Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments
Abstract
:1. Introduction
2. Results
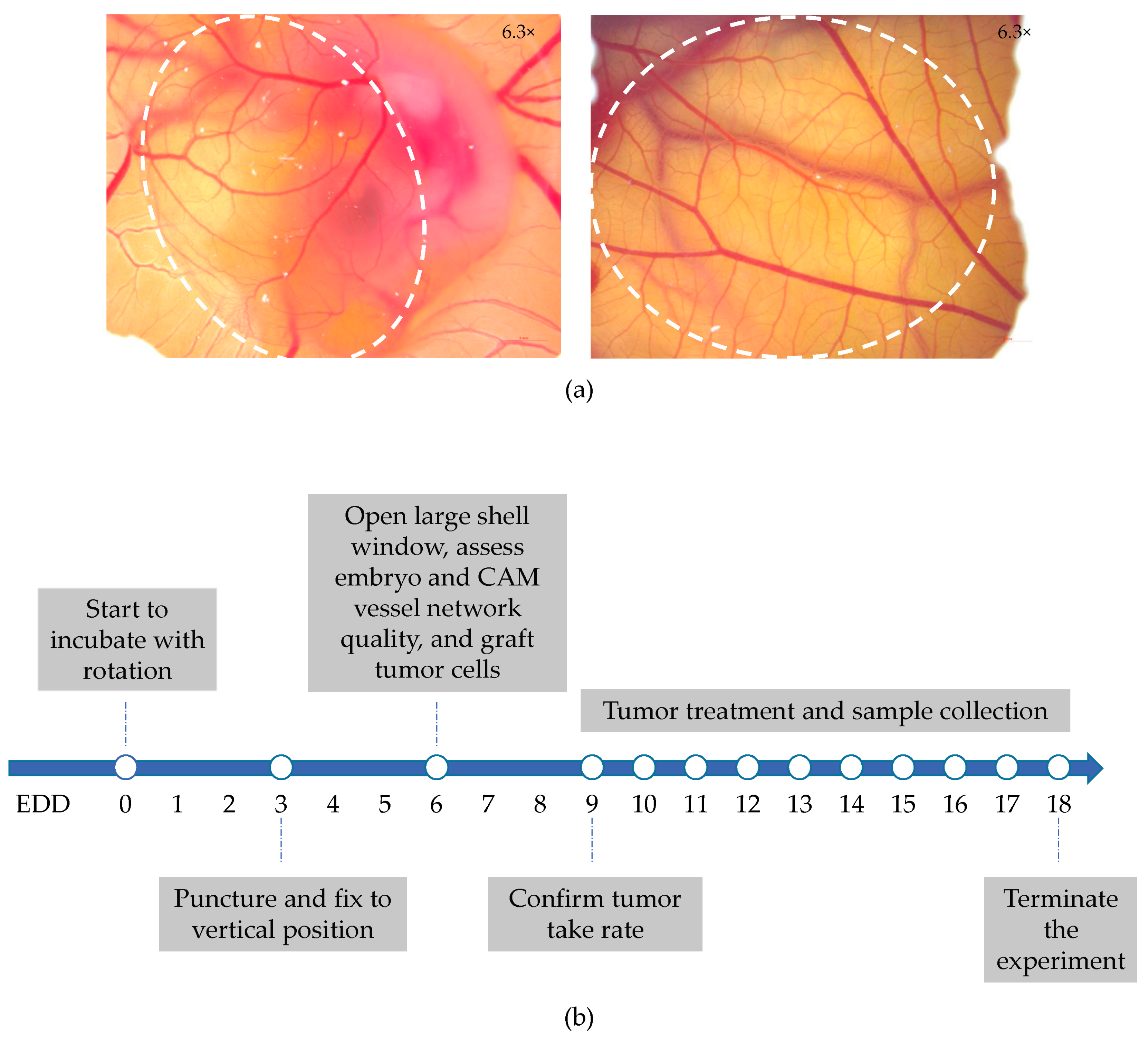
2.1. Establishment of Five Different Human NSCLC-CAM Tumor Models
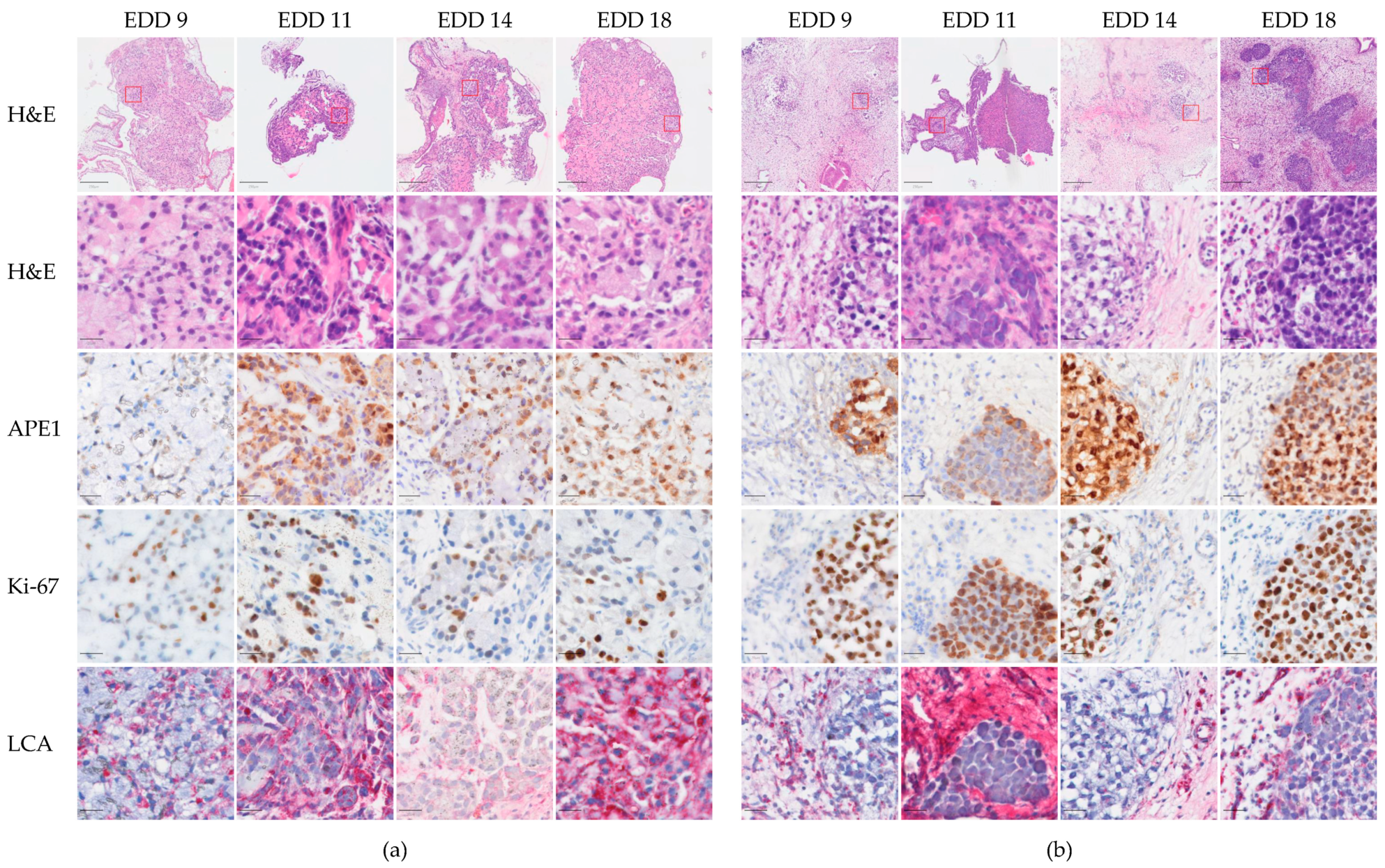
2.2. Histological Characteristics of A549-CAM and H460-CAM Tumor Tissues
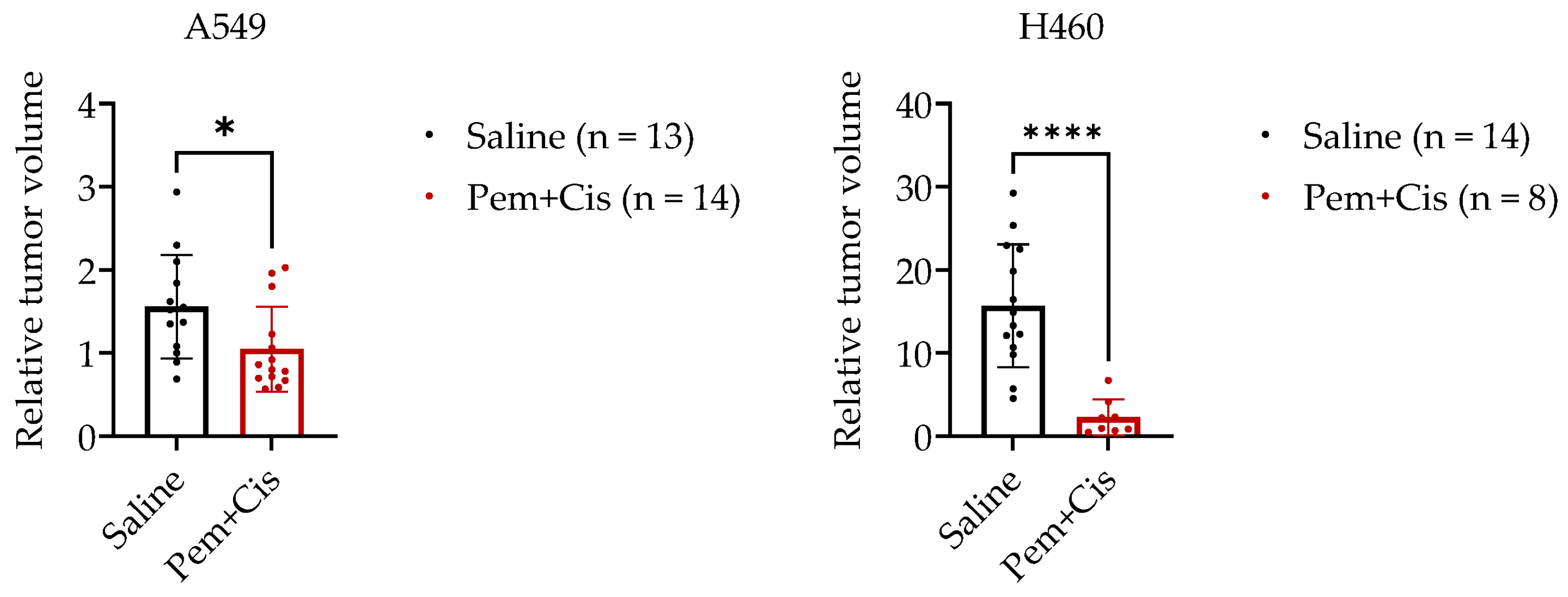
2.3. Chemotherapy Treatment of A549-CAM and H460-CAM Tumors
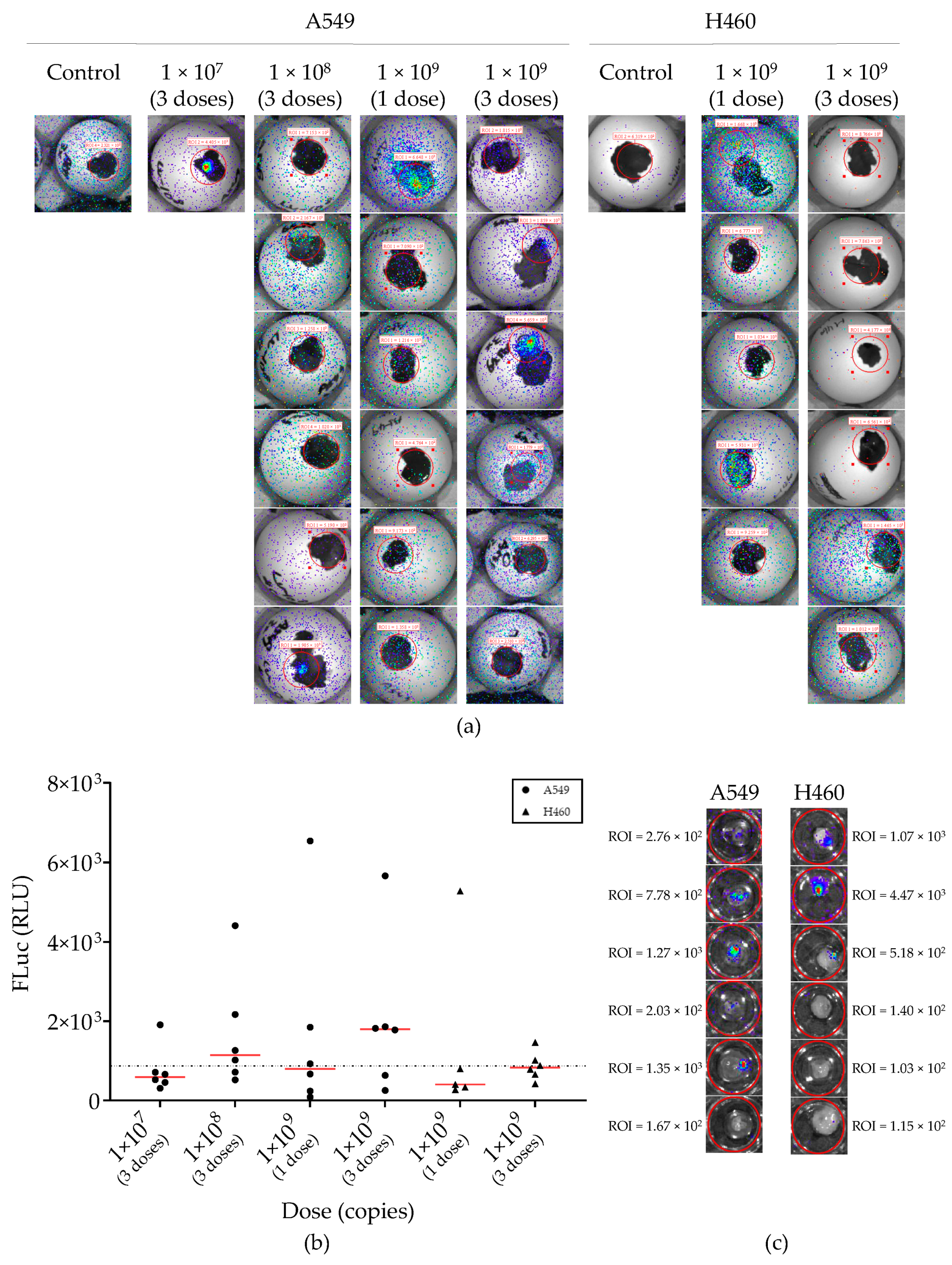
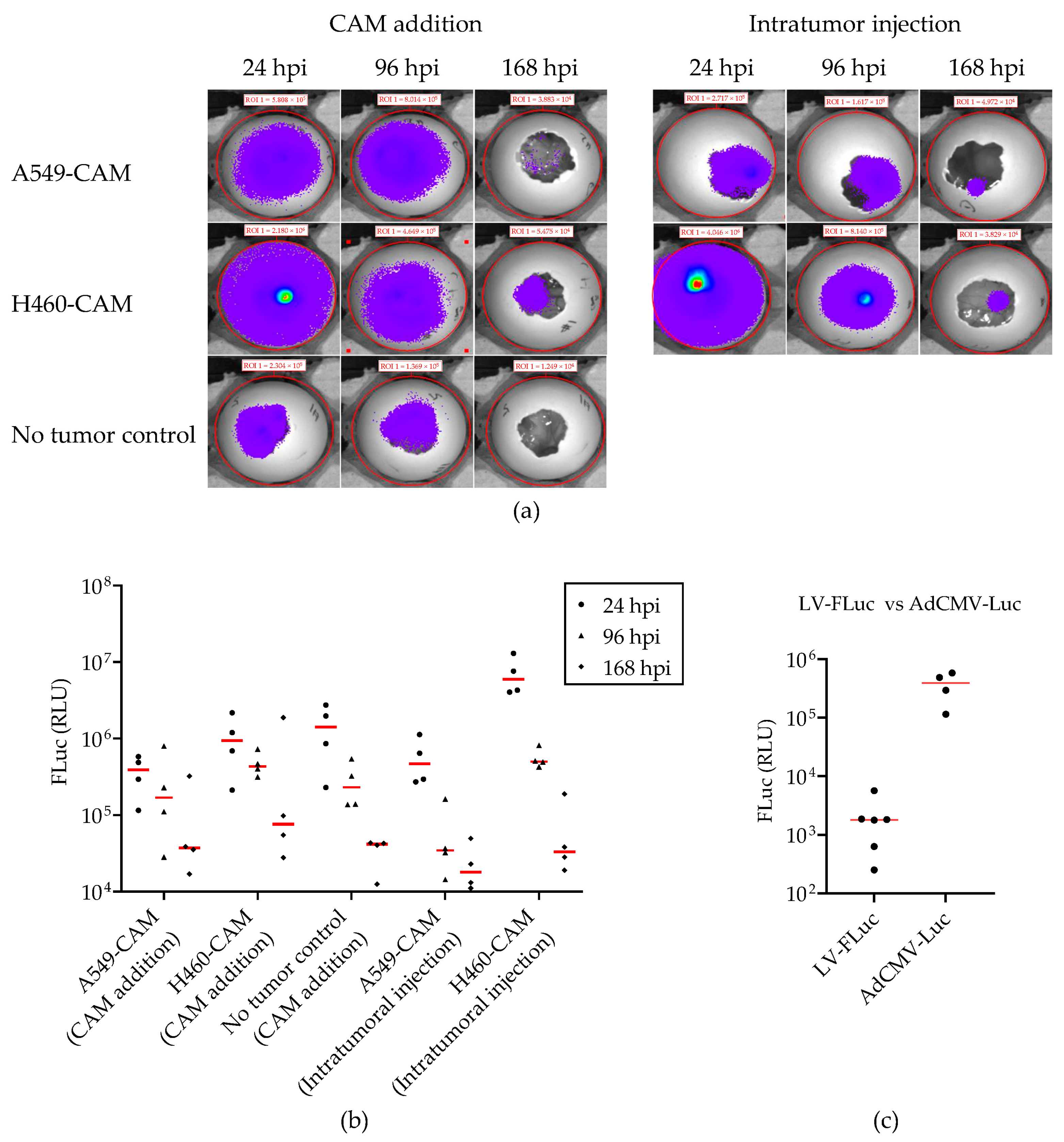
2.4. Lentiviral Vector-Mediated Gene Delivery into Established NSCLC-CAM Tumors
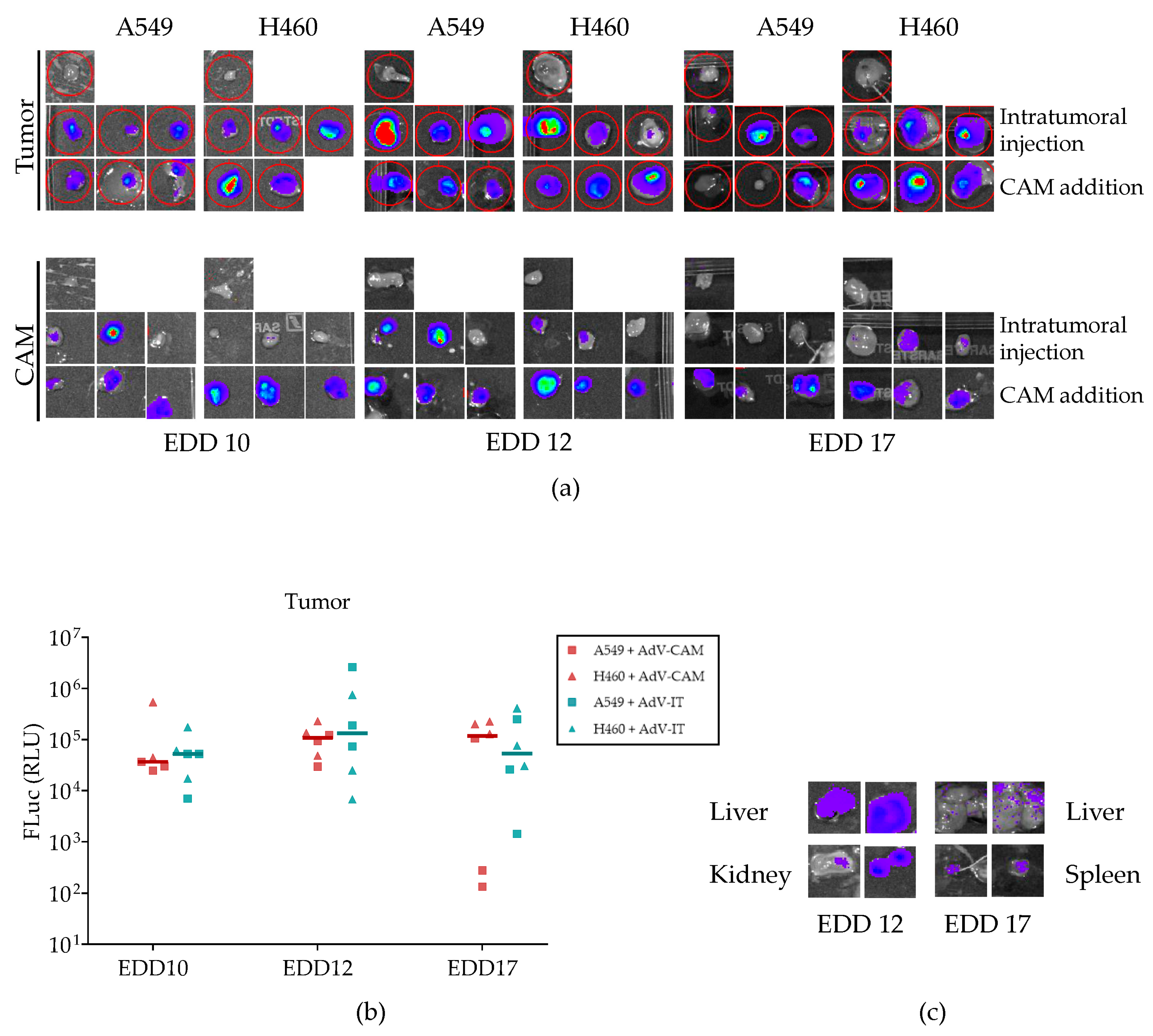
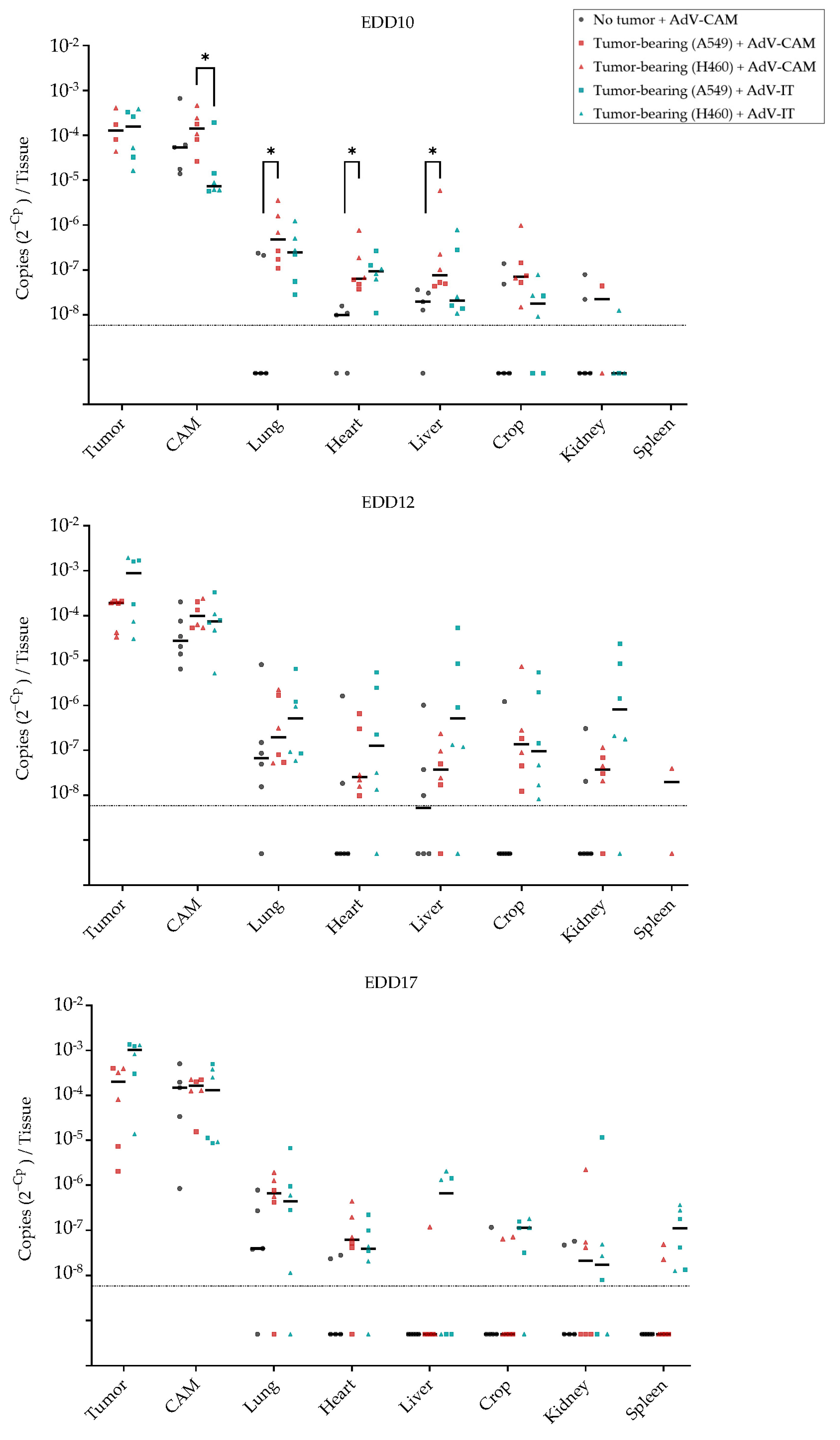
2.5. Adenovirus Vector-Mediated Gene Delivery into Established NSCLC-CAM Tumors and Host Chicken Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Viral Vector Production and Titration
4.3. NSCLC-CAM Tumor Assay
4.4. Histological Analysis of CAM Tumor Tissues
4.5. Bioluminescence Measurement via Live Imaging
4.6. Experimental Treatments of NSCLC-CAM Tumors
4.7. Quantification of Adenovirus Copy Numbers via qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.M.; Nagelkerke, A. Current developments in modelling the tumour microenvironment in vitro: Incorporation of biochemical and physical gradients. Organs-on-a-Chip 2021, 3, 100012. [Google Scholar] [CrossRef]
- Martinez-Pacheco, S.; O’Driscoll, L. Pre-Clinical In Vitro Models Used in Cancer Research: Results of a Worldwide Survey. Cancers 2021, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Could 3D models of cancer enhance drug screening? Biomaterials 2020, 232, 119744. [Google Scholar] [CrossRef] [PubMed]
- Onaciu, A.; Munteanu, R.; Munteanu, V.C.; Gulei, D.; Raduly, L.; Feder, R.I.; Pirlog, R.; Atanasov, A.G.; Korban, S.S.; Irimie, A.; et al. Spontaneous and Induced Animal Models for Cancer Research. Diagnostics 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Auerbach, R.; Kubai, L.; Knighton, D.; Folkman, J. A simple procedure for the long-term cultivation of chicken embryos. Dev. Biol. 1974, 41, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Wilting, J.; Neeff, H.; Christ, B. Embryonic lymphangiogenesis. Cell Tissue Res. 1999, 297, 1–11. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane in the study of tumor angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. [Google Scholar]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959. [Google Scholar] [CrossRef]
- Power, E.A.; Fernandez-Torres, J.; Zhang, L.; Yaun, R.; Lucien, F.; Daniels, D.J. Chorioallantoic membrane (CAM) assay to study treatment effects in diffuse intrinsic pontine glioma. PLoS ONE 2022, 17, e0263822. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Mouse Tumor Models for Advanced Cancer Immunotherapy. Int. J. Mol. Sci. 2020, 21, 4118. [Google Scholar] [CrossRef] [PubMed]
- Kleibeuker, E.A.; Ten Hooven, M.A.; Castricum, K.C.; Honeywell, R.; Griffioen, A.W.; Verheul, H.M.; Slotman, B.J.; Thijssen, V.L. Optimal treatment scheduling of ionizing radiation and sunitinib improves the antitumor activity and allows dose reduction. Cancer Med. 2015, 4, 1003–1015. [Google Scholar] [CrossRef]
- Rovithi, M.; Avan, A.; Funel, N.; Leon, L.G.; Gomez, V.E.; Wurdinger, T.; Griffioen, A.W.; Verheul, H.M.; Giovannetti, E. Development of bioluminescent chick chorioallantoic membrane (CAM) models for primary pancreatic cancer cells: A platform for drug testing. Sci. Rep. 2017, 7, 44686. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Scanlon, C.S.; Banerjee, R.; Russo, N.; Inglehart, R.C.; Willis, A.L.; Weiss, S.J.; D’Silva, N.J. The Histone Methyltransferase EZH2 Mediates Tumor Progression on the Chick Chorioallantoic Membrane Assay, a Novel Model of Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2013, 6, 273–281. [Google Scholar] [CrossRef]
- Jefferies, B.; Lenze, F.; Sathe, A.; Truong, N.; Anton, M.; von Eisenhart-Rothe, R.; Nawroth, R.; Mayer-Kuckuk, P. Non-invasive imaging of engineered human tumors in the living chicken embryo. Sci. Rep. 2017, 7, 4991. [Google Scholar] [CrossRef]
- Kunz, P.; Schenker, A.; Sähr, H.; Lehner, B.; Fellenberg, J. Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS ONE 2019, 14, e0215312. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiang, D.B.; Yang, X.Q.; Chen, L.S.; Li, M.X.; Zhong, Z.Y.; Zhang, Y.S. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer 2009, 66, 298–304. [Google Scholar] [CrossRef]
- Long, K.; Gu, L.; Li, L.; Zhang, Z.; Li, E.; Zhang, Y.; He, L.; Pan, F.; Guo, Z.; Hu, Z. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021, 12, 503. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Jilani, S.M.; Murphy, T.J.; Thai, S.N.; Eichmann, A.; Alva, J.A.; Iruela-Arispe, M.L. Selective binding of lectins to embryonic chicken vasculature. J. Histochem. Cytochem. 2003, 51, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Parikh, P.; von Pawel, J.; Biesma, B.; Vansteenkiste, J.; Manegold, C.; Serwatowski, P.; Gatzemeier, U.; Digumarti, R.; Zukin, M.; et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 3543–3551. [Google Scholar] [CrossRef]
- Wang, J.L.; Lan, Y.W.; Tsai, Y.T.; Chen, Y.C.; Staniczek, T.; Tsou, Y.A.; Yen, C.C.; Chen, C.M. Additive Antiproliferative and Antiangiogenic Effects of Metformin and Pemetrexed in a Non-Small-Cell Lung Cancer Xenograft Model. Front. Cell Dev. Biol. 2021, 9, 688062. [Google Scholar] [CrossRef]
- Diržiuvienė, R.; Šlekienė, L.; Palubinskienė, J.; Balnytė, I.; Lasienė, K.; Stakišaitis, D.; Valančiūtė, A. Tumors derived from lung cancer cells respond differently to treatment with sodium valproate (a HDAC inhibitor) in a chicken embryo chorioallantoic membrane model. Histol. Histopathol. 2022, 37, 1201–1212. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Pan, P.; Zhang, X.; Jia, Y.; Zhu, P.; Chen, X.; Jiao, Y.; Kang, G.; Zhang, L.; et al. α5-nAChR associated with Ly6E modulates cell migration via TGF-β1/Smad signaling in non-small cell lung cancer. Carcinogenesis 2022, 43, 393–404. [Google Scholar] [CrossRef]
- Sulaiman, S.; Arafat, K.; Al-Azawi, A.M.; AlMarzooqi, N.A.; Lootah, S.; Attoub, S. Butein and Frondoside-A Combination Exhibits Additive Anti-Cancer Effects on Tumor Cell Viability, Colony Growth, and Invasion and Synergism on Endothelial Cell Migration. Int. J. Mol. Sci. 2021, 23, 431. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the chick embryo chorioallantoic membrane (CAM) as a platform for anti-metastatic drug testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Tschanz, F.; Bender, S.; Telarovic, I.; Waller, V.; Speck, R.F.; Pruschy, M. The ADAM17-directed Inhibitory Antibody MEDI3622 Antagonizes Radiotherapy-induced VEGF Release and Sensitizes Non-Small Cell Lung Cancer for Radiotherapy. Cancer Res. Commun. 2021, 1, 164–177. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. BioMed Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Koh, A.P.; Antony, J.; Huang, R.Y. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Cavey, M.J. Development of the liver in the chicken embryo. I. Hepatic cords and sinusoids. Anat. Rec. 1992, 234, 555–567. [Google Scholar] [CrossRef]
- Wang, Y.; Rousset, X.; Prunier, C.; Garcia, P.; Dosda, E.; Leplus, E.; Viallet, J. PD-1/PD-L1 Checkpoint Inhibitors Are Active in the Chicken Embryo Model and Show Antitumor Efficacy In Ovo. Cancers 2022, 14, 3095. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.Y.; Ye, Z.; Cheng, L. Molecular imaging and stem cell research. Mol. Imaging 2011, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Gerard, R.D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. USA 1993, 90, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Tumor Take Rate on EDD 9 | Percent Measurable Tumors on EDD 9 | Embryo Survival Until EDD 18 * | Mean Relative Tumor Volume Increase from EDD 9–18 (SD) | Sample Size Needed Derived from Power Analysis # |
|---|---|---|---|---|---|
| SW1573 | 95% (40/42) | 55% (22/40) | 95% (21/22) | 3.3 (2.9) | 28 |
| A549 | 81% (34/42) | 65% (22/34) | 91% (20/22) | 3.7 (2.2) | 13 |
| H1299 | 91% (21/23) | 57% (12/21) | 100% (12/12) | 6.1 (6.4) | 37 |
| H292 | 95% (20/21) | 50% (10/20) | 100% (10/10) | 6.2 (5.9) | 31 |
| H460 | 90% (38/42) | 45% (17/38) | 88% (15/17) | 23.9 (17.7) | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Brachtlova, T.; van der Meulen-Muileman, I.H.; Kleerebezem, S.; Liu, C.; Li, P.; van Beusechem, V.W. Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 15425. https://doi.org/10.3390/ijms242015425
Li J, Brachtlova T, van der Meulen-Muileman IH, Kleerebezem S, Liu C, Li P, van Beusechem VW. Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments. International Journal of Molecular Sciences. 2023; 24(20):15425. https://doi.org/10.3390/ijms242015425
Chicago/Turabian StyleLi, Jing, Tereza Brachtlova, Ida H. van der Meulen-Muileman, Stijn Kleerebezem, Chang Liu, Peiyu Li, and Victor W. van Beusechem. 2023. "Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments" International Journal of Molecular Sciences 24, no. 20: 15425. https://doi.org/10.3390/ijms242015425
APA StyleLi, J., Brachtlova, T., van der Meulen-Muileman, I. H., Kleerebezem, S., Liu, C., Li, P., & van Beusechem, V. W. (2023). Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments. International Journal of Molecular Sciences, 24(20), 15425. https://doi.org/10.3390/ijms242015425







