Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian
Abstract
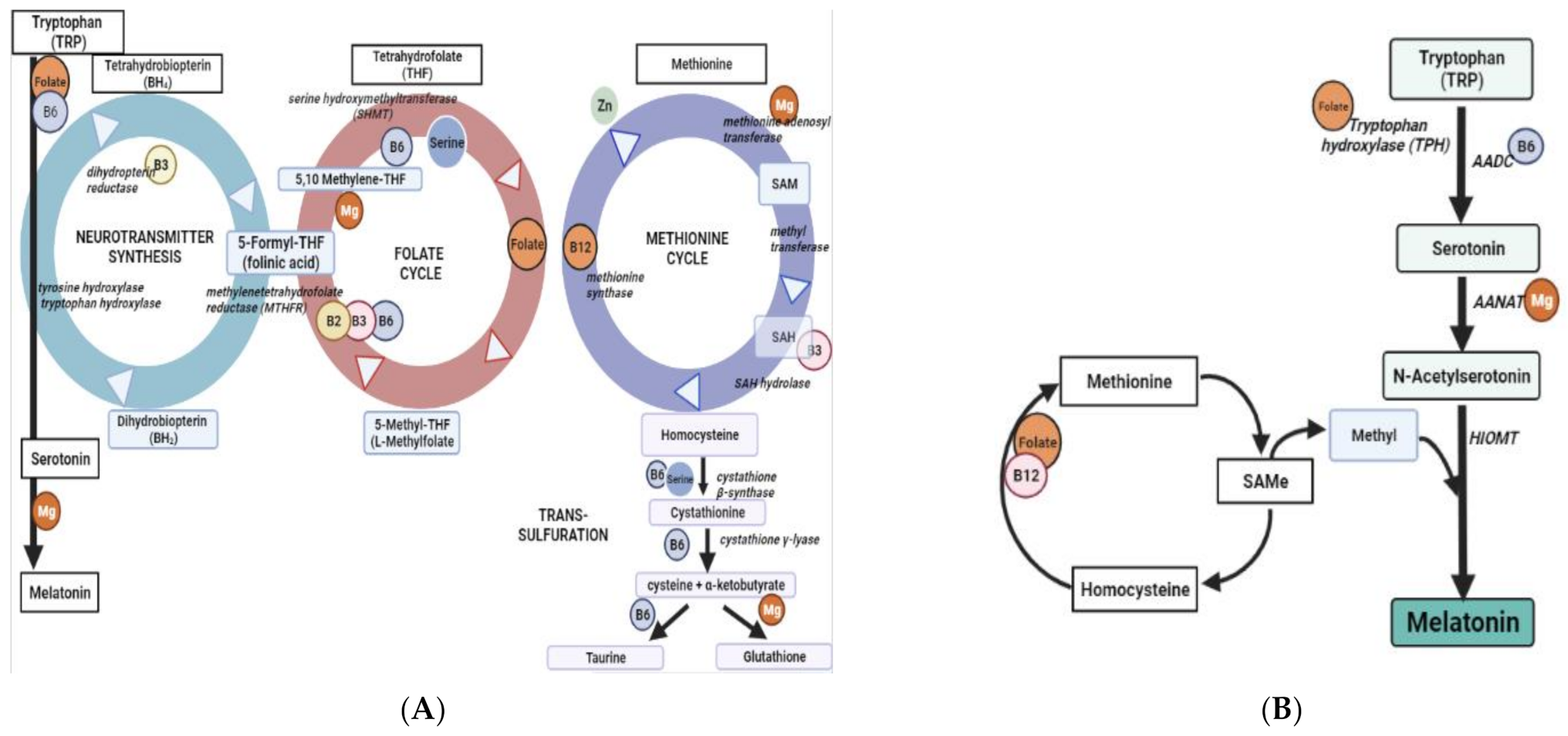
1. Introduction
2. Results
2.1. Study Participants
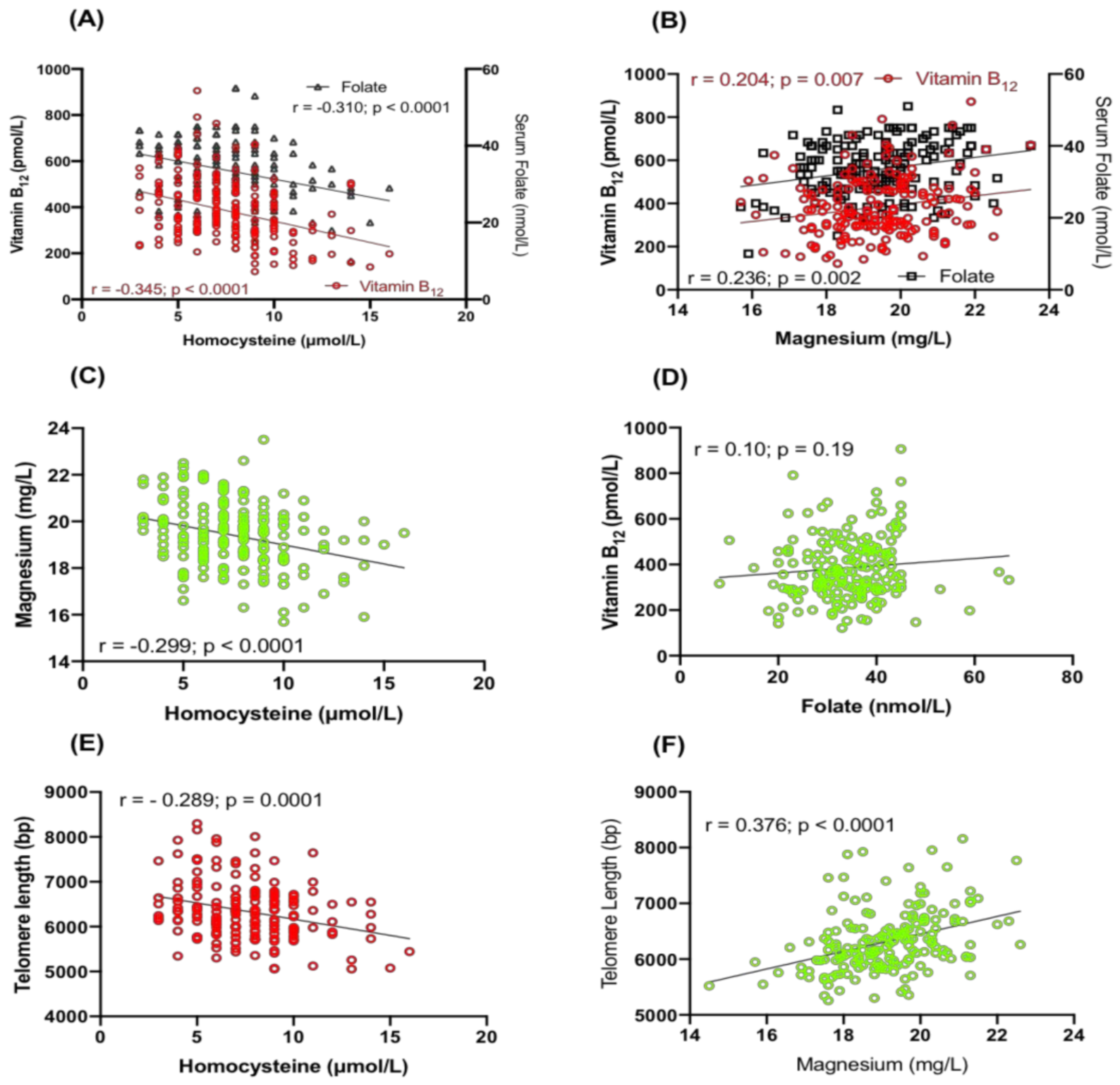
2.2. Relationship between Hcy, Serum Folate, Vitamin B12 and Magnesium
2.3. Correlation of TL with Serum Folate, Vitamin B12, Hcy and Magnesium
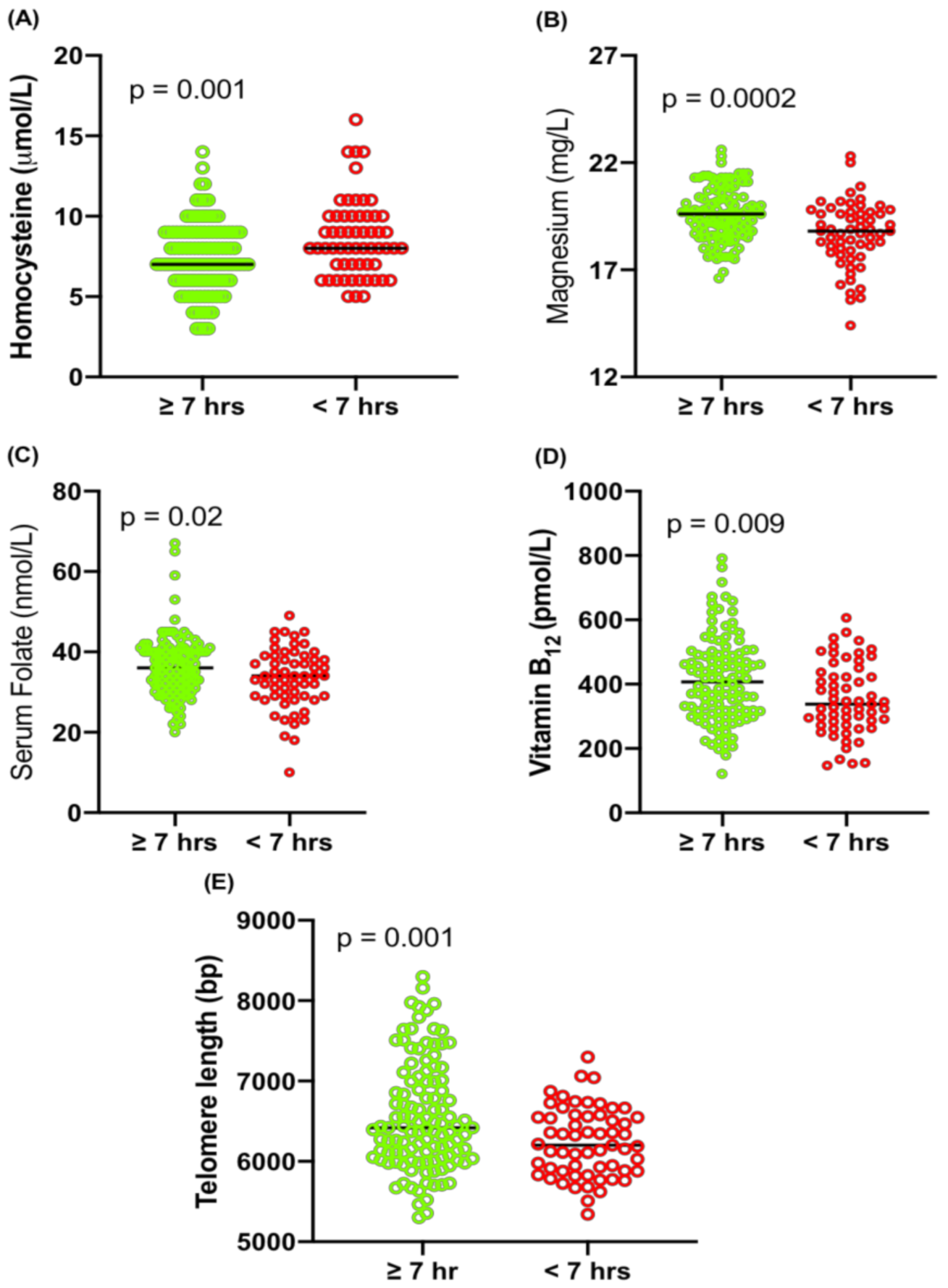
2.4. Association of Sleep with Hcy, Magnesium, Folate, Vitamin B12 and TL
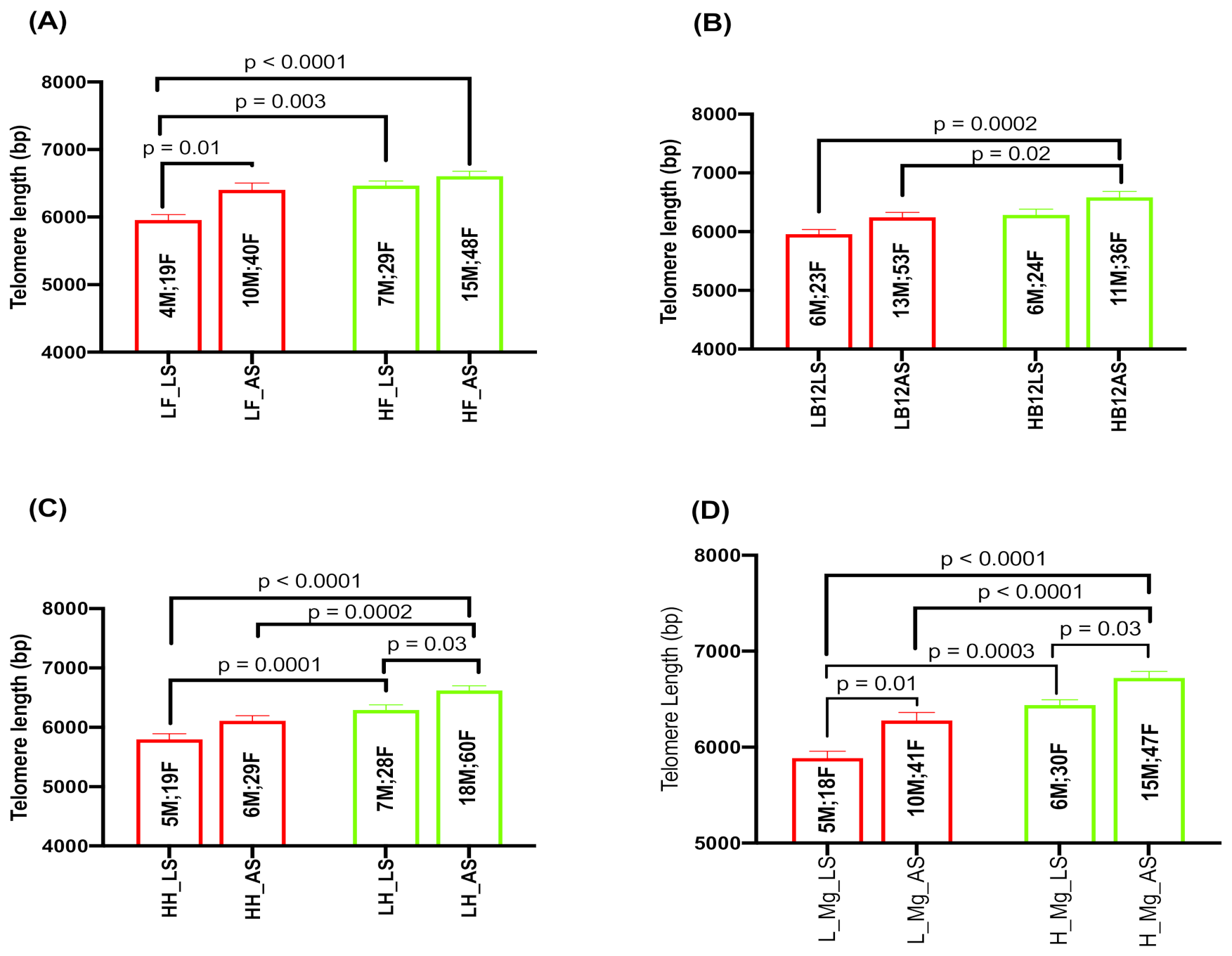
2.5. Effect of Serum Folate, Vitamin B12, Hcy, and Magnesium with Sleep Duration on TL
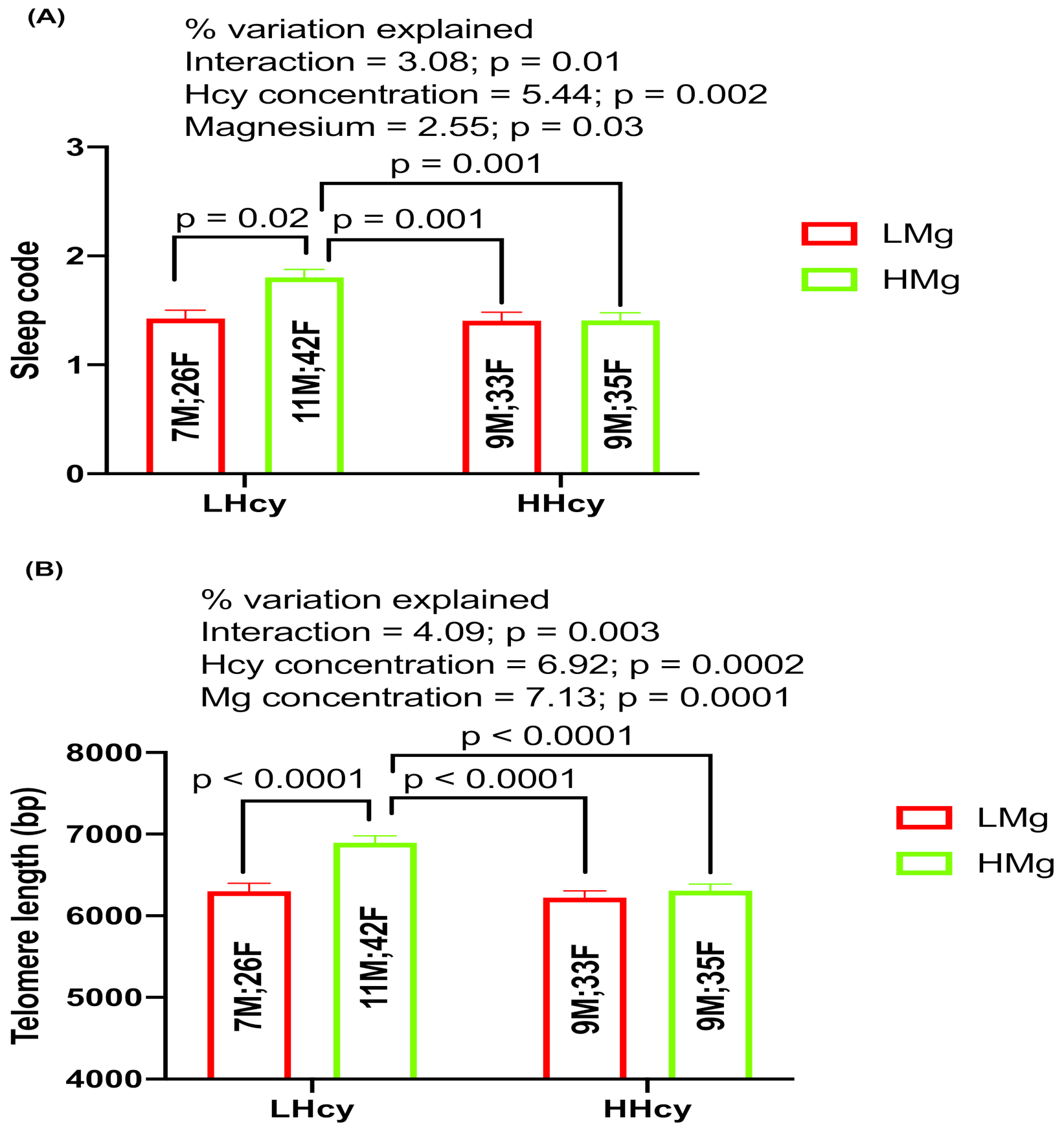
2.6. Interaction Effects of Hcy and Magnesium on Sleep and TL
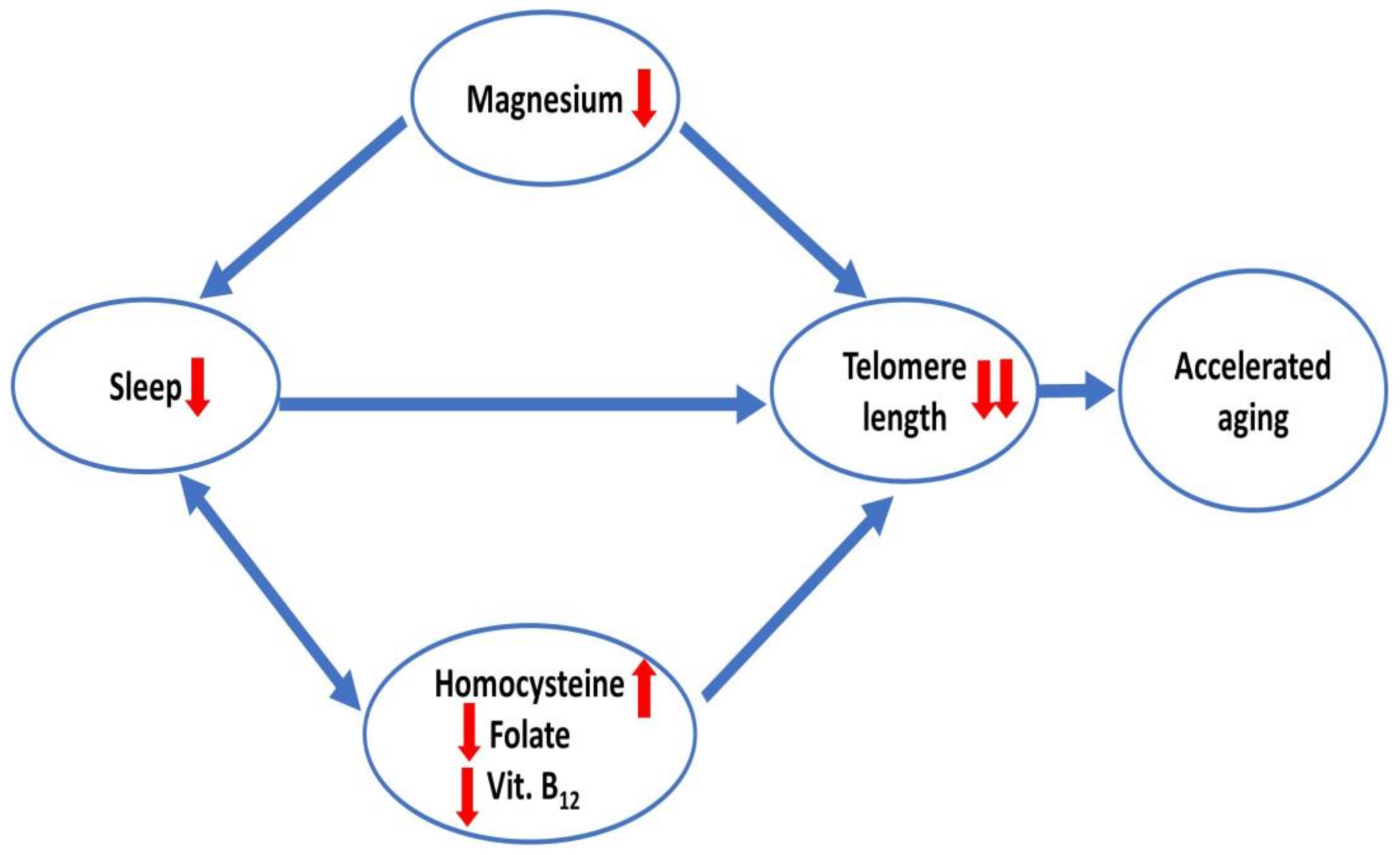
3. Discussion
4. Materials and Methods
4.1. Recruitment of Study Participants
4.2. DNA Isolation and Real-Time qPCR Assay for Telomere Length (TL) Measurement
4.3. Micronutrient Analyses
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, G.; Surana, U.; Clément, M.V. Replication stress-induced endogenous DNA damage drives cellular senescence induced by a sub-lethal oxidative stress. Nucleic Acids Res. 2017, 45, 10564–10582. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Duan, M.; Yadav, T.; Phoon, L.; Wang, X.; Zhang, J.M.; Zou, L.; Lan, L. An R-loop-initiated CSB-RAD52-POLD3 pathway suppresses ROS-induced telomeric DNA breaks. Nucleic Acids Res. 2020, 48, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; Verlaet, A.A.J.; Breynaert, A.; De Bruyne, T.; Hermans, N. Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef]
- Killilea, D.W.; Ames, B.N. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 5768–5773. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg(2+) in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mirza, I.A.; Berghuis, A.M.; Mackenzie, R.E. Magnesium and phosphate ions enable NAD binding to methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase. J. Biol. Chem. 2005, 280, 34316–34323. [Google Scholar] [CrossRef]
- Zhao, L.N.; Kaldis, P. The catalytic mechanism of the mitochondrial methylenetetrahydrofolate dehydrogenase/cyclohydrolase (MTHFD2). PLoS Comput. Biol. 2022, 18, e1010140. [Google Scholar] [CrossRef]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J. Clin. Investig. 2013, 123, 996–1002. [Google Scholar] [CrossRef]
- Neeley, W.L.; Essigmann, J.M. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 2006, 19, 491–505. [Google Scholar] [CrossRef]
- Rowe, W.J. Correcting magnesium deficiencies may prolong life. Clin. Interv. Aging 2012, 7, 51–54. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130.e6. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef]
- Miller, A.L. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern. Med. Rev. 2003, 8, 7–19. [Google Scholar]
- Pusceddu, I.; Herrmann, W.; Kleber, M.E.; Scharnagl, H.; Hoffmann, M.M.; Winklhofer-Roob, B.M.; März, W.; Herrmann, M. Subclinical inflammation, telomere shortening, homocysteine, vitamin B6, and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. J. Nutr. 2020, 59, 1399–1411. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Escobedo, J.O.; Lim, S.; Samoei, G.K.; Strongin, R.M. Homocystamides promote free-radical and oxidative damage to proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 551–554. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.M.; Ruan, M.H.; Zhou, A.H.; Qian, Y.; Chen, C. Homocysteine inhibits neural stem cells survival by inducing DNA interstrand cross-links via oxidative stress. Neurosci. Lett. 2016, 635, 24–32. [Google Scholar] [CrossRef]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44 (Suppl. 1), S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, F.M.; Oliver, A.J.S.; Bastos, P.C.; Guillén, L.S.; Domínguez, R. Sleep improvement in athletes: Use of nutritional supplements. Arch. Med. Deporte. 2017, 34, 93–99. [Google Scholar]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Rafie, N.; Amani, R.; Shirani, F. The Role of Magnesium in Sleep Health: A Systematic Review of Available Literature. Biol. Trace Elem. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Cao, Y.; Zhen, S.; Taylor, A.W.; Appleton, S.; Atlantis, E.; Shi, Z. Magnesium Intake and Sleep Disorder Symptoms: Findings from the Jiangsu Nutrition Study of Chinese Adults at Five-Year Follow-Up. Nutrients 2018, 10, 1354. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Chua, A.; Thomas, P.; Fenech, M. Sleep Duration, Health Promotion Index, sRAGE, and ApoE-ε4 Genotype Are Associated With Telomere Length in Healthy Australians. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 243–249. [Google Scholar] [CrossRef]
- Grieshober, L.; Wactawski-Wende, J.; Hageman Blair, R.; Mu, L.; Liu, J.; Nie, J.; Carty, C.L.; Hale, L.; Kroenke, C.H.; LaCroix, A.Z.; et al. A Cross-Sectional Analysis of Telomere Length and Sleep in the Women’s Health Initiative. Am. J. Epidemiol. 2019, 188, 1616–1626. [Google Scholar] [CrossRef]
- Jackowska, M.; Hamer, M.; Carvalho, L.A.; Erusalimsky, J.D.; Butcher, L.; Steptoe, A. Short sleep duration is associated with shorter telomere length in healthy men: Findings from the Whitehall II cohort study. PLoS ONE 2012, 7, e47292. [Google Scholar] [CrossRef]
- James, S.; McLanahan, S.; Brooks-Gunn, J.; Mitchell, C.; Schneper, L.; Wagner, B.; Notterman, D.A. Sleep Duration and Telomere Length in Children. J. Pediatr. 2017, 187, 247–252.e1. [Google Scholar] [CrossRef]
- Wynchank, D.; Bijlenga, D.; Penninx, B.W.; Lamers, F.; Beekman, A.T.; Kooij, J.J.S.; Verhoeven, J.E. Delayed sleep-onset and biological age: Late sleep-onset is associated with shorter telomere length. Sleep 2019, 42, zsz139. [Google Scholar] [CrossRef]
- Hu, L.; Bai, Y.; Hu, G.; Zhang, Y.; Han, X.; Li, J. Association of Dietary Magnesium Intake With Leukocyte Telomere Length in United States Middle-Aged and Elderly Adults. Front. Nutr. 2022, 9, 840804. [Google Scholar] [CrossRef]
- Maguire, D.; Neytchev, O.; Talwar, D.; McMillan, D.; Shiels, P.G. Telomere Homeostasis: Interplay with Magnesium. Int. J. Mol. Sci. 2018, 19, 157. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; He, S.; Li, P.; Ma, C.; Ma, M.; Liu, Y.; Lv, L.; Ping, F.; Zhang, H.; et al. Dietary Magnesium Intake and Leukocyte Telomere Attrition in Adults: The Regulatory Role of Serum Tumor Necrosis Factor α. Mediat. Inflamm. 2020, 2020, 7610436. [Google Scholar]
- O’Callaghan, N.J.; Bull, C.; Fenech, M. Elevated plasma magnesium and calcium may be associated with shorter telomeres in older South Australian women. J. Nutr. Health Aging 2014, 18, 131–136. [Google Scholar] [CrossRef]
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef]
- Rubio, M.A.; Davalos, A.R.; Campisi, J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp. Cell Res. 2004, 298, 17–27. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Pilger, R.; Sitte, N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic. Biol. Med. 2000, 28, 64–74. [Google Scholar] [CrossRef]
- Bell, L.T.; Branstrator, M.; Roux, C.; Hurley, L.S. Chromosomal abnormalities in maternal and fetal tissues of magnesium- or zinc-deficient rats. Teratology 1975, 12, 221–226. [Google Scholar] [CrossRef]
- MacGregor, J.T. Dietary factors affecting spontaneous chromosomal damage in man. Prog. Clin. Biol. Res. 1990, 347, 139–153. [Google Scholar]
- Martin, H.; Uring-Lambert, B.; Adrian, M.; Lahlou, A.; Bonet, A.; Demougeot, C.; Devaux, S.; Laurant, P.; Richert, L.; Berthelot, A. Effects of long-term dietary intake of magnesium on oxidative stress, apoptosis and ageing in rat liver. Magnes. Res. 2008, 21, 124–130. [Google Scholar] [PubMed]
- Martínez-Ezquerro, J.D.; Rodríguez-Castañeda, A.; Ortiz-Ramírez, M.; Sánchez-García, S.; Rosas-Vargas, H.; Sánchez-Arenas, R.; García-de la Torre, P. Oxidative stress, telomere length, and frailty in an old age population. Rev. Investig. Clin. 2019, 71, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, L.; Han, P.; Meng, F.; Jiao, C.; Zhang, H. Effect of the drug combination of magnesium sulfate and phentolamine on homocysteine and C-reactive protein in the serum of patients with pregnancy-induced hypertension syndrome. Exp. Ther. Med. 2019, 17, 3682–3688. [Google Scholar] [CrossRef] [PubMed]
- Van der Spek, A.; Broer, L.; Draisma, H.H.M.; Pool, R.; Albrecht, E.; Beekman, M.; Mangino, M.; Raag, M.; Nyholt, D.R.; Dharuri, H.K.; et al. Metabolomics reveals a link between homocysteine and lipid metabolism and leukocyte telomere length: The ENGAGE consortium. Sci. Rep. 2019, 9, 11623. [Google Scholar] [CrossRef] [PubMed]
- Billyard, A.J.; Eggett, D.L.; Franz, K.B. Dietary magnesium deficiency decreases plasma melatonin in rats. Magnes. Res. 2006, 19, 157–161. [Google Scholar]
- Abbasi, B.; Kimiagar, M.; Sadeghniiat, K.; Shirazi, M.M.; Hedayati, M.; Rashidkhani, B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012, 17, 1161–1169. [Google Scholar]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Lu, L.; Knutson, K.L.; Carnethon, M.R.; Fly, A.D.; Luo, J.; Haas, D.M.; Shikany, J.M.; Kahe, K. Association of magnesium intake with sleep duration and sleep quality: Findings from the CARDIA study. Sleep 2022, 45. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Chollet, D.; Franken, P.; Raffin, Y.; Malafosse, A.; Widmer, J.; Tafti, M. Blood and brain magnesium in inbred mice and their correlation with sleep quality. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2173-8. [Google Scholar] [CrossRef]
- Durlach, J.; Pagès, N.; Bac, P.; Bara, M.; Guiet-Bara, A.; Agrapart, C. Chronopathological forms of magnesium depletion with hypofunction or with hyperfunction of the biological clock. Magnes. Res. 2002, 15, 263–268. [Google Scholar]
- Carroll, J.E.; Esquivel, S.; Goldberg, A.; Seeman, T.E.; Effros, R.B.; Dock, J.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Insomnia and Telomere Length in Older Adults. Sleep 2016, 39, 559–564. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef]
- Lenz, O.; Xiong, J.; Nelson, M.D.; Raizen, D.M.; Williams, J.A. FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav. Immun. 2015, 47, 141–148. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Shockley, K.R.; Romer, M.A.; Galante, R.J.; Zimmerman, J.E.; Naidoo, N.; Baldwin, D.A.; Jensen, S.T.; Churchill, G.A.; Pack, A.I. Macromolecule biosynthesis: A key function of sleep. Physiol. Genom. 2007, 31, 441–457. [Google Scholar] [CrossRef]
- Singh, P.; Donlea, J.M. Bidirectional Regulation of Sleep and Synapse Pruning after Neural Injury. Curr. Biol. 2020, 30, 1063–1076.e3. [Google Scholar] [CrossRef]
- Stanhope, B.A.; Jaggard, J.B.; Gratton, M.; Brown, E.B.; Keene, A.C. Sleep Regulates Glial Plasticity and Expression of the Engulfment Receptor Draper Following Neural Injury. Curr. Biol. 2020, 30, 1092–1101.e3. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Cheung, V.; Yuen, V.M.; Wong, G.T.C.; Choi, S.W. The effect of sleep deprivation and disruption on DNA damage and health of doctors. Anaesthesia 2019, 74, 434–440. [Google Scholar] [CrossRef]
- DeBardeleben, H.K.; Lopes, L.E.; Nessel, M.P.; Raizen, D.M. Stress-Induced Sleep After Exposure to Ultraviolet Light Is Promoted by p53 in Caenorhabditis elegans. Genetics 2017, 207, 571–582. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021, 81, 4979–4993.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Geng, P.; Tang, X.; Xiang, L.; Lu, X.; Li, J.; Ruan, Z.; Chen, J.; Xie, G.; et al. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J. Pineal Res. 2017, 63, e12405. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Beneke, S. Regulation of chromatin structure by poly(ADP-ribosyl)ation. Front. Genet. 2012, 3, 169. [Google Scholar] [CrossRef]
- Kraus, W.L.; Hottiger, M.O. PARP-1 and gene regulation: Progress and puzzles. Mol. Asp. Med. 2013, 34, 1109–1123. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Tedim Ferreira, M.; Gagné, J.P.; Sharma, A.K.; Hendzel, M.J.; Masson, J.Y.; Poirier, G.G. Emerging roles of eraser enzymes in the dynamic control of protein ADP-ribosylation. Nat. Commun. 2019, 10, 1182. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Zabaras, D.; Almond, T.; Cavuoto, P.; James-Martin, G.; Fenech, M. Whey protein isolate improves vitamin B(12) and folate status in elderly Australians with subclinical deficiency of vitamin B(12). Mol. Nutr. Food Res. 2017, 61, 1600915. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Heyman, M.B.; Elwan, D.; Shiboski, S.; Lin, J.; Blackburn, E.; Epel, E. Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl. Psychiatry 2015, 5, e581. [Google Scholar] [CrossRef]
| Male | Female | |
|---|---|---|
| Total number (n =172) | 36 | 136 |
| Mean age (years) | 54.78 ± 1.2 | 53.79 ± 0.71 |
| BMI (kg/m2) | 27.32 ± 0.77 | 26.49 ± 0.49 |
| Magnesium (mg/L) | 19.48 ± 0.21 | 19.32 ± 0.12 |
| Serum folate (nm/L) | 33.78 ± 1.52 | 34.42 ± 0.85 |
| Vitamin B12 (pg/mL) | 414.3 ± 29.56 | 420.4 ± 18.21 |
| Homocysteine (μmol/L) | 8.98 ± 0.44 | 8.65 ± 0.21 |
| Telomere length (bp) | 6328 ± 56.76 | 6425 ± 60.66 |
| Exercise | ||
| At least once a week | 27 | 100 |
| Seldom/Never | 9 | 36 |
| Working Hours | ||
| Less than 9 h | 32 | 121 |
| 9 h or more | 4 | 15 |
| Stress | ||
| Light/Moderate | 32 | 119 |
| Excessive | 4 | 17 |
| Breakfast | ||
| Every morning | 33 | 128 |
| Often skip | 3 | 8 |
| Nutritional habit (3–4 serves of fruit/vegetable) | ||
| Good/Moderate | 34 | 127 |
| Poor | 2 | 9 |
| Independent Variable | Total Cohort | Male | Female | <7 h Sleep | ≥7 h Sleep |
|---|---|---|---|---|---|
| Gender | 0.113 | - | - | 0.121 | 0.11 |
| Age | −0.219 | −0.216 | −0.229 | −0.212 | −0.224 |
| BMI | −0.015 | −0.009 | −0.018 | −0.021 | −0.013 |
| Folate | 0.143 | 0.147 | 0.142 | 0.133 | 0.149 |
| B12 | 0.104 | 0.11 | 0.102 | 0.095 | 0.107 |
| Hcy | −0.312 * | −0.307 * | −0.314 * | −0.397 * | −0.296 |
| Mg | 0.298 * | 0.291 * | 0.299 * | 0.243 * | 0.342 |
| Multiple R | |||||
| 0.604 * | 0.597 * | 0.605 * | 0.433 | 0.463 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, V.S.; Deo, P.; Thomas, P.; Fenech, M. Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian. Int. J. Mol. Sci. 2023, 24, 982. https://doi.org/10.3390/ijms24020982
Dhillon VS, Deo P, Thomas P, Fenech M. Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian. International Journal of Molecular Sciences. 2023; 24(2):982. https://doi.org/10.3390/ijms24020982
Chicago/Turabian StyleDhillon, Varinderpal S., Permal Deo, Philip Thomas, and Michael Fenech. 2023. "Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian" International Journal of Molecular Sciences 24, no. 2: 982. https://doi.org/10.3390/ijms24020982
APA StyleDhillon, V. S., Deo, P., Thomas, P., & Fenech, M. (2023). Low Magnesium in Conjunction with High Homocysteine and Less Sleep Accelerates Telomere Attrition in Healthy Elderly Australian. International Journal of Molecular Sciences, 24(2), 982. https://doi.org/10.3390/ijms24020982






