On Associations between Fear-Induced Aggression, Bdnf Transcripts, and Serotonin Receptors in the Brains of Norway Rats: An Influence of Antiaggressive Drug TC-2153
Abstract
1. Introduction
2. Results
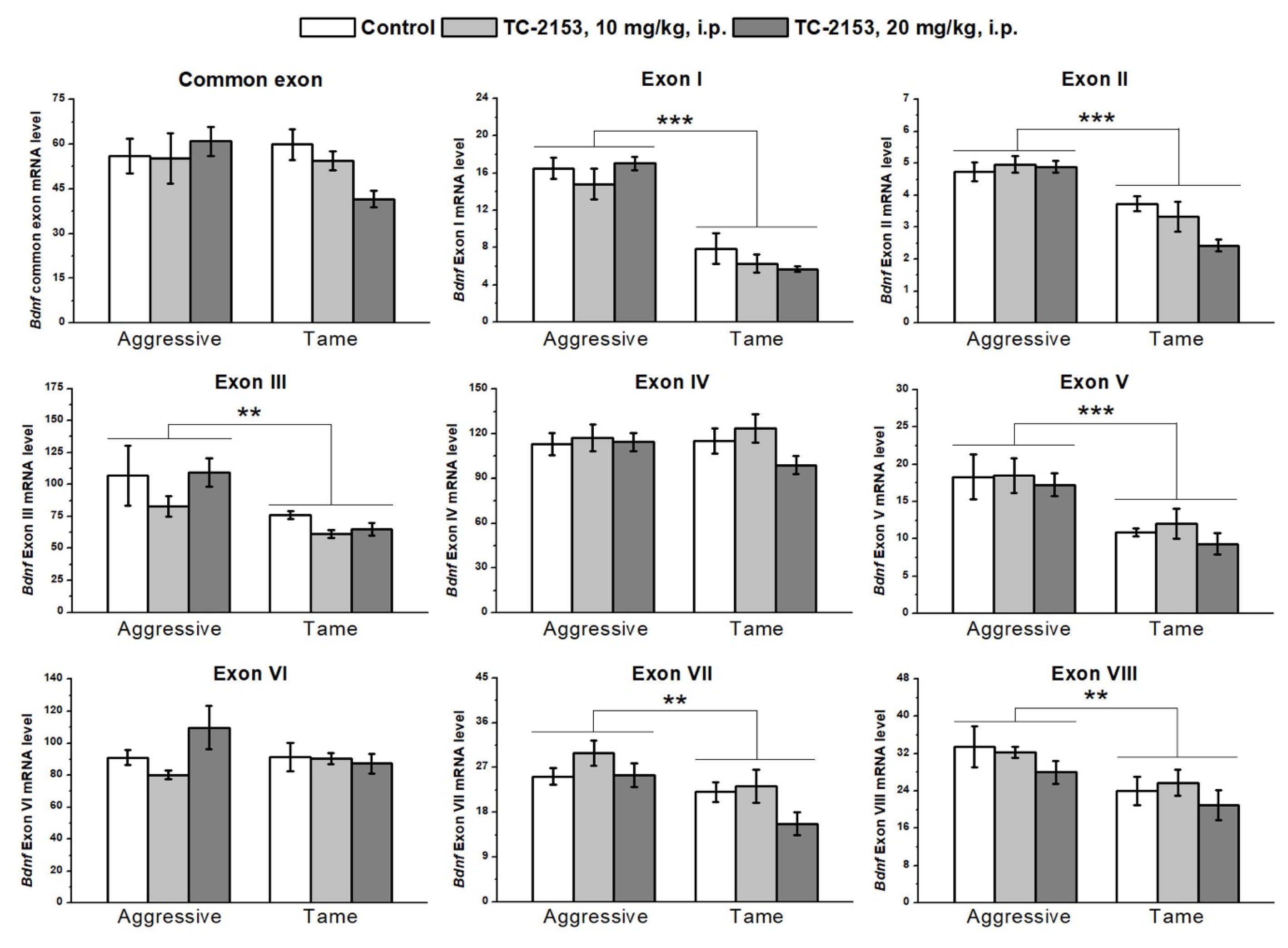
2.1. Effects of Acute TC-2153 Treatment on mRNA Levels of Bdnf Untranslated Exons I–VIII and Coding Exon IX in the Cortex of Aggressive and Tame Rats
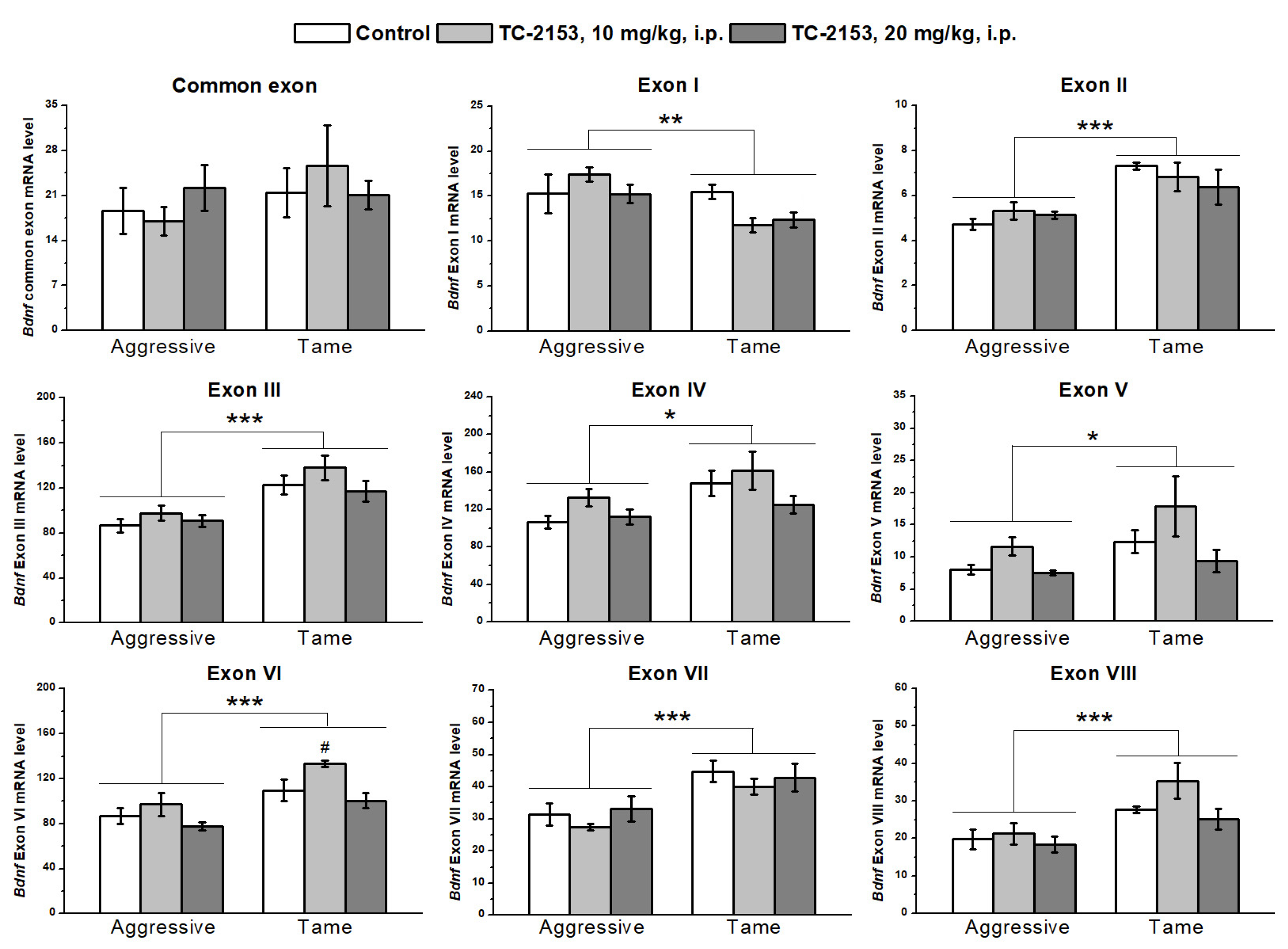
2.2. Effects of Acute TC-2153 Treatment on mRNA Levels of Bdnf Untranslated Exons I–VIII and Coding Exon IX in the Hippocampus of Aggressive and Tame Rats
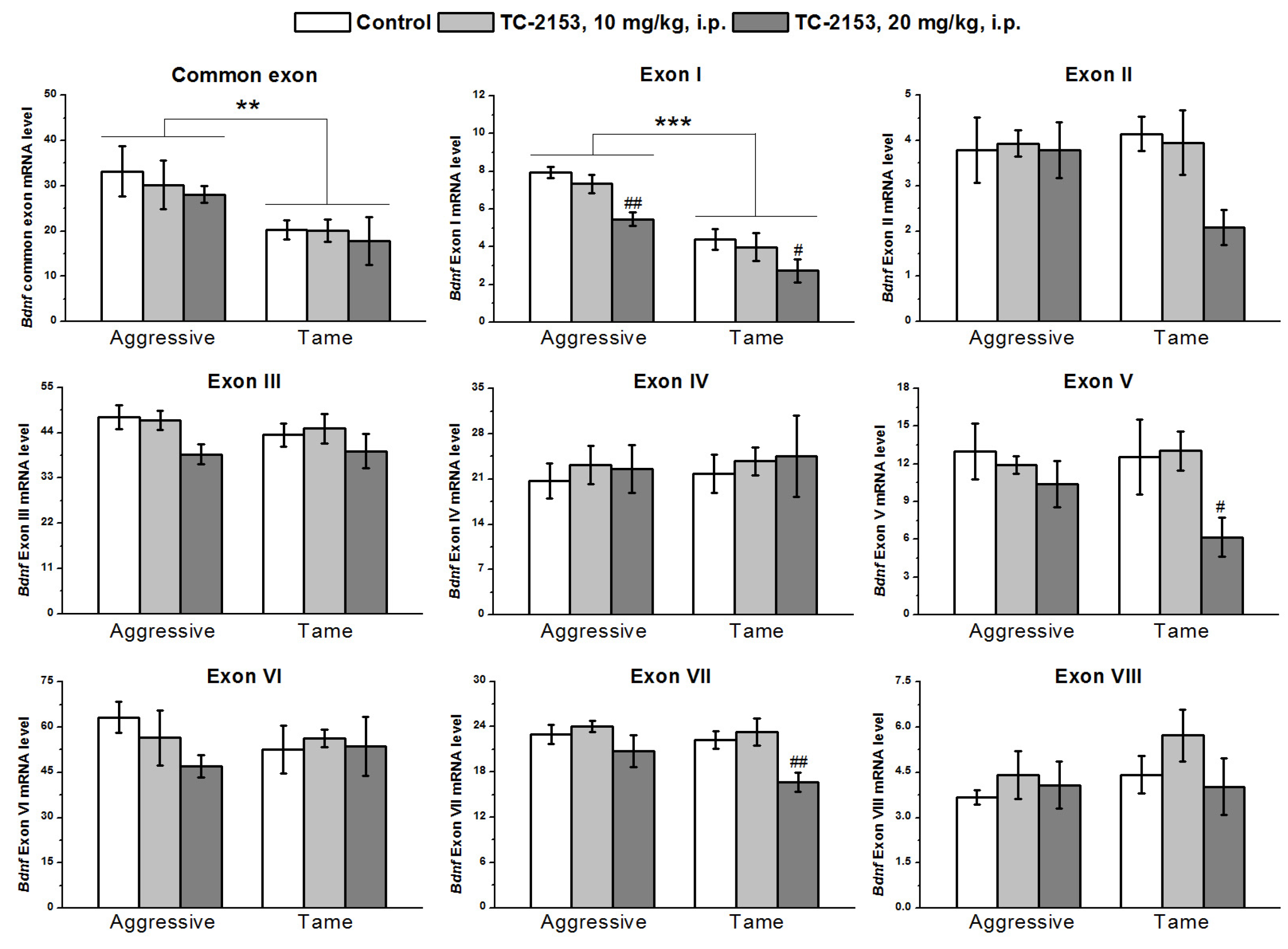
2.3. Effects of Acute TC-2153 Treatment on mRNA Levels of Bdnf Untranslated Exons I–VIII and Coding Exon IX in the Hypothalamus of Aggressive and Tame Rats
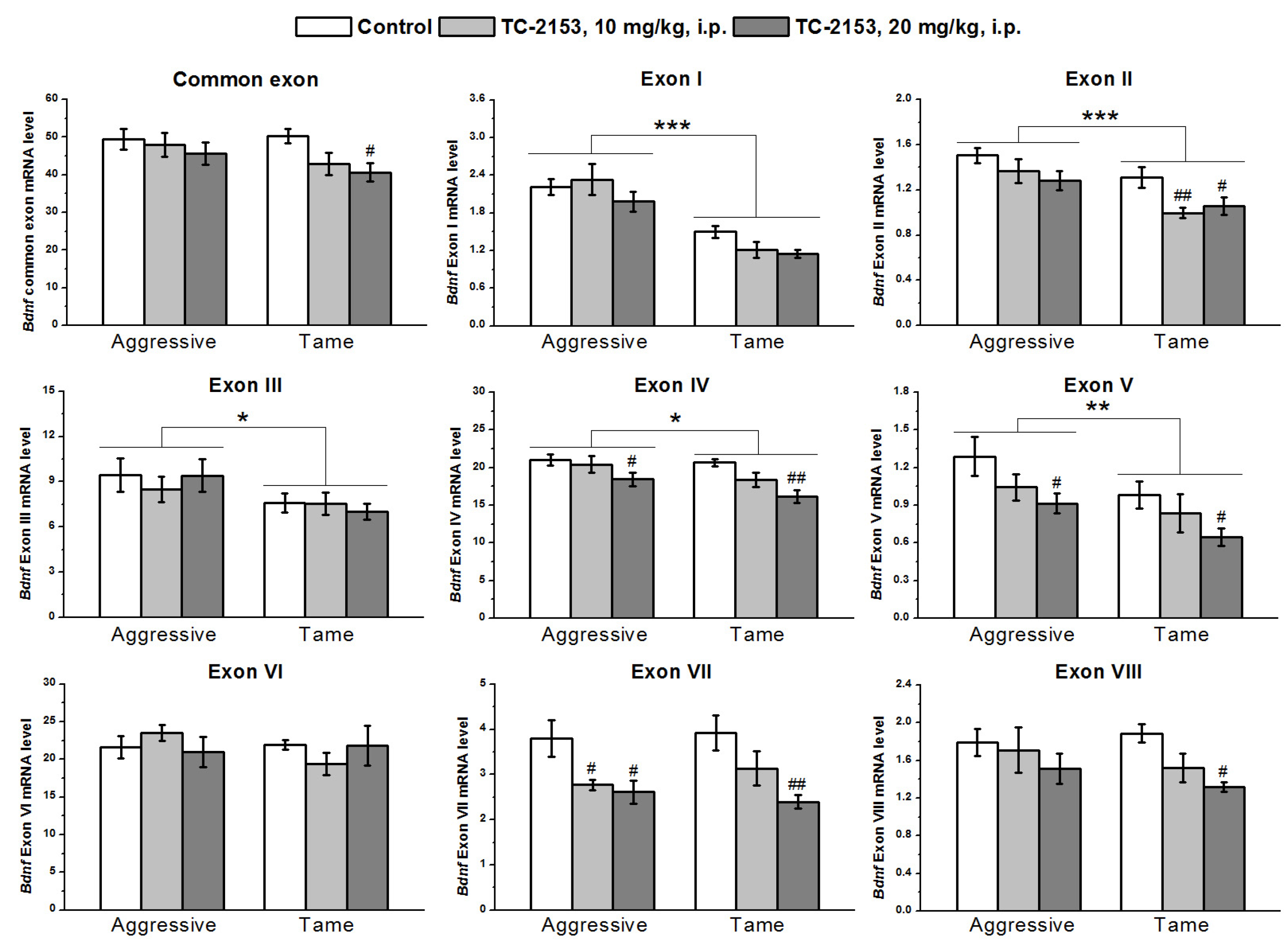
2.4. Effects of Acute TC-2153 Treatment on mRNA Levels of Bdnf Untranslated Exons I–VIII and Coding Exon IX in the Midbrain of Aggressive and Tame Rats
2.5. The Influence of Acute TC-2153 Treatment on the Htr1a mRNA Levels in Brain Structures of Aggressive and Tame Rats
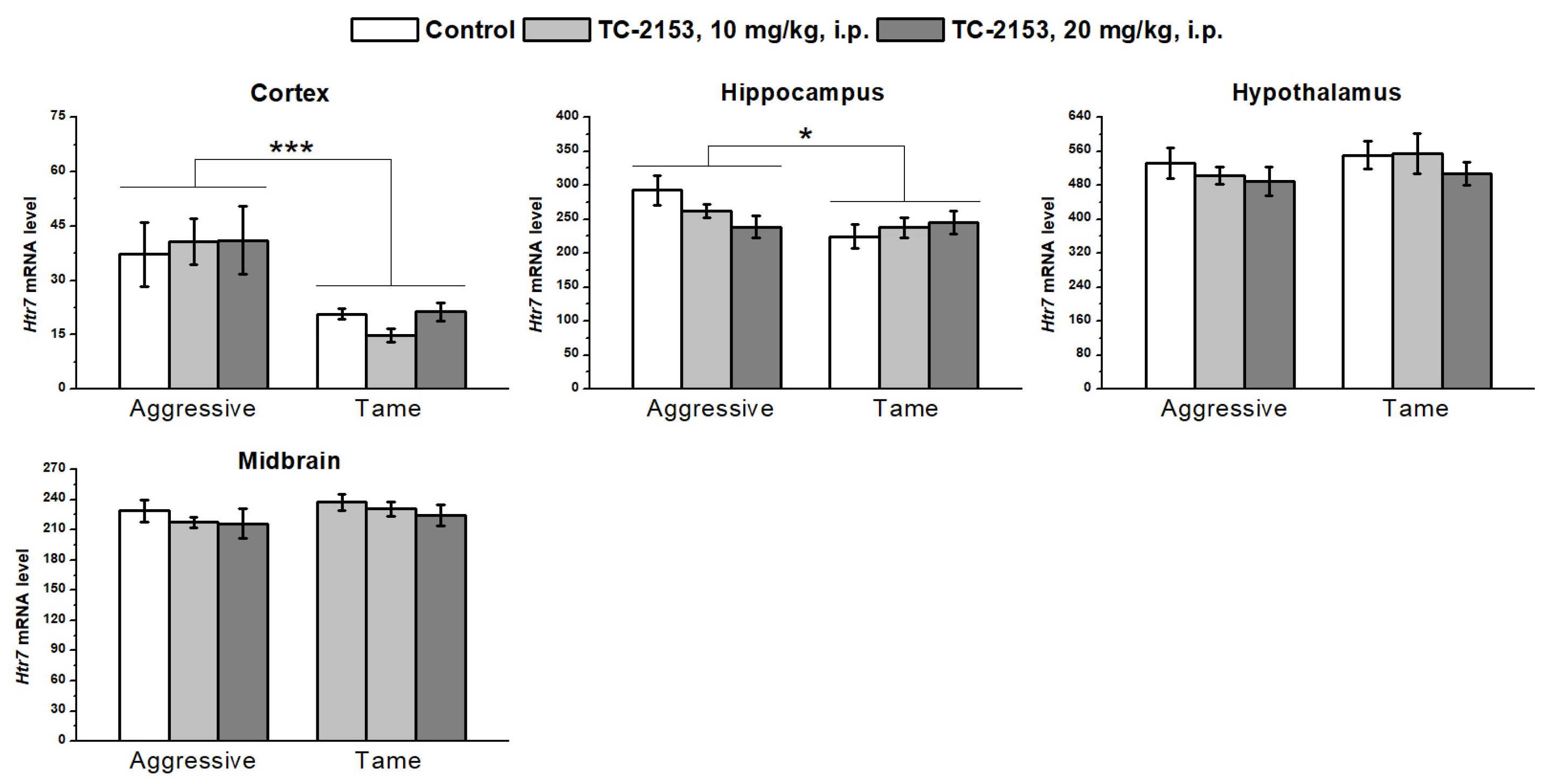
2.6. Effects of Acute TC-2153 Treatment on Htr7 mRNA Levels in Brain Structures of Aggressive and Tame Rats
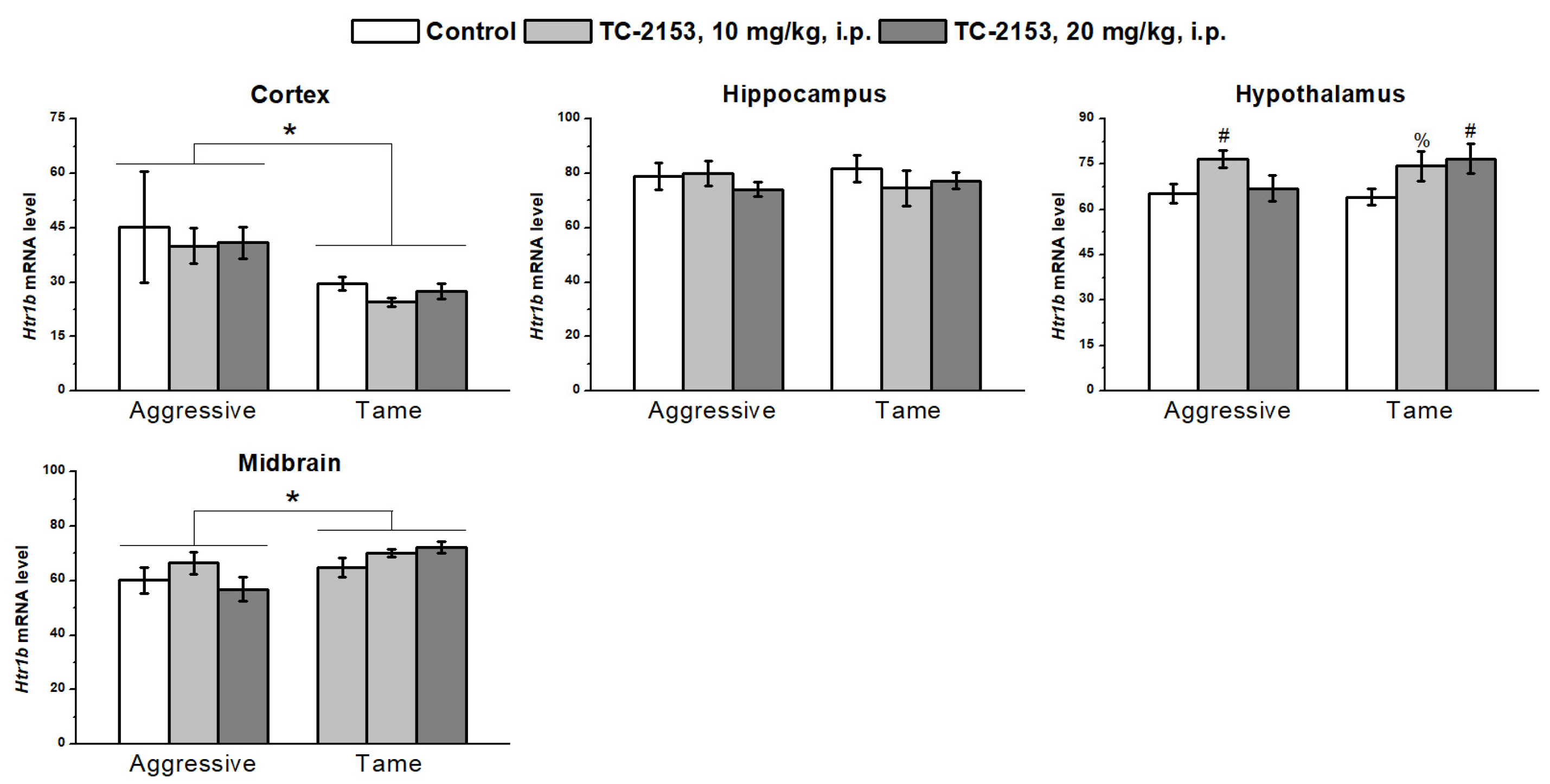
2.7. Effects of Acute TC-2153 Treatment on Htr1b mRNA Levels in Brain Structures of Aggressive and Tame Rats
2.8. Effects of Acute TC-2153 Treatment on Htr2a mRNA Levels in Brain Structures of Aggressive and Tame Rats
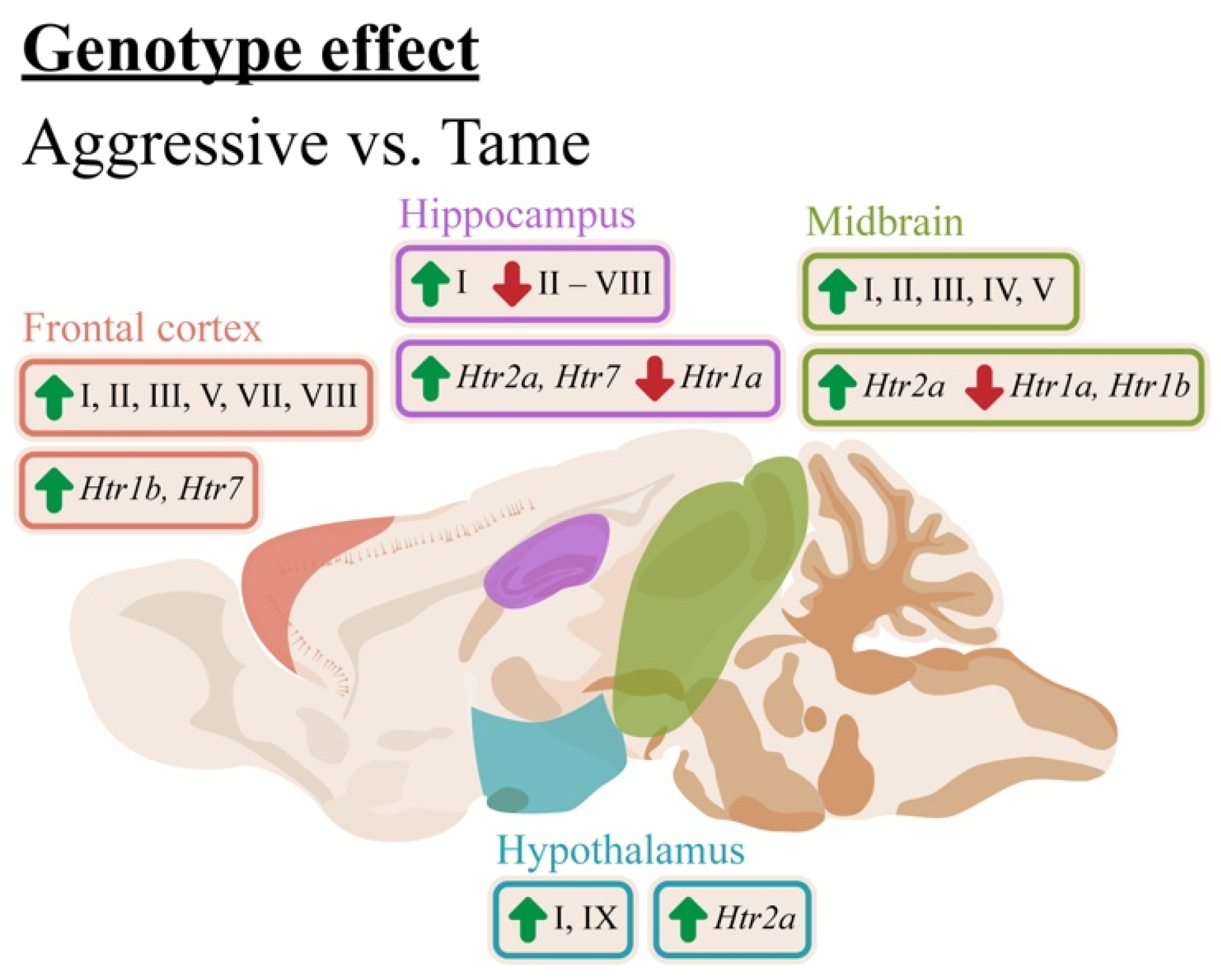
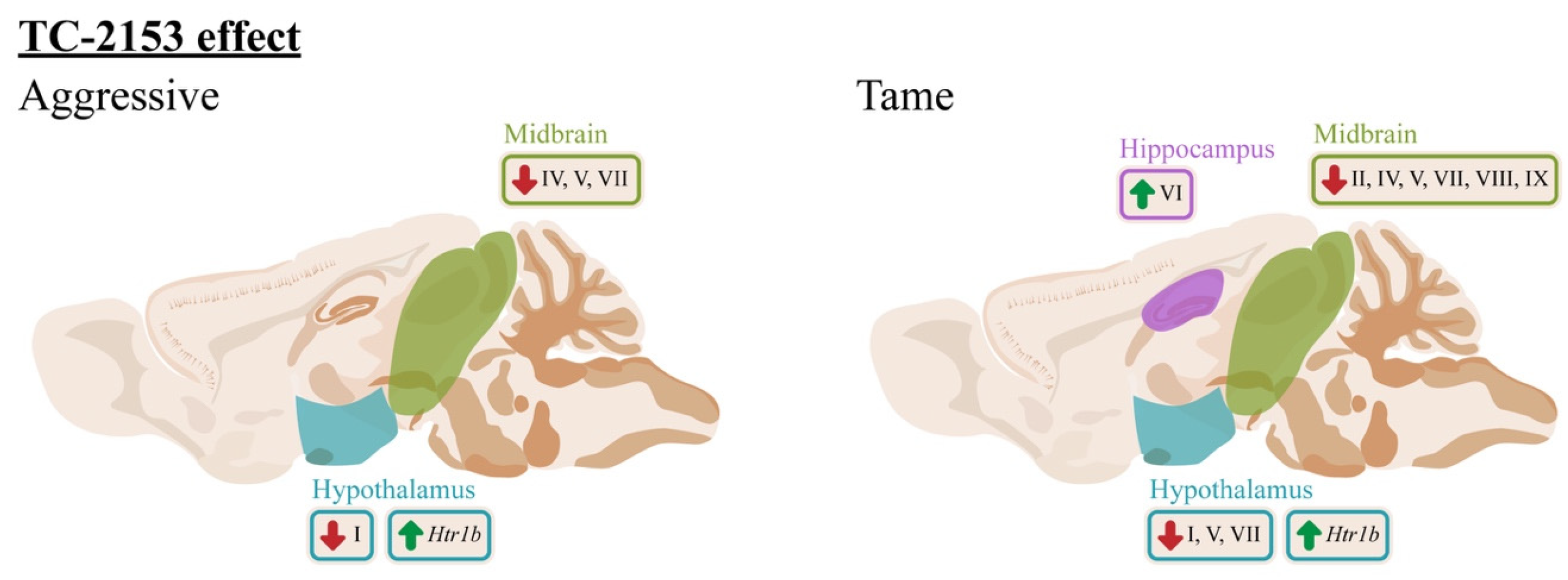
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedures
4.2. Real-Time PCR
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and ratBDNF gene structure and expression revisited. J. Neurosci. Res. 2006, 85, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Perovic, M.; Tesic, V.; Djordjevic, A.M.; Smiljanic, K.; Loncarevic-Vasiljkovic, N.; Ruzdijic, S.; Kanazir, S. BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. AGE 2012, 35, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Baj, G.; Leone, E.; Chao, M.V.; Tongiorgi, E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc. Natl. Acad. Sci. USA 2011, 108, 16813–16818. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 223–250. [Google Scholar] [CrossRef]
- Maynard, K.R.; Hobbs, J.W.; Sukumar, M.; Kardian, A.S.; Jimenez, D.V.; Schloesser, R.J.; Martinowich, K. Bdnf mRNA splice variants differentially impact CA1 and CA3 dendrite complexity and spine morphology in the hippocampus. Brain Struct. Funct. 2017, 222, 3295–3307. [Google Scholar] [CrossRef]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 6212983. [Google Scholar] [CrossRef] [PubMed]
- CZuccato, C.; Ciammola, A.; Rigamonti, D.; Leavitt, B.R.; Goffredo, D.; Conti, L.; MacDonald, M.E.; Friedlander, R.M.; Silani, V.; Hayden, M.R.; et al. Loss of Huntingtin-Mediated BDNF Gene Transcription in Huntington’s Disease. Science 2001, 293, 493–498. [Google Scholar] [CrossRef]
- Ou, L.-C.; Gean, P.-W. Transcriptional Regulation of Brain-Derived Neurotrophic Factor in the Amygdala during Consolidation of Fear Memory. Mol. Pharmacol. 2007, 72, 350–358. [Google Scholar] [CrossRef]
- Rattiner, L.M.; Davis, M.; Ressler, K.J. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn. Mem. 2004, 11, 727–731. [Google Scholar] [CrossRef]
- Fuchikami, M.; Yamamoto, S.; Morinobu, S.; Takei, S.; Yamawaki, S. Epigenetic Regulation of BDNF Gene in Response to Stress. Psychiatry Investig. 2010, 7, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Neeley, E.; Berger, R.; Koenig, J.; Leonard, S. Prenatal stress differentially alters brain-derived neurotrophic factor expression and signaling across rat strains. Neuroscience 2011, 187, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ilchibaeva, T.V.; Tsybko, A.S.; Kozhemyakina, R.V.; Kondaurova, E.; Popova, N.K.; Naumenko, V.S. Genetically defined fear-induced aggression: Focus on BDNF and its receptors. Behav. Brain Res. 2018, 343, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Günther, L.; Scheuch, K.; Klein, J.; Eckhart, S.; Hellweg, R.; Danker-Hopfe, H.; Oehler, J. Higher BDNF concentrations in the hippocampus and cortex of an aggressive mouse strain. Behav. Brain Res. 2009, 197, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kondaurova, E.M.; Bazovkina, D.V.; Tsybko, A.S.; Il’Chibaeva, T.V.; Popova, N.K. On the role of 5-HT1Areceptor gene in behavioral effect of brain-derived neurotrophic factor. J. Neurosci. Res. 2014, 92, 1035–1043. [Google Scholar] [CrossRef]
- Spalletta, G.; Morris, D.; Angelucci, F.; Rubino, I.; Spoletini, I.; Bria, P.; Martinotti, G.; Siracusano, A.; Bonaviri, G.; Bernardini, S.; et al. BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. Eur. Psychiatry 2010, 25, 311–313. [Google Scholar] [CrossRef]
- Yochum, C.; Doherty-Lyon, S.; Hoffman, C.; Hossain, M.M.; Zelikoff, J.T.; Richardson, J.R. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: Role of altered catecholamines and BDNF. Exp. Neurol. 2014, 254, 145–152. [Google Scholar] [CrossRef]
- Maynard, K.; Hill, J.; Calcaterra, N.E.; Palko, M.E.; Kardian, A.; Paredes, D.; Sukumar, M.; Adler, B.D.; Jimenez, D.V.; Schloesser, R.J.; et al. Functional Role of BDNF Production from Unique Promoters in Aggression and Serotonin Signaling. Neuropsychopharmacology 2015, 41, 1943–1955. [Google Scholar] [CrossRef]
- Popova, N.K. From genes to aggressive behavior: The role of serotonergic system. Bioessays 2006, 28, 495–503. [Google Scholar] [CrossRef]
- Ilchibaeva, T.V.; Tsybko, A.S.; Kondaurova, E.M.; Kovetskaya, A.I.; Kozhemyakina, R.V.; Naumenko, V.S. Expression Patterns of Serotonin Receptors 1A and 7 in the Brain of Rats with Genetically Determined Fear-Induced Aggressive Behavior or the Lack of Aggression. Neurochem. J. 2020, 14, 180–186. [Google Scholar] [CrossRef]
- Olivier, B.; van Oorschot, R. 5-HT1B receptors and aggression: A review. Eur. J. Pharmacol. 2005, 526, 207–217. [Google Scholar] [CrossRef]
- Sánchez, C.; Arnt, J.; Hyttel, J.; Moltzen, E.K. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology 1993, 110, 53–59. [Google Scholar] [CrossRef] [PubMed]
- van der Vegt, B.J.; Lieuwes, N.; van de Wall, E.H.E.M.; Kato, K.; Moya-Albiol, L.; Martínez-Sanchis, S.; de Boer, S.F.; Koolhaas, J.M. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav. Neurosci. 2003, 117, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.; Kondaurova, E.; Bazovkina, D.; Tsybko, A.; Tikhonova, M.; Kulikov, A.; Popova, N. Effect of brain-derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains. Neuroscience 2012, 214, 59–67. [Google Scholar] [CrossRef]
- Rumajogee, P.; Madeira, A.; Vergé, D.; Hamon, M.; Miquel, M.-C. Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J. Neurochem. 2002, 83, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Ilchibaeva, T.; Tsybko, A.; Zeug, A.; Müller, F.E.; Guseva, D.; Bischoff, S.; Ponimaskin, E.; Naumenko, V. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells 2022, 11, 2384. [Google Scholar] [CrossRef]
- Tsybko, A.S.; Ilchibaeva, T.V.; Filimonova, E.A.; Eremin, D.V.; Popova, N.K.; Naumenko, V.S. The Chronic Treatment With 5-HT2A Receptor Agonists Affects the Behavior and the BDNF System in Mice. Neurochem. Res. 2020, 45, 3059–3075. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2AReceptor-Mediated Regulation of Brain-Derived Neurotrophic Factor mRNA in the Hippocampus and the Neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kozhemjakina, R.V.; Plyusnina, I.Z.; Popova, N.K. Expression of serotonin transporter gene and startle response in rats with genetically determined fear-induced aggression. Bull. Exp. Biol. Med. 2009, 147, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Ilchibaeva, T.V.; Tsybko, A.S.; Kozhemyakina, R.V.; Konoshenko, M.Y.; Popova, N.K.; Naumenko, V.S. The relationship between different types of genetically defined aggressive behavior. J. Ethol. 2016, 35, 75–81. [Google Scholar] [CrossRef]
- Albert, F.W.; Shchepina, O.; Winter, C.; Römpler, H.; Teupser, D.; Palme, R.; Ceglarek, U.; Kratzsch, J.; Sohr, R.; Trut, L.N.; et al. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm. Behav. 2008, 53, 413–421. [Google Scholar] [CrossRef]
- Pliusnina, I.Z.; Shchepina, O.; Os’Kina, I.N.; Trut, L.N. Some features of learning in swimming Morris test in rats selected for behavior towards human. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2007, 57, 344–351. [Google Scholar]
- Ilchibaeva, T.V.; Kondaurova, E.M.; Tsybko, A.S.; Kozhemyakina, R.V.; Popova, N.K.; Naumenko, V.S. Brain-derived neurotrophic factor (BDNF) and its precursor (proBDNF) in genetically defined fear-induced aggression. Behav. Brain Res. 2015, 290, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kondaurova, E.M.; Ilchibaeva, T.V.; Tsybko, A.S.; Kozhemyakina, R.V.; Popova, N.K.; Naumenko, V.S. 5-HT1A receptor gene silencers Freud-1 and Freud-2 are differently expressed in the brain of rats with genetically determined high level of fear-induced aggression or its absence. Behav. Brain Res. 2016, 310, 20–25. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kozhemyakina, R.V.; Plyusnina, I.F.; Kulikov, A.V.; Popova, N.K. Serotonin 5-HT1A receptor in infancy-onset aggression: Comparison with genetically defined aggression in adult rats. Behav. Brain Res. 2013, 243, 97–101. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S.; Plyusnina, I.Z.; Kulikov, A.V. Reduction in 5-HT1A receptor density, 5-HT1A mRNA expression, and functional correlates for 5-HT1A receptors in genetically defined aggressive rats. J. Neurosci. Res. 2005, 80, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Kulikov, A.V.; Nikulina, E.M.; Kozlachkova, E.Y.; Maslova, G.B. Serotonin metabolism and 5-HT receptors in Norway rats selected for low aggressiveness to man. Aggr. Behav. 1991, 17, 207–213. [Google Scholar] [CrossRef]
- Voĭtenko, N.N.; Kolpakov, V.G. Brain monoamine oxidase and the predisposition for catalepsy in tame and aggressive Norway rats. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 1993, 43, 1000–1005. [Google Scholar]
- Moskaliuk, V.S.; Kozhemyakina, R.V.; Bazovkina, D.V.; Terenina, E.; Khomenko, T.M.; Volcho, K.P.; Salakhutdinov, N.F.; Kulikov, A.V.; Naumenko, V.S.; Kulikova, E. On an association between fear-induced aggression and striatal-enriched protein tyrosine phosphatase (STEP) in the brain of Norway rats. Biomed. Pharmacother. 2022, 147, 112667. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Tikhonova, M.A.; Kulikova, E.A.; Volcho, K.P.; Khomenko, T.M.; Salakhutdinov, N.F.; Popova, N.K. A new synthetic varacin analogue, 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin-6-amine hydrochloride (TC-2153), decreased hereditary catalepsy and increased the BDNF gene expression in the hippocampus in mice. Psychopharmacology 2011, 221, 469–478. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Tikhonova, M.A.; Kulikova, E.A.; Khomenko, T.M.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; Popova, N.K. Effect of new potential psychotropic drug, 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin-6-amine hydrochloride, on the expression of serotonin-related genes in mouse brain. Mol. Biol. 2011, 45, 282–288. [Google Scholar] [CrossRef]
- Kulikova, E.; Kulikov, A.; Kulikov, E.K.A.A. Striatal-enriched Tyrosine Protein Phosphatase (STEP) in the Mechanisms of Depressive Disorders. Curr. Protein Pept. Sci. 2017, 18, 1152–1162. [Google Scholar] [CrossRef]
- Kulikova, E.; Khotskin, N.; Illarionova, N.; Sorokin, I.; Bazhenova, E.; Kondaurova, E.; Volcho, K.; Khomenko, T.; Salakhutdinov, N.; Ponimaskin, E.; et al. Inhibitor of Striatal-Enriched Protein Tyrosine Phosphatase, 8-(Trifluoromethyl)-1,2,3,4,5-Benzopentathiepin-6-Amine hydrochloride (TC-2153), Produces Antidepressant-Like Effect and Decreases Functional Activity and Protein Level of 5-HT2A Receptor in the Brain. Neuroscience 2018, 394, 220–231. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Volcho, K.P.; Salakhutdinov, N.F.; Kulikov, A.V. Benzopentathiepine Derivative, 8-(Trifluoromethyl)-1,2,3,4,5-Benzopentathiepin- 6-Amine Hydrochloride (TC-2153), as a Promising Antidepressant of New Generation. Lett. Drug Des. Discov. 2017, 14, 974–984. [Google Scholar] [CrossRef]
- Dias, B.G. Differential regulation of Brain Derived Neurotrophic Factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology 2003, 45, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Pei, L.; Li, Y.-N.; Zheng, H.; Yang, S.; Wan, Y.; Mao, L.; Xia, Y.-P.; He, Q.-W.; Li, M.; et al. Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci. Rep. 2017, 7, 14926. [Google Scholar] [CrossRef]
- Fiore, M.; Amendola, T.; Triaca, V.; Tirassa, P.; Alleva, E.; Aloe, L. Agonistic encounters in aged male mouse potentiate the expression of endogenous brain NGF and BDNF: Possible implication for brain progenitor cells’ activation. Eur. J. Neurosci. 2003, 17, 1455–1464. [Google Scholar] [CrossRef][Green Version]
- Chan, J.; Unger, T.; Byrnes, J.; Rios, M. Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience 2006, 142, 49–58. [Google Scholar] [CrossRef]
- Ito, W.; Chehab, M.; Thakur, S.; Li, J.; Morozov, A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011, 10, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Eaton, M.J.; Staley, J.K.; Globus, M.Y.-T.; Whittemore, S.R. Developmental Regulation of Early Serotonergic Neuronal Differentiation: The Role of Brain-Derived Neurotrophic Factor and Membrane Depolarization. Dev. Biol. 1995, 170, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.; Hyman, C.; Studer, L.; Egli, M.; Evtouchenko, L.; Jackson, C.; Dahl-Jørgensen, A.; Lindsay, R.M.; Seiler, R.W. Effect of BDNF on Dopaminergic, Serotonergic, and GABAergic Neurons in Cultures of Human Fetal Ventral Mesencephalon. Exp. Neurol. 1995, 133, 50–63. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S. Neuronal and behavioral plasticity: The role of serotonin and BDNF systems tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef]
- De Boer, S.F.; Lesourd, M.; Mocaer, E.; Koolhaas, J.M. Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J. Pharmacol. Exp. Ther. 1999, 288, 1125–1133. [Google Scholar]
- Heisler, L.K.; Chu, H.-M.; Brennan, T.J.; Danao, J.A.; Bajwa, P.; Parsons, L.H.; Tecott, L.H. Elevated anxiety and antidepressant-like responses in serotonin 5-HT 1A receptor mutant mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15049–15054. [Google Scholar] [CrossRef] [PubMed]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef] [PubMed]
- Guscott, M.; Bristow, L.; Hadingham, K.; Rosahl, T.; Beer, M.; Stanton, J.; Bromidge, F.; Owens, A.; Huscroft, I.; Myers, J.; et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 2005, 48, 492–502. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Stachowicz, K.; Tatarczyńska, E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 2006, 51, 578–586. [Google Scholar] [CrossRef]
- Stiedl, O.; Pappa, E.; Konradsson-Geuken, Å.; Ögren, S.O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.M.M.; Miczek, K.A. Aggression Escalated by Social Instigation or by Discontinuation of Reinforcement (“Frustration”) in Mice Inhibition by Anpirtoline: A 5-HT1B Receptor Agonist. Neuropsychopharmacology 2002, 27, 171–181. [Google Scholar] [CrossRef]
- Saudou, F.; Amara, D.A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M.-C.; Hen, R. Enhanced Aggressive Behavior in Mice Lacking 5-HT 1B Receptor. Science 1994, 265, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Muehlenkamp, F.; Lucion, A.; Vogel, W. Effects of selective serotonergic agonists on aggressive behavior in rats. Pharmacol. Biochem. Behav. 1995, 50, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, M.; Ago, Y.; Sowa, C.; Sakamoto, Y.; Nishihara, B.; Koyama, Y.; Baba, A.; Matsuda, T. Modulation by 5-HT2A Receptors of Aggressive Behavior in Isolated Mice. Jpn. J. Pharmacol. 2002, 89, 89–92. [Google Scholar] [CrossRef]
- Altieri, S.C.; Garcia-Garcia, A.L.; Leonardo, E.D.; Andrews, A.M. Rethinking 5-HT1AReceptors: Emerging Modes of Inhibitory Feedback of Relevance to Emotion-Related Behavior. ACS Chem. Neurosci. 2013, 4, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Raote, I.; Bhattacharya, A.; Panicker, M.M. Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways. In Serotonin Receptors in Neurobiology; Chattopadhyay, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; Available online: http://www.ncbi.nlm.nih.gov/books/NBK1853/ (accessed on 8 October 2022).
- Aleyasin, H.; Flanigan, M.E.; Russo, S.J. Neurocircuitry of aggression and aggression seeking behavior: Nose poking into brain circuitry controlling aggression. Curr. Opin. Neurobiol. 2018, 49, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Luoni, A.; Guidotti, G.; Racagni, G.; Fumagalli, F.; Riva, M.A. Modulation of neuronal plasticity following chronic concomitant administration of the novel antipsychotic lurasidone with the mood stabilizer valproic acid. Psychopharmacology 2012, 226, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chatterjee, M.; Baguley, T.D.; Brouillette, J.; Kurup, P.; Ghosh, D.; Kanyo, J.; Zhang, Y.; Seyb, K.; Ononenyi, C.; et al. Inhibitor of the Tyrosine Phosphatase STEP Reverses Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. PLoS Biol. 2014, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kurup, P.; Baguley, T.D.; Foscue, E.; Ellman, J.A.; Nairn, A.C.; Lombroso, P.J. Inhibition of the tyrosine phosphatase STEP61 restores BDNF expression and reverses motor and cognitive deficits in phencyclidine-treated mice. Cell. Mol. Life Sci. 2016, 73, 1503–1514. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Sinyakova, N.A.; Kulikova, E.A.; Khomenko, T.M.; Salakhutdinov, N.F.; Kulikov, V.A.; Volcho, K.P. Effects of Acute and Chronic Treatment of Novel Psychotropic Drug, 8- (Trifluoromethyl)-1, 2, 3, 4, 5-benzopentathiepin-6-amine Hydrochloride (TC-2153), on the Behavior of Zebrafish (Danio Rerio): A Comparison with Fluoxetine. Lett. Drug Des. Discov. 2019, 16, 1321–1328. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Tikhonova, M.; Kulikova, E.A.; Volcho, K.P.; Khomenko, T.M.; Salakhutdinov, N.F.; Popova, N.K. Antidepressant Activity of 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin- 6-amine hydrochloride (TC-2153): Comparison with Classical Antidepressants. Lett. Drug Des. Discov. 2013, 11, 169–173. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Tikhonova, M.A.; Volcho, K.P.; Khomenko, T.M.; Salakhutdinov, N.F.; Kulikov, A.V.; Popova, N.K. Comparison of behavioral effects of fluoxetine, imipramine and new psychotropic drug TC-2153 on mice with hereditary predisposition to catalepsy. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2015, 65, 105–112. [Google Scholar] [CrossRef]
- Maroteaux, L.; Saudou, F.; Amlaiky, N.; Boschert, U.; Plassat, J.L.; Hen, R. Mouse 5HT1B serotonin receptor: Cloning, functional expression, and localization in motor control centers. Proc. Natl. Acad. Sci. USA 1992, 89, 3020–3024. [Google Scholar] [CrossRef]
- Naumenko, E.; Popova, N.; Nikulina, E.; Dygalo, N.; Shishkina, G.; Borodin, P.; Markel, A. Behavior, adrenocortical activity, and brain monoamines in Norway rats selected for reduced aggressiveness towards man. Pharmacol. Biochem. Behav. 1989, 33, 85–91. [Google Scholar] [CrossRef]
- Plyusnina, I.; Oskina, I. Behavioral and Adrenocortical Responses to Open-Field Test in Rats Selected for Reduced Aggressiveness Toward Humans. Physiol. Behav. 1997, 61, 381–385. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Naumenko, V.S.; Voronova, I.P.; Tikhonova, M.A.; Popova, N.K. Quantitative RT-PCR assay of 5-HT1A and 5-HT2A serotonin receptor mRNAs using genomic DNA as an external standard. J. Neurosci. Methods 2005, 141, 97–101. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Osipova, D.V.; Kostina, E.V.; Kulikov, A.V. Utilization of a two-standard system in real-time PCR for quantification of gene expression in the brain. J. Neurosci. Methods 2008, 170, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.S.; Kulikov, A.V. Quantitative assay of 5-HT(1A) serotonin receptor gene expression in the brain. Mol. Biol. 2006, 40, 37–44. [Google Scholar] [CrossRef]


| Gene | Sequence | Annealing Temperature, °C | Product Length, bp |
|---|---|---|---|
| Polr2a | F: 5′-TTGTCGGGCAGCAGAACGTG-3′ R: 5′-CAATGAGACCTTCTCGTCCTCCC-3′ | 63 | 186 |
| Bdnf common exon IX | F: 5′-TAGCAAAAAGAGAATTGGCTG-3′ R: 5′-TTTCTGGTCATGGATATGTCC-3′ | 59 | 257 |
| Bdnf exon I | F: 5′-TGGCGACAGGGAAATCTC-3′ R: 5′-GAATGAGCGAGGTTACCAATG-3′ | 60 | 242 |
| Bdnf exon II | F: 5′-CGTAAGGAAGTGGAAGAAACCGTCTA-3′ R: 5′-ATCTCAGTGTGAGCCGAACCTC-3′ | 65 | 253 |
| Bdnf exon III | F: 5′-GATTCTCGCTGGATAGTTCTTTATG-3′ R: 5′-GGAGGGAAAATAGAAAGGGGTAA-3′ | 61 | 99 |
| Bdnf exon IV | F: 5′-CCAGTCTCTGCCTAGATCAAATG-3′ R: 5′-GCCGATATGTACTCCTGTTCTTCAG-3′ | 63 | 121 |
| Bdnf exon V | F: 5′-AAACCATAACCCCGCACACT-3′ R: 5′-CCGCACCTTCCCGCAC-3′ | 64 | 77 |
| Bdnf exon VI | F: 5′-ACCAGGAGCGTGACAACAATG-3′ R: 5′-GTCCACACAAAGCTCTCGGATC-3′ | 65 | 59 |
| Bdnf exon VII | F: 5′-CTTCTTACAAGTCCAAGGTCAACA-3′ R: 5′-AAGTCAAAACTTTCACTTCCTCTG-3′ | 64 | 167 |
| Bdnf exon VIII | F: 5′-GGAACTTGGGATCATTCTTGTCAC-3′ R: 5′-TCTGGAGGATGCCTAAAGAGG-3′ | 63 | 117 |
| Htr1a | F: 5′-CTGTCACTCTCTGCCCTACTTCTG-3′ R: 5′-CCAGAGCACATAACCCAGAGTAGT-3′ | 65 | 175 |
| Htr1b | F: 5′-GTTCTTTCCCTGGTCCGCTC-3′ R: 5′-GTCCTGGTAAATGTAGTCGTCGG-3′ | 64 | 176 |
| Htr2a | F: 5′-GTATATCCATGCCAATCCCAG-3′ R: 5′-CTTGATAGTCAGGAAGTAGGTG-3′ | 60 | 164 |
| Htr7 | F: 5′-TGATCTCGGTGTGCTTCGTC-3′ R: 5′-GTGACACTAACGAAAGGCATGAC-3′ | 64 | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskaliuk, V.S.; Kozhemyakina, R.V.; Khomenko, T.M.; Volcho, K.P.; Salakhutdinov, N.F.; Kulikov, A.V.; Naumenko, V.S.; Kulikova, E.A. On Associations between Fear-Induced Aggression, Bdnf Transcripts, and Serotonin Receptors in the Brains of Norway Rats: An Influence of Antiaggressive Drug TC-2153. Int. J. Mol. Sci. 2023, 24, 983. https://doi.org/10.3390/ijms24020983
Moskaliuk VS, Kozhemyakina RV, Khomenko TM, Volcho KP, Salakhutdinov NF, Kulikov AV, Naumenko VS, Kulikova EA. On Associations between Fear-Induced Aggression, Bdnf Transcripts, and Serotonin Receptors in the Brains of Norway Rats: An Influence of Antiaggressive Drug TC-2153. International Journal of Molecular Sciences. 2023; 24(2):983. https://doi.org/10.3390/ijms24020983
Chicago/Turabian StyleMoskaliuk, Vitalii S., Rimma V. Kozhemyakina, Tatyana M. Khomenko, Konstantin P. Volcho, Nariman F. Salakhutdinov, Alexander V. Kulikov, Vladimir S. Naumenko, and Elizabeth A. Kulikova. 2023. "On Associations between Fear-Induced Aggression, Bdnf Transcripts, and Serotonin Receptors in the Brains of Norway Rats: An Influence of Antiaggressive Drug TC-2153" International Journal of Molecular Sciences 24, no. 2: 983. https://doi.org/10.3390/ijms24020983
APA StyleMoskaliuk, V. S., Kozhemyakina, R. V., Khomenko, T. M., Volcho, K. P., Salakhutdinov, N. F., Kulikov, A. V., Naumenko, V. S., & Kulikova, E. A. (2023). On Associations between Fear-Induced Aggression, Bdnf Transcripts, and Serotonin Receptors in the Brains of Norway Rats: An Influence of Antiaggressive Drug TC-2153. International Journal of Molecular Sciences, 24(2), 983. https://doi.org/10.3390/ijms24020983







