Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens
Abstract
1. Introduction
2. Results
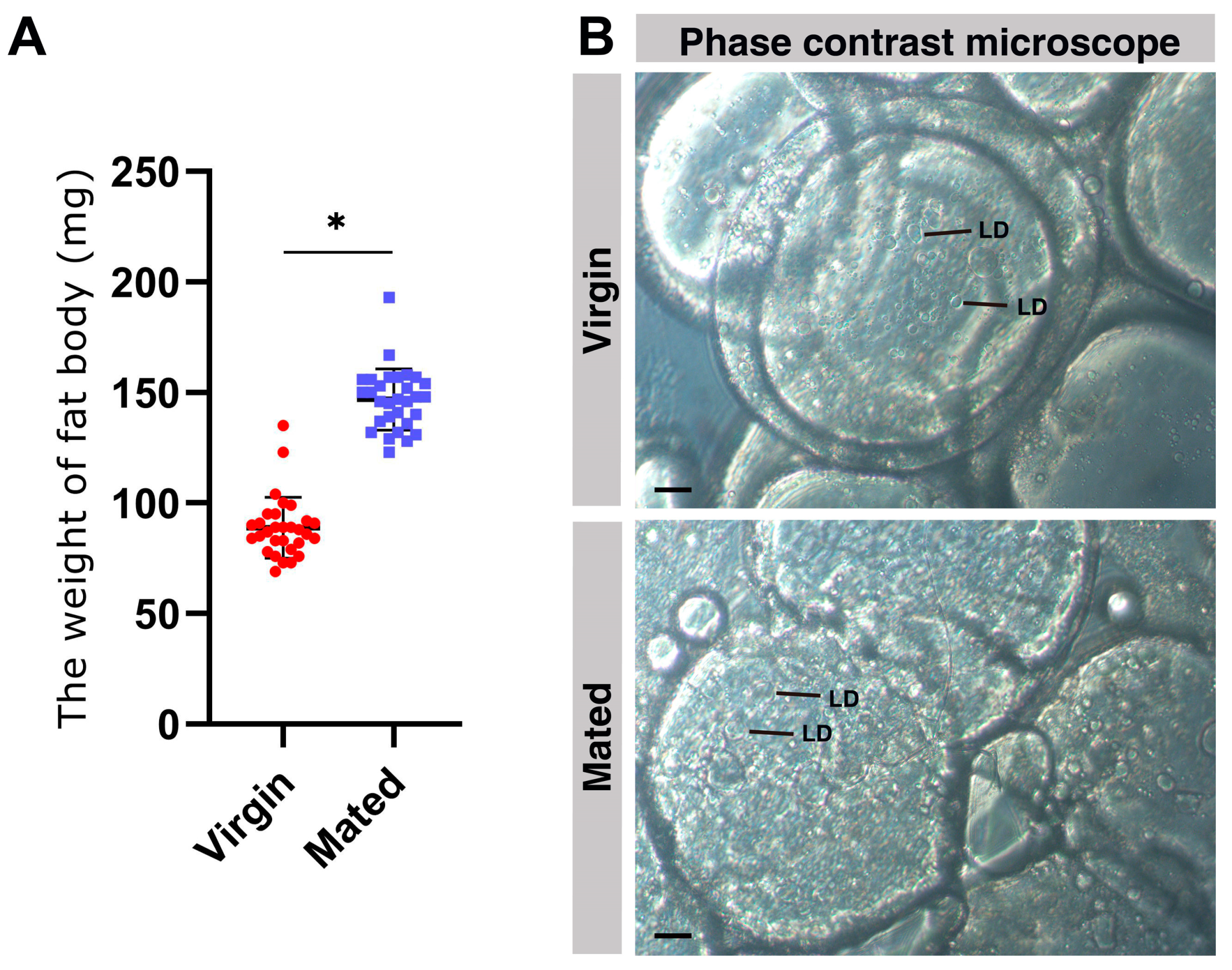
2.1. An Obvious Increase in the Fat Body Weight of Mated Queens
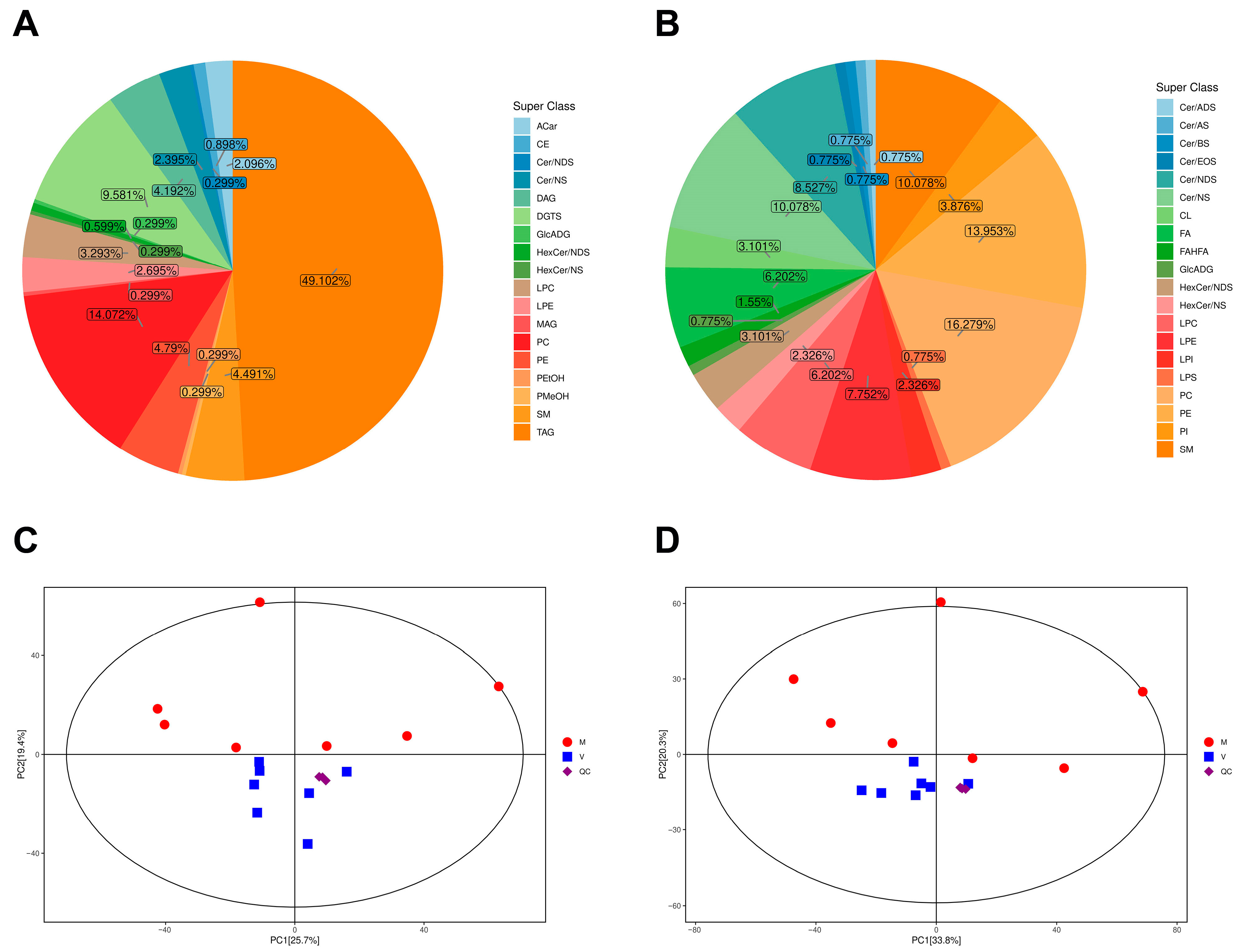
2.2. Metabolic Profiling of the Fat Bodies of Virgin and Mated Bumblebee Queens
2.3. Metabolite Analysis and Differential Accumulation of Metabolites
2.4. Principal Component Analysis of Differential Metabolites
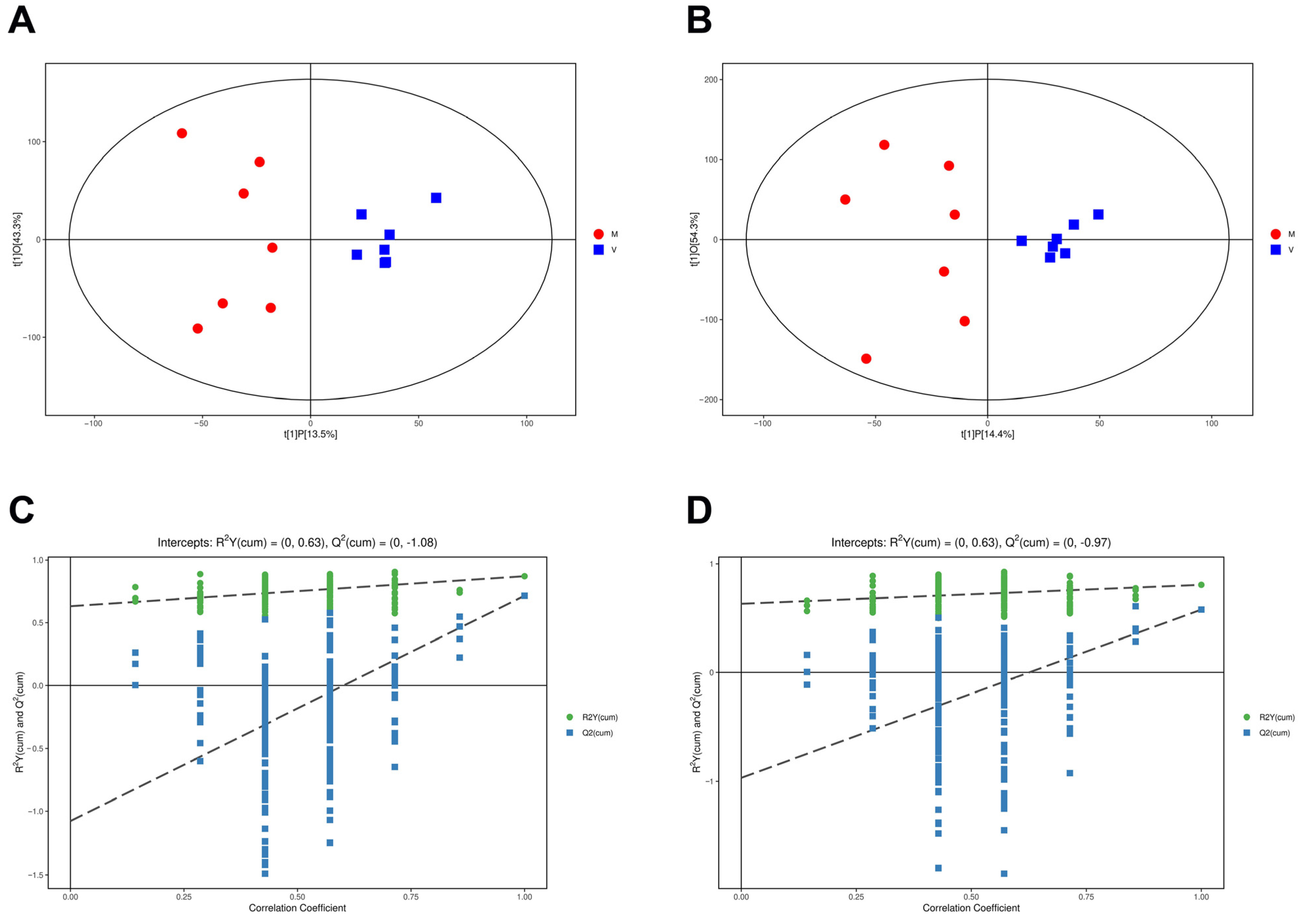
2.5. Orthogonal Partial Least Squares Discriminant Analysis of Differential Metabolites
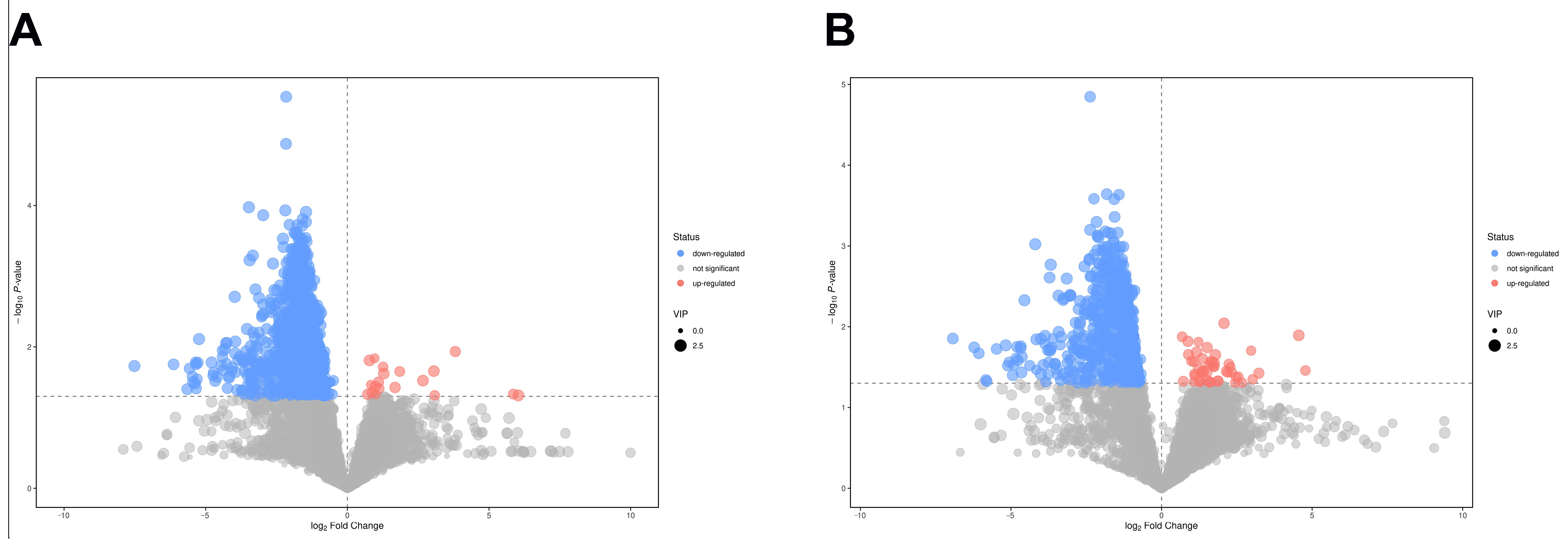
2.6. Screening of Differential Metabolites
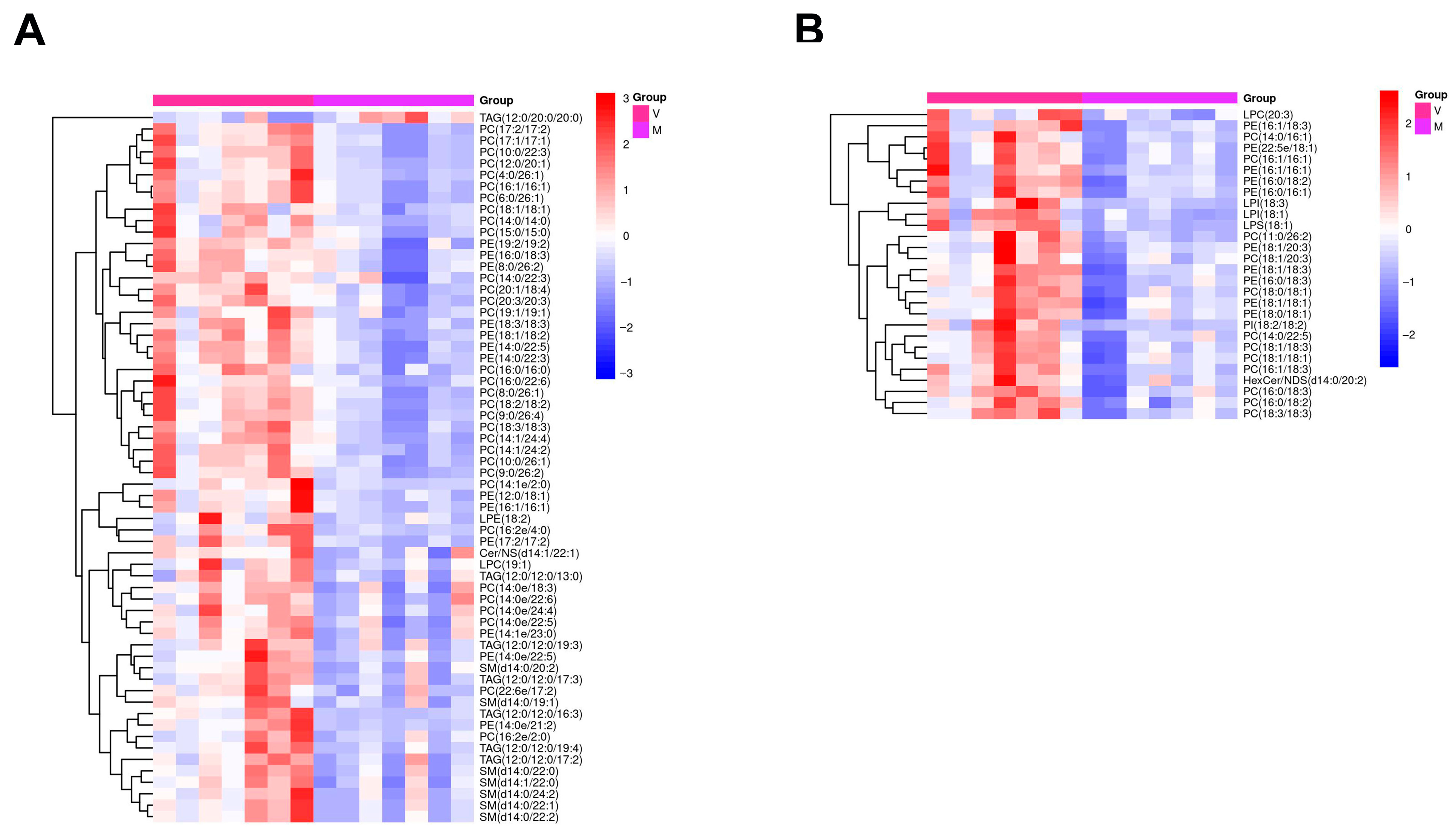
2.7. Hierarchical Cluster Analysis of Differential Metabolites
2.8. ROC Analysis of Metabolites
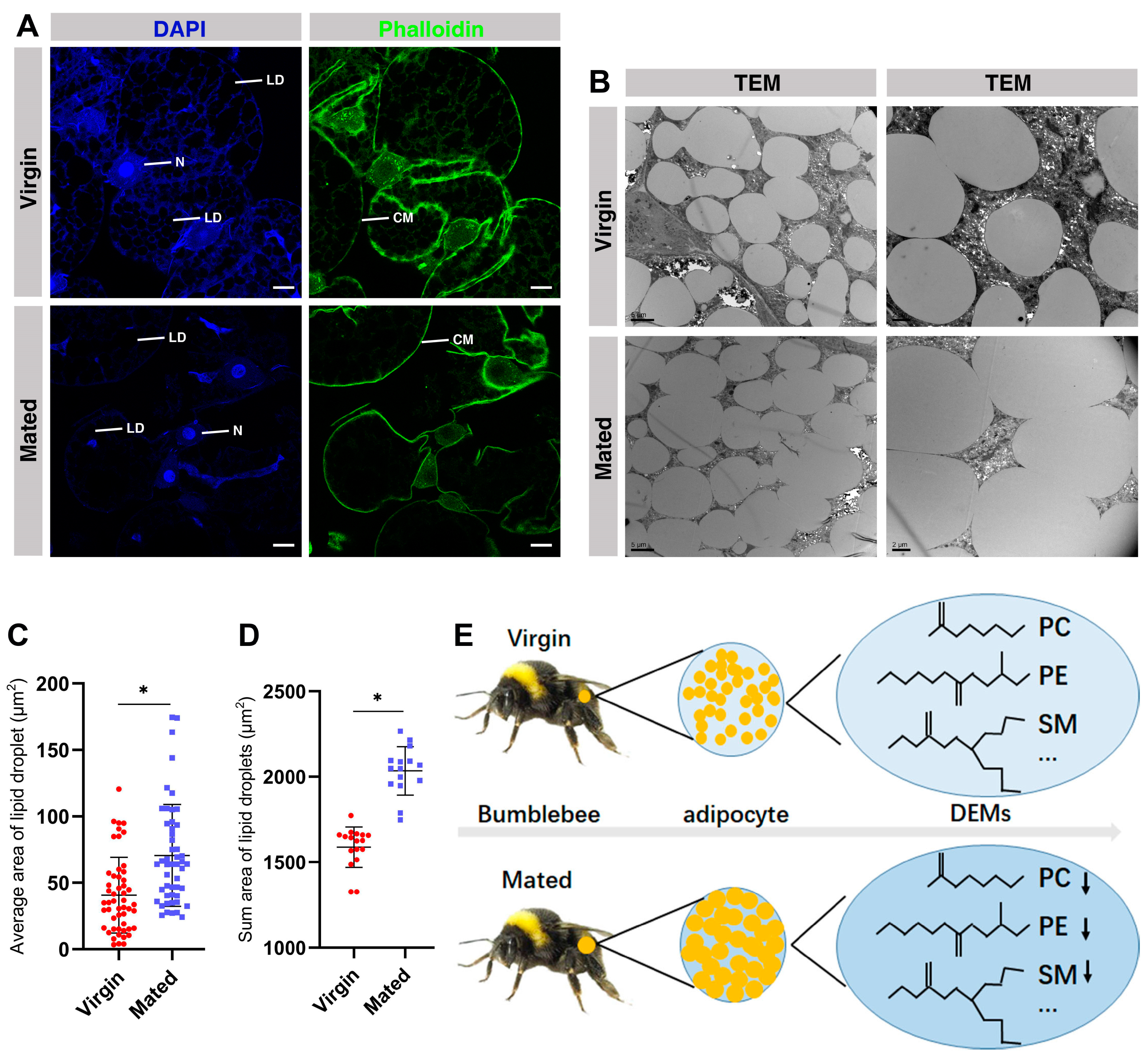
2.9. The Fusion of Lipid Droplets in the Fat Body of Mated Bumblebee Queens
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Metabolites Extraction
4.3. LC-MS/MS Analysis
4.4. Metabolome Data Preprocessing and Annotation
4.5. Phase Contrast Microscopy
4.6. Fluorescence Microscopy
4.7. Transmission Electron Microscopy (TEM)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Meeus, I.; Brown, M.J.; De Graaf, D.C.; Smagghe, G. Effects of invasive parasites on bumble bee declines. Conserv. Biol. 2011, 25, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Evison, S.E.; Roberts, K.E.; Laurenson, L.; Pietravalle, S.; Hui, J.; Biesmeijer, J.C.; Smith, J.E.; Budge, G.; Hughes, W.O. Pervasiveness of parasites in pollinators. PLoS ONE 2012, 7, e30641. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- An, J.; Huang, J.; Shao, Y.; Zhang, S.; Wang, B.; Liu, X.; Wu, J.; Williams, P.H. The bumblebees of North China (Apidae, Bombus Latreille). Zootaxa 2014, 3830, 1–89. [Google Scholar] [CrossRef]
- Mommaerts, V.; Jans, K.; Smagghe, G. Impact of Bacillus thuringiensis strains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci. 2010, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Teal, P.; Grozinger, C.M.; Hefetz, A. Precocene-I inhibits juvenile hormone biosynthesis, ovarian activation, aggression and alters sterility signal production in bumble bee (Bombus terrestris) workers. J. Exp. Biol. 2014, 217 Pt 17, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.; Li, K.; Sadd, B.M.; Guo, Y.; Zhuang, D.; Zhang, Z.; Chen, Y.; Evans, J.D.; Guo, J.; et al. Dynamic changes of gut microbial communities of bumble bee queens through important life stages. mSystems 2019, 4, e00631-19. [Google Scholar] [CrossRef] [PubMed]
- Treanore, E.D.; Amsalem, E. Examining the individual and additive effects of cold storage and CO2 narcosis on queen survival and reproduction in bumble bees. J. Insect Physiol. 2022, 139, 104394. [Google Scholar] [CrossRef]
- Amsalem, E.; Padilla, M.; Schreiber, P.M.; Altman, N.S.; Hefetz, A.; Grozinger, C.M. Do bumble bee, Bombus impatiens, queens signal their reproductive and mating status to their workers? J. Chem. Ecol. 2017, 43, 563–572. [Google Scholar] [CrossRef]
- Krams, R.; Gudra, D.; Popovs, S.; Willow, J.; Krama, T.; Munkevics, M.; Megnis, K.; Joers, P.; Fridmanis, D.; Contreras Garduno, J.; et al. Dominance of fructose-associated Fructobacillus in the gut microbiome of bumblebees (Bombus terrestris) inhabiting natural forest meadows. Insects 2022, 13, 98. [Google Scholar] [CrossRef]
- Gomulski, L.M.; Dimopoulos, G.; Xi, Z.; Scolari, F.; Gabrieli, P.; Siciliano, P.; Clarke, A.R.; Malacrida, A.R.; Gasperi, G. Transcriptome profiling of sexual maturation and mating in the Mediterranean fruit fly, Ceratitis capitata. PLoS ONE 2012, 7, e30857. [Google Scholar] [CrossRef]
- Short, S.M.; Lazzaro, B.P. Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster. G3 2013, 3, 827–840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alfonso-Parra, C.; Ahmed-Braimah, Y.H.; Degner, E.C.; Avila, F.W.; Villarreal, S.M.; Pleiss, J.A.; Wolfner, M.F.; Harrington, L.C. Mating-induced transcriptome changes in the reproductive tract of female Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004451. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Liu, Y.; Yang, J.; Xie, W.; Wang, S.; Wu, Q.; Zhou, X.; Pang, B.; Zhang, Y. Transcriptomic analysis of mating responses in Bemisia tabaci MED females. Insects 2020, 11, 308. [Google Scholar] [CrossRef]
- Rangel, J.; Shepherd, T.F.; Gonzalez, A.N.; Hillhouse, A.; Konganti, K.; Ing, N.H. Transcriptomic analysis of the honey bee (Apis mellifera) queen spermathecae reveals genes that may be involved in sperm storage after mating. PLoS ONE 2021, 16, e0244648. [Google Scholar] [CrossRef]
- Veiga, J.C.; Ruiz, G.R.S.; Carvalho-Zilse, G.A.; Menezes, C.; Contrera, F.A.L. Queens remate despite traumatic mating in stingless bees. Curr. Zool. 2022, 68, 81–92. [Google Scholar] [CrossRef]
- Baer, B.; Eubel, H.; Taylor, N.L.; O’Toole, N.; Millar, A.H. Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 2009, 10, R67. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Q.; Hu, X.; Pang, C.; Li, J.; Huang, J. Mating stimulates the immune response and sperm storage-related genes expression in spermathecae of bumblebee (Bombus terrestris) queen. Front. Genet. 2021, 12, 795669. [Google Scholar] [CrossRef]
- Colgan, T.J.; Finlay, S.; Brown, M.J.F.; Carolan, J.C. Mating precedes selective immune priming which is maintained throughout bumblebee queen diapause. BMC Genom. 2019, 20, 959. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Lattorff, H.M.G. Tissue specificity in social context-dependent lysozyme expression in bumblebees. Antibiotics 2020, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Furtado, W.C.; Azevedo, D.O.; Martins, G.F.; Zanuncio, J.C.; Serrao, J.E. Histochemistry and ultrastructure of urocytes in the pupae of the stingless bee Melipona quadrifasciata (Hymenoptera: Meliponini). Microsc. Microanal. 2013, 19, 1502–1510. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef] [PubMed]
- Dostalkova, S.; Dobes, P.; Kunc, M.; Hurychova, J.; Skrabisova, M.; Petrivalsky, M.; Titera, D.; Havlik, J.; Hyrsl, P.; Danihlik, J. Winter honeybee (Apis mellifera) populations show greater potential to induce immune responses than summer populations after immune stimuli. J. Exp. Biol. 2021, 224 Pt 3, jeb232595. [Google Scholar] [CrossRef]
- Gillette, C.M.; Hazegh, K.E.; Nemkov, T.; Stefanoni, D.; D’Alessandro, A.; Taliaferro, J.M.; Reis, T. Gene-diet interactions: Dietary rescue of metabolic effects in spen-depleted Drosophila melanogaster. Genetics 2020, 214, 961–975. [Google Scholar] [CrossRef]
- Meschi, E.; Delanoue, R. Adipokine and fat body in flies: Connecting organs. Mol. Cell. Endocrinol. 2021, 533, 111339. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Ratnaparkhi, A.; Sudhakaran, J. Neural pathways in nutrient sensing and insulin signaling. Front. Physiol. 2022, 13, 1002183. [Google Scholar] [CrossRef]
- Matsuoka, S.; Armstrong, A.R.; Sampson, L.L.; Laws, K.M.; Drummond-Barbosa, D. Adipocyte metabolic pathways regulated by diet control the female germline stem cell lineage in Drosophila melanogaster. Genetics 2017, 206, 953–971. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Boggs, C.L. Antibody development to identify components of IIS and mTOR signaling pathways in lepidopteran species, a set of non-model insects. MicroPubl. Biol. 2023, 2023, 10.17912. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Drummond-Barbosa, D. Insulin signaling acts in adult adipocytes via GSK-3beta and independently of FOXO to control Drosophila female germline stem cell numbers. Dev. Biol. 2018, 440, 31–39. [Google Scholar] [CrossRef]
- Lockett, G.A.; Almond, E.J.; Huggins, T.J.; Parker, J.D.; Bourke, A.F. Gene expression differences in relation to age and social environment in queen and worker bumble bees. Exp. Gerontol. 2016, 77, 52–61. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.Y.; Wan, H.; Li, J.; Jin, B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018, 85, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Duennes, M.A.; Fisher, K.; Der, J.P.; Watrous, K.M.; Okamoto, N.; Yamanaka, N.; Woodard, S.H. Transcriptome analysis reveals nutrition- and age-related patterns of gene expression in the fat body of pre-overwintering bumble bee queens. Mol. Ecol. 2020, 29, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Barie, K.; Levin, E.; Amsalem, E. CO2 narcosis induces a metabolic shift mediated via juvenile hormone in Bombus impatiens gynes. Insect Biochem. Mol. Biol. 2022, 149, 103831. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yang, B.J.; Wu, Y.; Gao, H.L.; Lin, X.M.; Zou, J.Z.; Liu, Z.W. Characterization of neutral lipases revealed the tissue-specific triacylglycerol hydrolytic activity in Nilaparvata lugens. Insect Sci. 2022, 30, 693–704. [Google Scholar] [CrossRef]
- Heier, C.; Kuhnlein, R.P. Triacylglycerol metabolism in Drosophila melanogaster. Genetics 2018, 210, 1163–1184. [Google Scholar] [CrossRef]
- Ziegler, R.; Van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Omics approaches for food authentication. Electrophoresis 2018, 39, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, H.; Zhu, Y.; Guo, Y.; Tang, Y.; Xie, K.; Zhang, G.; Dai, G.; Gao, Y.; Zhang, T. Untargeted and targeted metabolomics identify metabolite biomarkers for Salmonella enteritidis in chicken meat. Food Chem. 2023, 409, 135294. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, J.; Li, M.; Xiao, Y.; Yi, Y. Prior infection of Galleria mellonella with sublethal dose of Bt elicits immune priming responses but incurs metabolic changes. J. Insect Physiol. 2022, 139, 104401. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Haufs-Brusberg, S.; Puetz, L.; Feickert, M.; Jaeckstein, M.Y.; Hoffmann, A.; Zurkovic, J.; Heine, M.; Trautmann, E.M.; Muller, C.E.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef]
- Luke, T.D.W.; Pryce, J.E.; Elkins, A.C.; Wales, W.J.; Rochfort, S.J. Use of large and diverse datasets for 1H NMR serum metabolic profiling of early lactation dairy cows. Metabolites 2020, 10, 180. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, W.; Huo, Y.; Kong, Y.; Zhang, W.; Li, S.; Zhou, W.; Wu, X.; Qin, F.; Hu, X. The effects of short-term dietary exposure to SiO2 nanoparticle on the domesticated lepidopteran insect model silkworm (Bombyx mori): Evidence from the perspective of multi-omics. Chemosphere 2023, 323, 138257. [Google Scholar] [CrossRef]
- Haider, A.; Wei, Y.C.; Lim, K.; Barbosa, A.D.; Liu, C.H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A regulates phosphatidylcholine homeostasis from the inner nuclear membrane in response to membrane stored curvature elastic stress. Dev. Cell 2018, 45, 481–495. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Lingrell, S.; McCloskey, N.; LeBlond, N.D.; Galleguillos, D.; Zhao, Y.Y.; Curtis, J.M.; Sipione, S.; Fullerton, M.D.; Vance, D.E.; et al. A role for phosphatidylcholine and phosphatidylethanolamine in hepatic insulin signaling. FASEB J. 2019, 33, 5045–5057. [Google Scholar] [CrossRef]
- Kamarudin, A.N.; Cox, T.; Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Xu, J.; Yang, W.; Li, Q.; Zhong, Y.; Cao, Y.; Yu, X.Q.; Deng, X. Regulation of antimicrobial peptide genes via insulin-like signaling pathway in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2018, 103, 12–21. [Google Scholar] [CrossRef]
- Prado, A.; Brunet, J.L.; Peruzzi, M.; Bonnet, M.; Bordier, C.; Crauser, D.; Le Conte, Y.; Alaux, C. Warmer winters are associated with lower levels of the cryoprotectant glycerol, a slower decrease in vitellogenin expression and reduced virus infections in winter honeybees. J. Insect Physiol. 2022, 136, 104348. [Google Scholar] [CrossRef] [PubMed]
- DiAngelo, J.R.; Bland, M.L.; Bambina, S.; Cherry, S.; Birnbaum, M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20853–20858. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef]
- Musselman, L.P.; Kuhnlein, R.P. Drosophila as a model to study obesity and metabolic disease. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb163881. [Google Scholar] [CrossRef]
- Trenti, F.; Sandron, T.; Guella, G.; Lencioni, V. Insect cold-tolerance and lipidome: Membrane lipid composition of two chironomid species differently adapted to cold. Cryobiology 2022, 106, 84–90. [Google Scholar] [CrossRef]
- Yeh, L.H.; Bajpai, R.K.; Sun, G.Y. Membrane lipid metabolism and phospholipase activity in insect Spodoptera frugiperda 9 ovarian cells. Lipids 1997, 32, 481–487. [Google Scholar] [CrossRef]
- Guo, Y.; Walther, T.C.; Rao, M.; Stuurman, N.; Goshima, G.; Terayama, K.; Wong, J.S.; Vale, R.D.; Walter, P.; Farese, R.V. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 2008, 453, 657–661. [Google Scholar] [CrossRef]
- Lee, J.; Ridgway, N.D. Phosphatidylcholine synthesis regulates triglyceride storage and chylomicron secretion by Caco2 cells. J. Lipid Res. 2018, 59, 1940–1950. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, J.; Wang, J.; Yin, Y.S.; Zhu, Y.; Li, L.L.; Huang, S.; Peng, S.; Xue, B.; Liao, R.; et al. A gel-like condensation of Cidec generates lipid-permeable plates for lipid droplet fusion. Dev. Cell 2021, 56, 2592–2606 e7. [Google Scholar] [CrossRef]
- Sun, Z.; Gong, J.; Wu, L.; Li, P. Imaging lipid droplet fusion and growth. Methods Cell Biol 2013, 116, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Gurel, F.; Gosterit, A. Effects of different stimulation methods on colony initiation and development of Bombus terrestris L. (Hymenoptera: Apidae) queens. Appl. Entomol. Zool. 2008, 43, 113–117. [Google Scholar] [CrossRef][Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, F.; Guo, Y.; Qu, Y.; Zhang, Z.; Yao, J.; Xu, J.; Li, J. Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens. Int. J. Mol. Sci. 2023, 24, 15408. https://doi.org/10.3390/ijms242015408
Guo Y, Liu F, Guo Y, Qu Y, Zhang Z, Yao J, Xu J, Li J. Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens. International Journal of Molecular Sciences. 2023; 24(20):15408. https://doi.org/10.3390/ijms242015408
Chicago/Turabian StyleGuo, Yueqin, Fugang Liu, Yulong Guo, Yingping Qu, Zhengyi Zhang, Jun Yao, Jin Xu, and Jilian Li. 2023. "Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens" International Journal of Molecular Sciences 24, no. 20: 15408. https://doi.org/10.3390/ijms242015408
APA StyleGuo, Y., Liu, F., Guo, Y., Qu, Y., Zhang, Z., Yao, J., Xu, J., & Li, J. (2023). Untargeted Lipidomics Analysis Unravels the Different Metabolites in the Fat Body of Mated Bumblebee (Bombus terrestris) Queens. International Journal of Molecular Sciences, 24(20), 15408. https://doi.org/10.3390/ijms242015408





