Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior
Abstract
1. Introduction
2. TRPC6 as Calcium Permeable Channel
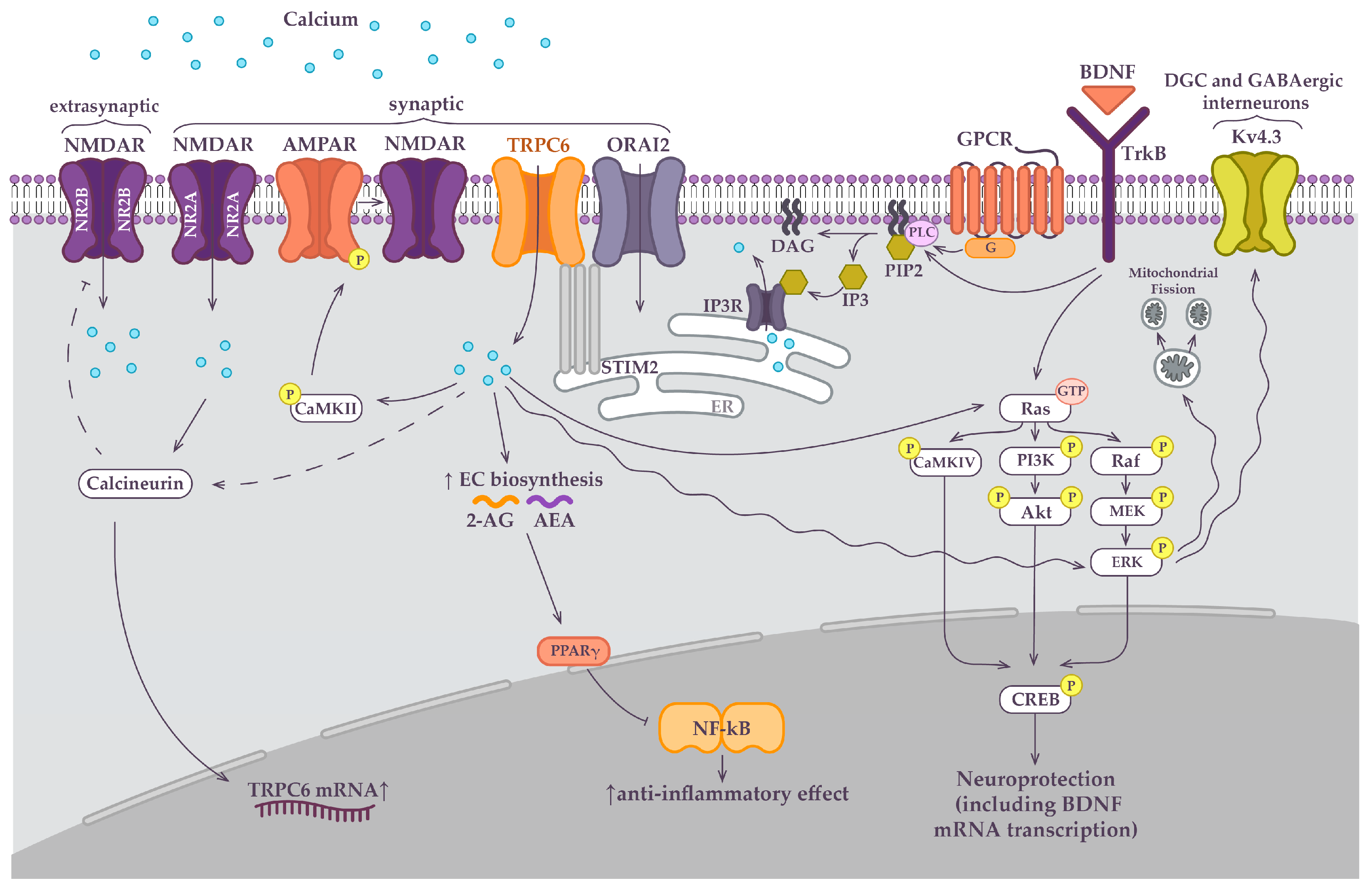
3. TRPC6-Mediated Synapse Development
4. TRPC6-Mediated Neuroprotective Intracellular Signaling Pathways
5. TRPC6 Dependent Signaling Pathways in Hippocampal DGC and GABAergic Interneuron
6. Influence of TRPC6 Agonists on Animal Behavior Including Memory Formation and Storage
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, J.; Harris, K.M. Do Thin Spines Learn to Be Mushroom Spines That Remember? Curr. Opin. Neurobiol. 2007, 17, 381–386. [Google Scholar] [CrossRef]
- Tackenberg, C.; Ghori, A.; Brandt, R. Thin, Stubby or Mushroom: Spine Pathology in Alzheimers Disease. Curr. Alzheimer Res. 2009, 6, 261–268. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced Synaptic STIM2 Expression and Impaired Store-Operated Calcium Entry Cause Destabilization of Mature Spines in Mutant Presenilin Mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef]
- Price, K.A.; Varghese, M.; Sowa, A.; Yuk, F.; Brautigam, H.; Ehrlich, M.E.; Dickstein, D.L. Altered Synaptic Structure in the Hippocampus in a Mouse Model of Alzheimer’s Disease with Soluble Amyloid-β Oligomers and No Plaque Pathology. Mol. Neurodegener. 2014, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, W.; Zhou, K.; Tai, Y.; Yao, H.; Jia, Y.; Ding, Y.; Wang, Y. Critical Role of TRPC6 Channels in the Formation of Excitatory Synapses. Nat. Neurosci. 2008, 11, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Griesi-Oliveira, K.; Acab, A.; Gupta, A.R.; Sunaga, D.Y.; Chailangkarn, T.; Nicol, X.; Nunez, Y.; Walker, M.F.; Murdoch, J.D.; Sanders, S.J.; et al. Modeling Non-Syndromic Autism and the Impact of TRPC6 Disruption in Human Neurons. Mol. Psychiatry 2014, 20, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kang, T.C. The Role of TRPC6 in Seizure Susceptibility and Seizure-Related Neuronal Damage in the Rat Dentate Gyrus. Neuroscience 2015, 307, 215–230. [Google Scholar] [CrossRef]
- Thapak, P.; Vaidya, B.; Joshi, H.C.; Singh, J.N.; Sharma, S.S. Therapeutic Potential of Pharmacological Agents Targeting TRP Channels in CNS Disorders. Pharmacol. Res. 2020, 159, 105026. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Bu, F.; Sun, G.; Tian, J.B.; Ting, S.M.; Li, J.; Aronowski, J.; Birnbaumer, L.; Freichel, M.; Zhu, M.X. Contribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic Stroke. Front. Cell Dev. Biol. 2021, 8, 618663. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. The Dysregulation of Intracellular Calcium in Alzheimer Disease. Cell Calcium 2010, 47, 183–189. [Google Scholar]
- Bezprozvanny, I. Calcium Signaling and Neurodegenerative Diseases. Trends Mol. Med. 2009, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer's Association Calcium Hypothesis Workgroup; Khachaturian, Z.S. Calcium Hypothesis of Alzheimer’s Disease and Brain Aging: A Framework for Integrating New Evidence into a Comprehensive Theory of Pathogenesis. Alzheimer’s Dement. 2017, 13, 178–182.e17. [Google Scholar] [CrossRef]
- Thomas, S.J.; Grossberg, G.T. Memantine: A Review of Studies into Its Safety and Efficacy in Treating Alzheimer’s Disease and Other Dementias. Clin. Interv. Aging 2009, 367. [Google Scholar] [CrossRef]
- Hong, C.; Jeong, B.; Park, H.J.; Chung, J.Y.; Lee, J.E.; Kim, J.; Shin, Y.C.; So, I. TRP Channels as Emerging Therapeutic Targets for Neurodegenerative Diseases. Front. Physiol. 2020, 11, 521830. [Google Scholar] [CrossRef]
- Lu, R.; Wang, J.; Tao, R.; Wang, J.; Zhu, T.; Guo, W.; Sun, Y.; Li, H.; Gao, Y.; Zhang, W.; et al. Reduced TRPC6 MRNA Levels in the Blood Cells of Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Mol. Psychiatry 2017, 23, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Li, Q.W.; Liu, J.S.; Jiang, G.X.; Liu, J.R.; Chen, S.D.; Cheng, Q. TRPC6 MRNA Levels in Peripheral Leucocytes of Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Case-Control Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019, 92, 279–284. [Google Scholar] [CrossRef]
- Tao, R.; Lu, R.; Wang, J.; Zeng, S.; Zhang, T.; Guo, W.; Zhang, X.; Cheng, Q.; Yue, C.; Wang, Y.; et al. Probing the Therapeutic Potential of TRPC6 for Alzheimer’s Disease in Live Neurons from Patient-Specific IPSCs. J. Mol. Cell Biol. 2020, 12, 807–816. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Fon Tacer, K.; Bezprozvanny, I. Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer’s Disease Treatment. J. Neurosci. 2016, 36, 11837–11850. [Google Scholar] [CrossRef]
- Popugaeva, E.; Chernyuk, D.; Zhang, H.; Postnikova, T.Y.; Pats, K.; Fedorova, E.; Poroikov, V.; Zaitsev, A.V.; Bezprozvanny, I. Derivatives of Piperazines as Potential Therapeutic Agents for Alzheimer’s Disease. Mol. Pharmacol. 2019, 95, 337–348. [Google Scholar] [CrossRef]
- Zernov, N.; Veselovsky, A.V.; Poroikov, V.V.; Melentieva, D.; Bolshakova, A.; Popugaeva, E. New Positive TRPC6 Modulator Penetrates Blood–Brain Barrier, Eliminates Synaptic Deficiency and Restores Memory Deficit in 5xFAD Mice. Int. J. Mol. Sci. 2022, 23, 13552. [Google Scholar] [CrossRef]
- Häfner, S.; Urban, N.; Schaefer, M. Discovery and Characterization of a Positive Allosteric Modulator of Transient Receptor Potential Canonical 6 (TRPC6) Channels. Cell Calcium 2019, 78, 26–34. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, M.; Peyton, M.; Boulay, G.; Hurst, R.; Stefani, E.; Birnbaumer, L. Trp, a Novel Mammalian Gene Family Essential for Agonist-Activated Capacitative Ca2+ Entry. Cell 1996, 85, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Inoue, R.; Yamazaki, K.; Maeda, A.; Kurosaki, T.; Yamakuni, T.; Tanaka, I.; Shimizu, S.; Ikenaka, K.; Imoto, K.; et al. Molecular and Functional Characterization of a Novel Mouse Transient Receptor Potential Protein Homologue TRP7: Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999, 274, 27359–27370. [Google Scholar] [CrossRef] [PubMed]
- Boulay, G.; Zhu, X.; Peyton, M.; Jiang, M.; Hurst, R.; Stefani, E.; Birnbaumer, L. Cloning and Expression of a Novel Mammalian Homolog of Drosophila Transient Receptor Potential (Trp) Involved in Calcium Entry Secondary to Activation of Receptors Coupled by the Gq Class of G Protein. J. Biol. Chem. 1997, 272, 29672–29680. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct Activation of Human TRPC6 and TRPC3 Channels by Diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The Trp Ion Channel Family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Guo, W.; Zheng, L.; Wu, J.X.; Liu, M.; Zhou, X.; Zhang, X.; Chen, L. Structure of the Receptor-Activated Human TRPC6 and TRPC3 Ion Channels. Cell Res. 2018, 28, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, J.; Tai, Y.; Wang, Y. TRPC Channels Promote Cerebellar Granule Neuron Survival. Nat. Neurosci. 2007, 10, 559–567. [Google Scholar] [CrossRef]
- Dietrich, A.; Gudermann, T. TRPC6: Physiological Function and Pathophysiological Relevance. Handb. Exp. Pharmacol. 2014, 222, 157–188. [Google Scholar] [CrossRef]
- Cayouette, S.; Lussier, M.P.; Mathieu, E.L.; Bousquet, S.M.; Boulay, G. Exocytotic Insertion of TRPC6 Channel into the Plasma Membrane upon Gq Protein-Coupled Receptor Activation. J. Biol. Chem. 2004, 279, 7241–7246. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Du, W.; Jia, C.; Yao, H.; Wang, Y. TRPC6 Inhibited NMDA Receptor Activities and Protected Neurons from Ischemic Excitotoxicity. J. Neurochem. 2012, 123, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Barria, A.; Muller, D.; Derkach, V.; Griffith, L.C.; Soderling, T.R. Regulatory Phosphorylation of AMPA-Type Glutamate Receptors by CaM-KII during Long-Term Potentiation. Science 1997, 276, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Malinow, R.; Malenka, R.C. AMPA Receptor Trafficking and Synaptic Plasticity. Annu. Rev. Neurosci. 2003, 25, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Isaac, J.T.R.; Yu, T.W. Receptor Trafficking and Synaptic Plasticity. Nat. Rev. Neurosci. 2004, 5, 952–962. [Google Scholar] [CrossRef]
- Zernov, N.; Bezprozvanny, I.; Popugaeva, E. CaMKIIβ Knockdown Decreases Store-Operated Calcium Entry in Hippocampal Dendritic Spines. IBRO Neurosci. Reports 2022, 12, 90. [Google Scholar] [CrossRef]
- Shi, J.; Mori, E.; Mori, Y.; Mori, M.; Li, J.; Ito, Y.; Inoue, R. Multiple Regulation by Calcium of Murine Homologues of Transient Receptor Potential Proteins TRPC6 and TRPC7 Expressed in HEK293 Cells. J. Physiol. 2004, 561, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Geshi, N.; Takahashi, S.; Kiyonaka, S.; Ichikawa, J.; Hu, Y.; Mori, Y.; Ito, Y.; Inoue, R. Molecular Determinants for Cardiovascular TRPC6 Channel Regulation by Ca2+/Calmodulin-Dependent Kinase II. J. Physiol. 2013, 591, 2851–2866. [Google Scholar] [CrossRef]
- Sattler, R.; Charlton, M.P.; Hafner, M.; Tymianski, M. Distinct Influx Pathways, Not Calcium Load, Determine Neuronal Vulnerability to Calcium Neurotoxicity. J. Neurochem. 1998, 71, 2349–2364. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, Ischemia and Excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Xia, P.; Chen, H.S.V.; Zhang, D.; Lipton, S.A. Memantine Preferentially Blocks Extrasynaptic over Synaptic NMDA Receptor Currents in Hippocampal Autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Stocca, G.; Vicini, S. Increased Contribution of NR2A Subunit to Synaptic NMDA Receptors in Developing Rat Cortical Neurons. J. Physiol. 1998, 507 Pt 1, 13–24. [Google Scholar] [CrossRef]
- Rumbaugh, G.; Vicini, S. Distinct Synaptic and Extrasynaptic NMDA Receptors in Developing Cerebellar Granule Neurons. J. Neurosci. 1999, 19, 10603–10610. [Google Scholar] [CrossRef]
- Tovar, K.R.; Westbrook, G.L. Mobile NMDA Receptors at Hippocampal Synapses. Neuron 2002, 34, 255–264. [Google Scholar] [CrossRef]
- Chen, M.; Lu, T.J.; Chen, X.J.; Zhou, Y.; Chen, Q.; Feng, X.Y.; Xu, L.; Duan, W.H.; Xiong, Z.Q. Differential Roles of NMDA Receptor Subtypes in Ischemic Neuronal Cell Death and Ischemic Tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, Y.; Li, X.; Wu, L.; Wang, Y. TRPC6 Expression in Neurons Is Differentially Regulated by NR2A- and NR2B-Containing NMDA Receptors. J. Neurochem. 2017, 143, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pan, J.; Pan, L.; Zhang, N. TRPC6 Inhibited NMDA Current in Cultured Hippocampal Neurons. Neuromolecular Med. 2013, 15, 389–395. [Google Scholar] [CrossRef]
- Courjaret, R.; Prakriya, M.; Machaca, K. SOCE as a Regulator of Neuronal Activity. J. Physiol. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Barbarosie, M.; Kameyama, K.; Bear, M.F.; Huganir, R.L. Regulation of Distinct AMPA Receptor Phosphorylation Sites during Bidirectional Synaptic Plasticity. Nature 2000, 405, 955–959. [Google Scholar] [CrossRef]
- Walton, M.R.; Dragunow, M. Is CREB a Key to Neuronal Survival? Trends Neurosci. 2000, 23, 48–53. [Google Scholar] [CrossRef]
- Tai, Y.; Feng, S.; Ge, R.; Du, W.; Zhang, X.; He, Z.; Wang, Y. TRPC6 Channels Promote Dendritic Growth via the CaMKIV-CREB Pathway. J Cell Sci. 2008, 2301–2307. [Google Scholar] [CrossRef]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK Pathway by Ca2+, and Calmodulin. Cell. Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef]
- Heiser, J.H.; Schuwald, A.M.; Sillani, G.; Ye, L.; Müller, W.E.; Leuner, K. TRPC6 Channel-Mediated Neurite Outgrowth in PC12 Cells and Hippocampal Neurons Involves Activation of RAS/MEK/ERK, PI3K, and CAMKIV Signaling. J. Neurochem. 2013, 127, 303–313. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E.; Zuccato, C.; Cattaneo, E. Brain-Derived Neurotrophic Factor in Neurodegenerative Diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, L.; Li, A.; Ge, J.; Laties, A.M.; Zhang, X. TRPC6 Channel Protects Retinal Ganglion Cells in a Rat Model of Retinal Ischemia/Reperfusion-Induced Cell Death. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5751–5758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hillard, C.J. Biochemistry and Pharmacology of the Endocannabinoids Arachidonylethanolamide and 2-Arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000, 61, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bardell, T.K.; Barker, E.L. Activation of TRPC6 Channels Promotes Endocannabinoid Biosynthesis in Neuronal CAD Cells. Neurochem. Int. 2010, 57, 76–83. [Google Scholar] [CrossRef]
- Bisogno, T.; Di Marzo, V. Cannabinoid Receptors and Endocannabinoids: Role in Neuroinflammatory and Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2010, 9, 564–573. [Google Scholar] [CrossRef]
- Xu, J.Y.; Chen, C. Endocannabinoids in Synaptic Plasticity and Neuroprotection. Neuroscientist 2015, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Shohami, E.; Cohen-Yeshurun, A.; Magid, L.; Algali, M.; Mechoulam, R. Endocannabinoids and Traumatic Brain Injury. Br. J. Pharmacol. 2011, 163, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; De Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The Endocannabinoid System Is Dysregulated in Multiple Sclerosis and in Experimental Autoimmune Encephalomyelitis. Brain 2007, 130, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Tontonoz, P. Integration of Metabolism and Inflammation by Lipid-Activated Nuclear Receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, P.; Guo, H.; Tian, B.; Liu, X.; Bittner, A.; Roland, B.; Salunga, R.; Ma, X.J.; Kamme, F.; Meurers, B.; et al. Nuclei and Subnuclei Gene Expression Profiling in Mammalian Brain. Brain Res. 2002, 943, 38–47. [Google Scholar] [CrossRef]
- Liu, L.; Chen, M.; Lin, K.; Xiang, X.; Yang, J.; Zheng, Y.; Xiong, X.; Zhu, S. TRPC6 Attenuates Cortical Astrocytic Apoptosis and Inflammation in Cerebral Ischemic/Reperfusion Injury. Front. Cell Dev. Biol. 2021, 8, 594283. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Huang, J.; Yao, H.; Zhou, K.; Duan, B.; Wang, Y. Inhibition of TRPC6 Degradation Suppresses Ischemic Brain Damage in Rats. J. Clin. Investig. 2010, 120, 3480–3492. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Medhurst, A.D.; Mattei, C.; Kelsell, R.E.; Calver, A.R.; Randall, A.D.; Benham, C.D.; Pangalos, M.N. MRNA Distribution Analysis of Human TRPC Family in CNS and Peripheral Tissues. Mol. Brain Res. 2002, 109, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.A.; Botond, G.; Borhegyi, Z.; Plummer, N.W.; Freund, T.F.; Hájos, N. DAG-Sensitive and Ca 21 Permeable TRPC6 Channels Are Expressed in Dentate Granule Cells and Interneurons in the Hippocampal Formation. Hippocampus 2013, 23, 221–232. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Z.; Liu, T.; Xiao, R.; Lv, K.; Wu, C.; Luo, Y.; Cai, Y.; Fan, X. AAV Delivery of ShRNA Against TRPC6 in Mouse Hippocampus Impairs Cognitive Function. Front. Cell Dev. Biol. 2021, 9, 688655. [Google Scholar] [CrossRef]
- Leutgeb, J.K.; Leutgeb, S.; Moser, M.B.; Moser, E.I. Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science 2007, 315, 961–966. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal Neurogenesis Confers Stress Resilience by Inhibiting the Ventral Dentate Gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Senzai, Y. Function of Local Circuits in the Hippocampal Dentate Gyrus-CA3 System. Neurosci. Res. 2019, 140, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hainmueller, T.; Bartos, M. Dentate Gyrus Circuits for Encoding, Retrieval and Discrimination of Episodic Memories. Nat. Rev. Neurosci. 2020, 21, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Jonas, P.; Lisman, J. Structure, Function, and Plasticity of Hippocampal Dentate Gyrus Microcircuits. Front. Neural Circuits 2014, 8, 110127. [Google Scholar] [CrossRef]
- Hoffman, D.A.; Magee, J.C.; Colbert, C.M.; Johnston, D. K+ Channel Regulation of Signal Propagation in Dendrites of Hippocampal Pyramidal Neurons. Nature 1997, 387, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Anderson, A.; Becker, A.; Poolos, N.P.; Deck, H.; Johnston, D. Acquired Dendritic Channelopathy in Temporal Lobe Epilepsy. Science 2004, 305, 532–535. [Google Scholar] [CrossRef]
- Birnbaum, S.G.; Varga, A.W.; Yuan, L.L.; Anderson, A.E.; Sweatt, J.D.; Schrader, L.A. Structure and Function of Kv4-Family Transient Potassium Channels. Physiol. Rev. 2004, 84, 803–833. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, J.Y.; Kang, T.C. TRPC6-Mediated ERK1/2 Activation Regulates Neuronal Excitability via Subcellular Kv4.3 Localization in the Rat Hippocampus. Front. Cell. Neurosci. 2017, 11, 316500. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Choi, S.H.; Kong, M.J.; Kang, T.C. TRPC6-Mediated ERK1/2 Activation Increases Dentate Granule Cell Resistance to Status Epilepticus via Regulating Lon Protease-1 Expression and Mitochondrial Dynamics. Cells 2019, 8, 1376. [Google Scholar] [CrossRef]
- Beis, D.; Schwarting, R.K.W.; Dietrich, A. Evidence for a Supportive Role of Classical Transient Receptor Potential 6 (TRPC6) in the Exploration Behavior of Mice. Physiol. Behav. 2011, 102, 245–250. [Google Scholar] [CrossRef]
- Cross, J.L.; Meloni, B.P.; Bakker, A.J.; Lee, S.; Knuckey, N.W. Modes of Neuronal Calcium Entry and Homeostasis Following Cerebral Ischemia. Int. J. Alzheimers. Dis. 2010. [Google Scholar] [CrossRef]
- Basora, N.; Boulay, G.; Bilodeau, L.; Rousseau, E.; Payet, M.D. 20-Hydroxyeicosatetraenoic Acid (20-HETE) Activates Mouse TRPC6 Channels Expressed in HEK293 Cells. J. Biol. Chem. 2003, 278, 31709–31716. [Google Scholar] [CrossRef]
- Aires, V.; Hichami, A.; Boulay, G.; Khan, N.A. Activation of TRPC6 Calcium Channels by Diacylglycerol (DAG)-Containing Arachidonic Acid: A Comparative Study with DAG-Containing Docosahexaenoic Acid. Biochimie 2007, 89, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Eady, T.N.; Hong, S.; Lu, Y.; Obenaus, A.; Bazan, N.G. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Transl. Stroke Res. 2011, 2, 33–41. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, J.; Chen, F.; Lin, Y. Neuroprotectin D1 Attenuates Brain Damage Induced by Transient Middle Cerebral Artery Occlusion in Rats through TRPC6/CREB Pathways. Mol. Med. Rep. 2013, 8, 543–550. [Google Scholar] [CrossRef]
- Guinamard, R.; Simard, C.; Del Negro, C. Flufenamic Acid as an Ion Channel Modulator. Pharmacol. Ther. 2013, 138, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, S.; Hatano, M.; Takada, Y.; Hino, K.; Kawamura, T.; Tanikawa, J.; Nakagawa, H.; Hase, H.; Nakao, A.; Hirano, M.; et al. Screening of Transient Receptor Potential Canonical Channel Activators Identifies Novel Neurotrophic Piperazine Compounds. Mol. Pharmacol. 2016, 89, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Tiapko, O.; Shrestha, N.; Lindinger, S.; Guedes De La Cruz, G.; Graziani, A.; Klec, C.; Butorac, C.; Graier, W.F.; Kubista, H.; Freichel, M.; et al. Lipid-Independent Control of Endothelial and Neuronal TRPC3 Channels by Light. Chem. Sci. 2019, 10, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, F.; Zhang, J.; Wang, T.; Wei, X.; Wu, J.; Feng, Y.; Dai, Z.; Wu, Q. Neuroprotective Effect of Resveratrol on Ischemia/Reperfusion Injury in Rats through TRPC6/CREB Pathways. J. Mol. Neurosci. 2013, 50, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Tong, L.; Xi, M.; Yang, H.; Dong, H.; Wen, A. Neuroprotective Effect of Calycosin on Cerebral Ischemia and Reperfusion Injury in Rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, J.; Liu, G.; Chen, F.; Lin, Y. Neuroprotection by (-)-Epigallocatechin-3-Gallate in a Rat Model of Stroke Is Mediated through Inhibition of Endoplasmic Reticulum Stress. Mol. Med. Rep. 2014, 9, 69–72. [Google Scholar] [CrossRef]
- Kumar, N.; Husain, G.M.; Singh, P.N.; Kumar, V. Antiaggressive Activity of Hyperforin: A Preclinical Study. Drug Discover. Ther. 2009, 3, 162–167. [Google Scholar]
- Dinamarca, M.C.; Cerpa, W.; Garrido, J.; Hancke, J.L.; Inestrosa, N.C. Hyperforin Prevents β-Amyloid Neurotoxicity and Spatial Memory Impairments by Disaggregation of Alzheimer’s Amyloid-β-Deposits. Mol. Psychiatry 2006, 11, 1032–1048. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, C.; Qin, X.; Yang, Z. Hyperforin Alleviates Mood Deficits of Adult Rats Suffered from Early Separation. Neurosci. Lett. 2015, 608, 1–5. [Google Scholar] [CrossRef]
- Klusa, V.; Germane, S.; Nöldner, M.; Chatterjee, S.S. Hypericum Extract and Hyperforin: Memory-Enhancing Properties in Rodents. Pharmacopsychiatry 2001, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Gaid, M.; Biedermann, E.; Füller, J.; Haas, P.; Behrends, S.; Krull, R.; Scholl, S.; Wittstock, U.; Müller-Goymann, C.; Beerhues, L. Biotechnological Production of Hyperforin for Pharmaceutical Formulation. Eur. J. Pharm. Biopharm. 2018, 126, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Sell, T.S.; Belkacemi, T.; Flockerzi, V.; Beck, A. Protonophore Properties of Hyperforin Are Essential for Its Pharmacological Activity. Sci. Rep. 2014, 4, 7500. [Google Scholar] [CrossRef]
- El Hamdaoui, Y.; Zheng, F.; Fritz, N.; Ye, L.; Tran, M.A.; Schwickert, K.; Schirmeister, T.; Braeuning, A.; Lichtenstein, D.; Hellmich, U.A.; et al. Analysis of Hyperforin (St. John’s Wort) Action at TRPC6 Channel Leads to the Development of a New Class of Antidepressant Drugs. Mol. Psychiatry 2022, 27, 5070–5085. [Google Scholar] [CrossRef]
- Cerpa, W.; Hancke, J.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Marin, P.; Inestrosa, N. The Hyperforin Derivative IDN5706 Occludes Spatial Memory Impairments and Neuropathological Changes in a Double Transgenic Alzheimers Mouse Model. Curr. Alzheimer Res. 2010, 7, 126–133. [Google Scholar] [CrossRef]
- Callizot, N.; Estrella, C.; Burlet, S.; Henriques, A.; Brantis, C.; Barrier, M.; Campanari, M.L.; Verwaerde, P. AZP2006, a New Promising Treatment for Alzheimer’s and Related Diseases. Sci. Rep. 2021, 11, 16806. [Google Scholar] [CrossRef]
- Singh, A.; Bodakhe, S.H. Resveratrol Attenuates Behavioural Impairment Associated with Learning and Memory in Rats with Diabetes Induced by a High-Fat Diet and Streptozotocin. Br. J. Pharmacol. 2022, 179, 4673–4691. [Google Scholar] [CrossRef]
- Kim, J.J.; Fanselow, M.S. Modality-Specific Retrograde Amnesia of Fear. Science 1992, 256, 675–677. [Google Scholar] [CrossRef]
- Krabbe, S.; Gründemann, J.; Lüthi, A. Amygdala Inhibitory Circuits Regulate Associative Fear Conditioning. Biol. Psychiatry 2018, 83, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Li, Y.; Moon, J.; Kim, K.S.; Smith, K.S.; Rudolph, U.; Gapon, S.; Yao, G.L.; Tsvetkov, E.; Rodig, S.J.; et al. Essential Role for TRPC5 in Amygdala Function and Fear-Related Behavior. Cell 2009, 137, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Strübing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of Novel TRPC Channels by Complex Subunit Interactions in Embryonic Brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris Water Maze in the Study of Learning and Memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. Neuropsychiatric Sequelae of Early Nutritional Modifications: A Beginner’s Guide to Behavioral Analysis. Methods Mol. Biol. 2018, 1735, 403–420. [Google Scholar] [CrossRef]
- Ma, H.; Li, C.; Wang, J.; Zhang, X.; Li, M.; Zhang, R.; Huang, Z.; Zhang, Y. Amygdala-Hippocampal Innervation Modulates Stress-Induced Depressive-like Behaviors through AMPA Receptors. Proc. Natl. Acad. Sci. USA 2021, 118, e2019409118. [Google Scholar] [CrossRef]
- Maren, S. Emotional Learning: Animals. In Learning and Memory: A Comprehensive Reference; Elsevier: Oxford, UK, 2008; pp. 475–502. [Google Scholar] [CrossRef]
- Cinalli, D.A.; Cohen, S.J.; Guthrie, K.; Stackman, R.W. Object Recognition Memory: Distinct Yet Complementary Roles of the Mouse CA1 and Perirhinal Cortex. Front. Mol. Neurosci. 2020, 13, 527543. [Google Scholar] [CrossRef]
- Mazarati, A.M. Behavioral and Cognitive Testing Procedures in Animal Models of Epilepsy. In Models of Seizures and Epilepsy, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 181–196. [Google Scholar] [CrossRef]
- Bin Kim, W.; Cho, J.-H. Encoding of Contextual Fear Memory in Hippocampal-Amygdala Circuit. Nat. Commun. 2020, 11, 1382. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Gardner-Medwin, A.R. Long-Lasting Potentiation of Synaptic Transmission in the Dentate Area of the Unanaestetized Rabbit Following Stimulation of the Perforant Path. J. Physiol. 1973, 232, 357–374. [Google Scholar] [CrossRef]
- Ito, M. Long-Term Depression. Annu. Rev. Neurosci. 1989, 12, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Sakurai, M.; Tongroach, P. Climbing Fibre Induced Depression of Both Mossy Fibre Responsiveness and Glutamate Sensitivity of Cerebellar Purkinje Cells. J. Physiol. 1982, 324, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Dudek, S.M.; Bear, M.F. Homosynaptic Long-Term Depression in Area CA1 of Hippocampus and Effects of N-Methyl-D-Aspartate Receptor Blockade. Proc. Natl. Acad. Sci. USA 1992, 89, 4363–4367. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Staruschenko, A. TRPC6 Channel as an Emerging Determinant of the Podocyte Injury Susceptibility in Kidney Diseases. Am. J. Physiol.—Ren. Physiol. 2015, 309, F393–F397. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. A Mutation in the TRPC6 Cation Channel Causes Familial Focal Segmental Glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef]
- Staruschenko, A.; Spires, D.; Palygin, O. Role of TRPC6 in Progression of Diabetic Kidney Disease. Curr. Hypertens. Rep. 2019, 21, 48. [Google Scholar] [CrossRef]
- Woelk, H.; Burkard, G.; Grünwald, J. Benefits and Risks of the Hypericum Extract LI 160: Drug Monitoring Study with 3250 Patients. J. Geriatr. Psychiatry Neurol. 1994, 7 (Suppl. 1), 34–38. [Google Scholar] [CrossRef]
| TRPC6 Positive Modulator | Behavioral Test | Daily Dose, Method | Duration | Animal Model (Disease Model) | Therapeutic Effect | Brain Region/System Involved | Reference |
|---|---|---|---|---|---|---|---|
| Hyperforin | Morris water maze test | 6 µM, intracerebral injection | 4–18 days | Sprague–Dawley rats injected with Aβ (AD) | Improvement of spatial memory | Hippocampus, striatum, basal forebrain, cerebellum and cerebral cortex [105] | [92] |
| Open field test | 3 mg/kg, intragastric administration | 14 days | Early separated from parents Wistar rats (depression) | Anxiolytic effect | Mesolimbic/nigrostriatal dopamine systems [106] | [93] | |
| Novelty suppressed feeding test | Anxiolytic effect and antidepressant effects | Amygdala, hippocampus [107] | |||||
| Forced swimming test | Antidepressant effect | Amygdala, hippocampus [106] | |||||
| Conditioned avoidance test | 1.25 mg/kg, oral administration | 7 consecutive days and day 17 (i.e., after 9 days without treatment) | Wistar rats (depression) | Antidepressant effect and improvement of memory | Hippocampus/amygdala [108] | [94] | |
| Passive avoidance tests | 3 times for 1 day (1 h before, 1 h and 23 h after training) | Antidepressant effect and improvement of memory | Hippocampus/amygdala [108] | ||||
| Hyp13 | Open field test | 5 mg/kg, intraperitoneal injection | Once (20 min before the test) | TRPC6 KO mice (depression) | Anxiolytic effect | Mesolimbic/nigrostriatal dopamine systems [106] | [97] |
| Novelty suppressed feeding test | Anxiolytic effect and antidepressant effects | Amygdala, hippocampus [107] | |||||
| Forced swimming test | Antidepressant effect | Amygdala, hippocampus [106] | |||||
| IDN5706 | Morris water maze test | 2 mg/kg, intraperitoneal injection | 4 weeks | APPPSEN1deltaE9 (AD) | Improvement of spatial memory | Hippocampus, striatum, basal forebrain, cerebellum and cerebral cortex [105] | [98] |
| AZP2006 | Y-maze test | 3 mg/kg, oral administration | 4–8 months | C57B/6Rj mice injected with Aβ (AD) | Improvement of spatial memory | Hippocampus [106] | [99] |
| Passive avoidance test | Antidepressant effect and improvement of memory | Hippocampus/amygdala [108] | |||||
| Resveratrol | Novel object recognition test | 50 or 100 mg/kg, intraperitoneal injection | 4 weeks | Sprague–Dawley rats fed a high-fat diet (type 2 diabetes mellitus) | Anxiolytic effect and improvement of memory | Hippocampus and perirhinal cortex [109] | [100] |
| Elevated plus maze test, | Improvement of acquisition and retention memory | Amygdala [106] | |||||
| Light–dark passive avoidance test | Improvement of emotional memory | Hippocampus/amygdala [108] | |||||
| Radial arm maze | Improvement of working and reference memories | Hippocampus, frontal cortex, and forebrain cholinergic pathways [110] | |||||
| Nest building | Restore of cognitive function | General (including hippocampus) [106] | |||||
| C20 | Cued fear conditioning test | 10 mg/kg, intraperitoneal injection | 14 days | 5xFAD mice (AD) | Improvement of cued memory | Amygdala [102] | [20] |
| Context fear conditioning test | Improvement of context memory | Hippocampus, amygdala [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zernov, N.; Popugaeva, E. Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior. Int. J. Mol. Sci. 2023, 24, 15415. https://doi.org/10.3390/ijms242015415
Zernov N, Popugaeva E. Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior. International Journal of Molecular Sciences. 2023; 24(20):15415. https://doi.org/10.3390/ijms242015415
Chicago/Turabian StyleZernov, Nikita, and Elena Popugaeva. 2023. "Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior" International Journal of Molecular Sciences 24, no. 20: 15415. https://doi.org/10.3390/ijms242015415
APA StyleZernov, N., & Popugaeva, E. (2023). Role of Neuronal TRPC6 Channels in Synapse Development, Memory Formation and Animal Behavior. International Journal of Molecular Sciences, 24(20), 15415. https://doi.org/10.3390/ijms242015415






