, 2025–2036. [Google Scholar] [CrossRef]
- Wei, R.; Lai, N.; Zhao, L.; Zhang, Z.; Zhu, X.; Guo, Q.; Chu, C.; Fu, X.; Li, X. Dendritic cells in pregnancy and pregnancy-associated diseases. Biomed. Pharmacother. 2021, 133, 110921. [Google Scholar] [CrossRef]
- Collins, M.K.; Tay, C.S.; Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Investig. 2009, 119, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J. Reprod. Immunol. 2017, 119, 91–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Zhou, Y.; Fu, B.; Wei, H. Uterine NK cell functions at maternal-fetal interface. Biol. Reprod. 2022, 107, 327–338. [Google Scholar] [CrossRef]
- Wells, A.I.; Coyne, C.B. Uterine NK cell education: Learning the ropes in pregnancy. Immunity 2021, 54, 1102–1104. [Google Scholar] [CrossRef]
- Alexandrova, M.; Manchorova, D.; Dimova, T. Immunity at maternal-fetal interface: KIR/HLA (Allo)recognition. Immunol. Rev. 2022, 308, 55–76. [Google Scholar] [CrossRef]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef]
- Amodio, G.; Gregori, S. Dendritic cells a double-edge sword in autoimmune responses. Front. Immunol. 2012, 3, 233. [Google Scholar] [CrossRef] [PubMed]
- Laskarin, G.; Redzovic, A.; Rubesa, Z.; Mantovani, A.; Allavena, P.; Haller, H.; Vlastelic, I.; Rukavina, D. Decidual natural killer cell tuning by autologous dendritic cells. Am. J. Reprod. Immunol. 2008, 59, 433–445. [Google Scholar] [CrossRef]
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal–fetal interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef]
- Hughes, G.C.; Clark, E.A. Regulation of dendritic cells by female sex steroids: Relevance to immunity and autoimmunity. Autoimmunity 2007, 40, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Hierweger, A.M.; Wieczorek, A.; Arck, P.C.; Thiele, K. Disruption of Glucocorticoid Action on CD11c+ Dendritic Cells Favors the Generation of CD4+ Regulatory T Cells and Improves Fetal Development in Mice. Front. Immunol. 2021, 12, 729742. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Amit, I.; Winter, D.R.; Jung, S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol. 2016, 17, 18–25. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Cai, T.; Du, P.; Suo, L.; Jiang, X.; Qin, Q.; Song, R.; Yang, X.; Jiang, Y.; Zhang, J.-A. High iodine promotes autoimmune thyroid disease by activating hexokinase 3 and inducing polarization of macrophages towards M1. Front. Immunol. 2022, 13, 1009932. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Gentek, R.; Molawi, K.; Sieweke, M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014, 262, 56–73. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, N.K.; Ljunggren, H.G.; Michaelsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Perricone, R.; Perricone, C.; De Carolis, C.; Shoenfeld, Y. NK cells in autoimmunity: A two-edg’d weapon of the immune system. Autoimmun. Rev. 2008, 7, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Konova, E. The role of NK cells in the autoimmune thyroid disease-associated pregnancy loss. Clin. Rev. Allergy Immunol. 2010, 39, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Sunwoo, J.B. Natural Killer Cells and Thyroid Diseases. Endocrinol. Metab. 2019, 34, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Hashemi, E.; Malarkannan, S. Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance. Cancers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Shi, F.D.; Van Kaer, L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat. Rev. Immunol. 2006, 6, 751–760. [Google Scholar] [CrossRef]
- Wisgalla, A.; Ramien, C.; Streitz, M.; Schlickeiser, S.; Lupu, A.-R.; Diemert, A.; Tolosa, E.; Arck, P.C.; Bellmann-Strobl, J.; Siebert, N.; et al. Alterations of NK Cell Phenotype during Pregnancy in Multiple Sclerosis. Front. Immunol. 2022, 13, 907994. [Google Scholar] [CrossRef]
- Klecha, A.J.; Genaro, A.M.; Gorelik, G.; Barreiro Arcos, M.L.; Silberman, D.M.; Schuman, M.; Garcia, S.I.; Pirola, C.; Cremaschi, G.A. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: Thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 2006, 189, 45–55. [Google Scholar] [CrossRef]
- Ortega-Rodríguez, A.C.; Martínez-Hernández, R.; Monsiváis-Urenda, A.; Serrano-Somavilla, A.; Sánchez-Gutiérrez, R.; González-Amaro, R.; Marazuela, M. Quantitative and Functional Analysis of PD-1+ NK Cells in Patients with Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2020, 105, dgaa569. [Google Scholar] [CrossRef] [PubMed]
- Solerte, S.B.; Precerutti, S.; Gazzaruso, C.; Locatelli, E.; Zamboni, M.; Schifino, N.; Bonacasa, R.; Rondanelli, M.; Taccani, D.; Ferrari, E.; et al. Defect of a subpopulation of natural killer immune cells in Graves’ disease and Hashimoto’s thyroiditis: Normalizing effect of dehydroepiandrosterone sulfate. Eur. J. Endocrinol. 2005, 152, 703–712. [Google Scholar] [CrossRef]
- Ciampolillo, A.; Guastamacchia, E.; Amati, L.; Magrone, T.; Munno, I.; Jirillo, E.; Triggiani, V.; Fallacara, R.; Tafaro, E. Modifications of the immune responsiveness in patients with autoimmune thyroiditis: Evidence for a systemic immune alteration. Curr. Pharm. Des. 2003, 9, 1946–1950. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, Y.; Amino, N.; Iwatani, Y.; Kaneda, T.; Nasu, M.; Mitsuda, N.; Tanizawa, O.; Miyai, K. Increase in peripheral natural killer cell activity in patients with autoimmune thyroid disease. Autoimmunity 1992, 11, 239–246. [Google Scholar] [CrossRef]
- Murakami, S.; Okubo, K.; Tsuji, Y.; Sakata, H.; Kikuchi, M.; Takahashi, T.; Kato, T.; Hirayama, R. Serum levels of interleukin-12 in Graves’ disease and their dynamic changes after surgery. Surg. Today 2005, 35, 1016–1020. [Google Scholar] [CrossRef]
- Corrales, J.J.; Orfao, A.; Lopez, A.; Ciudad, J.; Mories, M.T. Serial analysis of the effects of methimazole therapy on circulating B cell subsets in Graves’ disease. J. Endocrinol. 1996, 151, 231–240. [Google Scholar] [CrossRef]
- Weetman, A.P. Immunity, thyroid function and pregnancy: Molecular mechanisms. Nat. Rev. Endocrinol. 2010, 6, 311–318. [Google Scholar] [CrossRef]
- Taylor, E.B.; Sasser, J.M. Natural killer cells and T lymphocytes in pregnancy and pre-eclampsia. Clin. Sci. 2017, 131, 2911–2917. [Google Scholar] [CrossRef]
- Saito, S.; Shigeru, S.; Nakashima, A.; Akitoshi, N.; Myojo-Higuma, S.; Subaru, M.H.; Shiozaki, A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J. Reprod. Immunol. 2008, 77, 14–22. [Google Scholar] [CrossRef]
- Mahajan, D.; Sharma, N.R.; Kancharla, S.; Kolli, P.; Tripathy, A.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Borzychowski, A.M.; Croy, B.A.; Chan, W.L.; Redman, C.W.; Sargent, I.L. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur. J. Immunol. 2005, 35, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, D.; Rubesa, G.; Gudelj, L.; Haller, H.; Podack, E.R. Characteristics of perforin expressing lymphocytes within the first trimester decidua of human pregnancy. Am. J. Reprod. Immunol. 1995, 33, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Crncic, T.B.; Laskarin, G.; Frankovic, K.J.; Tokmadzic, V.S.; Strbo, N.; Bedenicki, I.; Le Bouteiller, P.; Tabiasco, J.; Rukavina, D. Early pregnancy decidual lymphocytes beside perforin use Fas ligand (FasL) mediated cytotoxicity. J. Reprod. Immunol. 2007, 73, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Le Bouteiller, P.; Piccinni, M.P. Human NK cells in pregnant uterus: Why there? Am. J. Reprod. Immunol. 2008, 59, 401–406. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Allan, D.S.; Bowen, M.; Powis, S.J.; Joseph, S.; Verma, S.; Hiby, S.E.; McMichael, A.J.; Loke, Y.W.; Braud, V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 2000, 30, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Ntrivalas, E.I.; Kwak-Kim, J.Y.; Gilman-Sachs, A.; Chung-Bang, H.; Ng, S.C.; Beaman, K.D.; Mantouvalos, H.P.; Beer, A.E. Status of peripheral blood natural killer cells in women with recurrent spontaneous abortions and infertility of unknown aetiology. Hum. Reprod. 2001, 16, 855–861. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Kronenberg, M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef]
- Slauenwhite, D.; Johnston, B. Regulation of NKT cell localization in homeostasis and infection. Front. Immunol. 2015, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.R.; Brennan, P.J.; Shay, T.; Watts, G.F.; Brigl, M.; Kang, J.; Brenner, M.B. ImmGen Project C. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat. Immunol. 2013, 14, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Van Kaer, L. Natural killer T cells in health and disease. Front. Biosci. 2011, 3, 236–251. [Google Scholar] [CrossRef]
- Wu, L.; Van Kaer, L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 2009, 9, 4–14. [Google Scholar] [CrossRef]
- Devendra, D.; Eisenbarth, G.S. Immunologic endocrine disorders. J. Allergy Clin. Immunol. 2003, 111 (Suppl. 2), S624–S636. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakamura, Y.; Matsuzuka, F.; Takamura, Y.; Miyauchi, A.; Iwatani, Y. Decrease of intrathyroidal CD161+Valpha24+Vbeta11+ NKT cells in Graves’ disease. Endocr. J. 2008, 55, 199–203. [Google Scholar] [CrossRef]
- Roman-Gonzalez, A.; Moreno, M.E.; Alfaro, J.M.; Uribe, F.; Latorre-Sierra, G.; Rugeles, M.T.; Montoya, C.J. Frequency and function of circulating invariant NKT cells in autoimmune diabetes mellitus and thyroid diseases in Colombian patients. Hum. Immunol. 2009, 70, 262–268. [Google Scholar] [CrossRef]
- Boyson, J.E.; Rybalov, B.; Koopman, L.A.; Exley, M.; Balk, S.P.; Racke, F.K.; Schatz, F.; Masch, R.; Wilson, S.B.; Strominger, J.L. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2002, 99, 13741–13746. [Google Scholar] [CrossRef]
- Ichikawa, T.; Negishi, Y.; Shimizu, M.; Takeshita, T.; Takahashi, H. α-Galactosylceramide-activated murine NK1.1(+) invariant-NKT cells in the myometrium induce miscarriages in mice. Eur. J. Immunol. 2016, 46, 1867–1877. [Google Scholar] [CrossRef]
- Southcombe, J.; Redman, C.; Sargent, I. Peripheral blood invariant natural killer T cells throughout pregnancy and in preeclamptic women. J. Reprod. Immunol. 2010, 87, 52–59. [Google Scholar] [CrossRef][Green Version]
- Miko, E.; Meggyes, M.; Doba, K.; Farkas, N.; Bogar, B.; Barakonyi, A.; Szereday, L.; Szekeres-Bartho, J.; Mezosi, E. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J. Reprod. Immunol. 2017, 124, 62–70. [Google Scholar] [CrossRef]
- Boyson, J.E.; Aktan, I.; Barkhuff, D.A.; Chant, A. NKT cells at the maternal-fetal interface. Immunol. Investig. 2008, 37, 565–582. [Google Scholar] [CrossRef][Green Version]
- Robertson, F.C.; Berzofsky, J.A.; Terabe, M. NKT cell networks in the regulation of tumor immunity. Front. Immunol. 2014, 5, 543. [Google Scholar] [CrossRef]
- Luty, J.; Ruckemann-Dziurdzińska, K.; Witkowski, J.M.; Bryl, E. Immunological aspects of autoimmune thyroid disease—Complex interplay between cells and cytokines. Cytokine 2019, 116, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Stramazzo, I.; Capriello, S.; Filardo, S.; Centanni, M.; Virili, C. Microbiota and Thyroid Disease: An Updated Systematic Review. Adv. Exp. Med. Biol. 2023, 1370, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Xie, G.; Zou, G.; Li, S.; Zhang, J.; Cai, W.; Xu, J. Correlation between gut microbiota and the development of Graves’ disease: A prospective study. iScience 2023, 26, 107188. [Google Scholar] [CrossRef]
- Calcaterra, V.; Mameli, C.; Rossi, V.; Magenes, V.C.; Massini, G.; Perazzi, C.; Verduci, E.; Zuccotti, G. What we know about the relationship between autoimmune thyroid diseases and gut microbiota: A perspective on the role of probiotics on pediatric endocrinology. Minerva Pediatr. 2022, 74, 650–671. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, W.; Li, J.; Shan, Z. Emerging trends and hot spots in autoimmune thyroiditis research from 2000 to 2022: A bibliometric analysis. Front. Immunol. 2022, 13, 953465. [Google Scholar] [CrossRef]
- Aversano, L.; Bernardi, M.L.; Cimitile, M.; Maiellaro, A.; Pecori, R. A systematic review on artificial intelligence techniques for detecting thyroid diseases. PeerJ Comput. Sci. 2023, 9, e1394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogović Crnčić, T.; Girotto, N.; Ilić Tomaš, M.; Krištofić, I.; Klobučar, S.; Batičić, L.; Ćurko-Cofek, B.; Sotošek, V. Innate Immunity in Autoimmune Thyroid Disease during Pregnancy. Int. J. Mol. Sci. 2023, 24, 15442. https://doi.org/10.3390/ijms242015442
Bogović Crnčić T, Girotto N, Ilić Tomaš M, Krištofić I, Klobučar S, Batičić L, Ćurko-Cofek B, Sotošek V. Innate Immunity in Autoimmune Thyroid Disease during Pregnancy. International Journal of Molecular Sciences. 2023; 24(20):15442. https://doi.org/10.3390/ijms242015442
Chicago/Turabian StyleBogović Crnčić, Tatjana, Neva Girotto, Maja Ilić Tomaš, Ines Krištofić, Sanja Klobučar, Lara Batičić, Božena Ćurko-Cofek, and Vlatka Sotošek. 2023. "Innate Immunity in Autoimmune Thyroid Disease during Pregnancy" International Journal of Molecular Sciences 24, no. 20: 15442. https://doi.org/10.3390/ijms242015442
APA StyleBogović Crnčić, T., Girotto, N., Ilić Tomaš, M., Krištofić, I., Klobučar, S., Batičić, L., Ćurko-Cofek, B., & Sotošek, V. (2023). Innate Immunity in Autoimmune Thyroid Disease during Pregnancy. International Journal of Molecular Sciences, 24(20), 15442. https://doi.org/10.3390/ijms242015442






