Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications
Abstract
1. Introduction
2. Results and Discussion
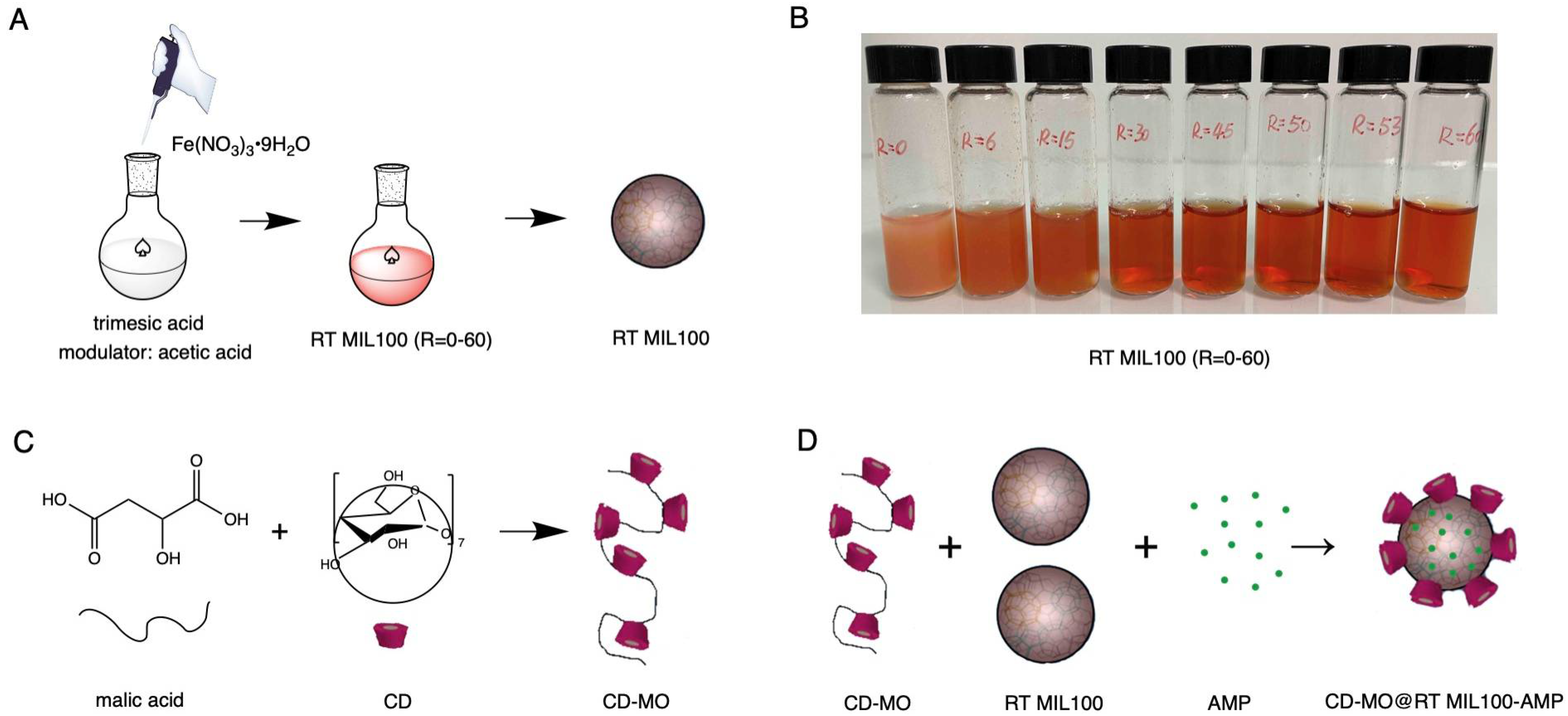
2.1. Preparation of RT MIL100: Effect of the Acetic Acid Modulator
2.1.1. Synthesis of RT MIL100 without Modulators
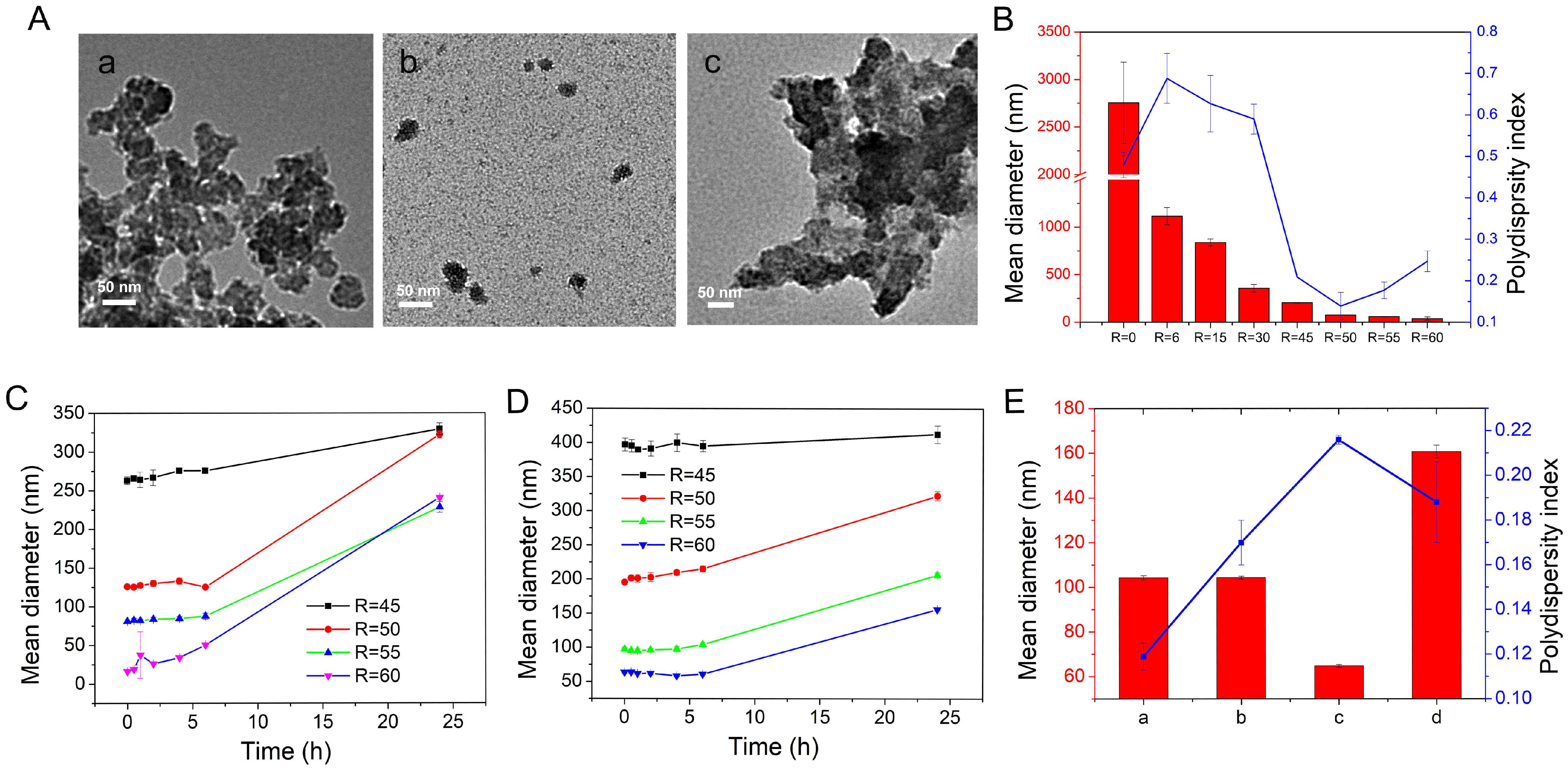
2.1.2. Synthesis of Modulated RT MIL100
2.1.3. Purification of Modulated RT MIL100
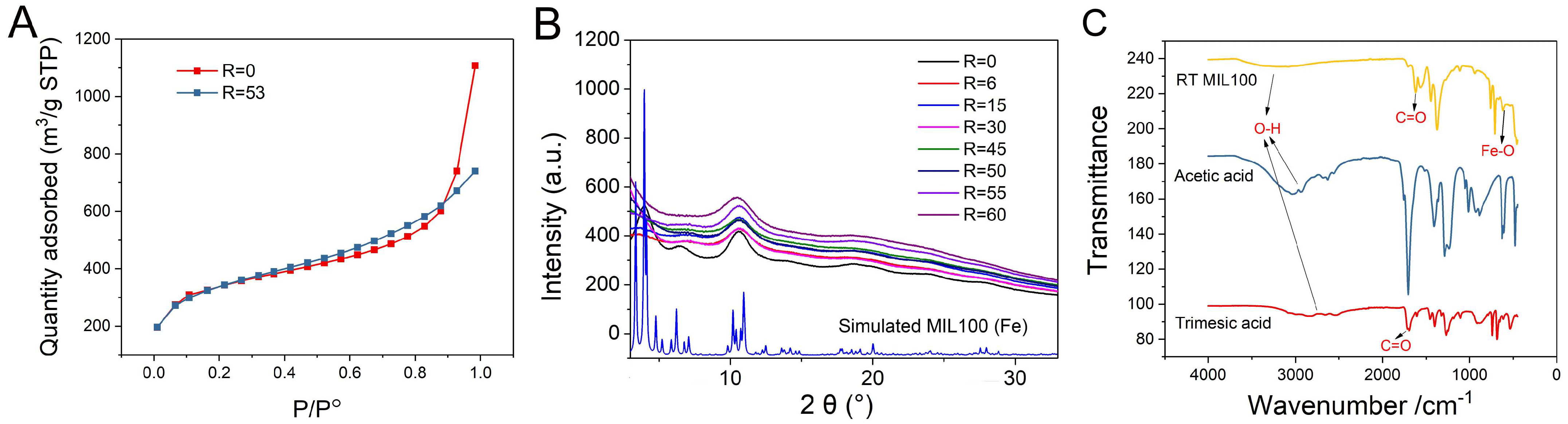
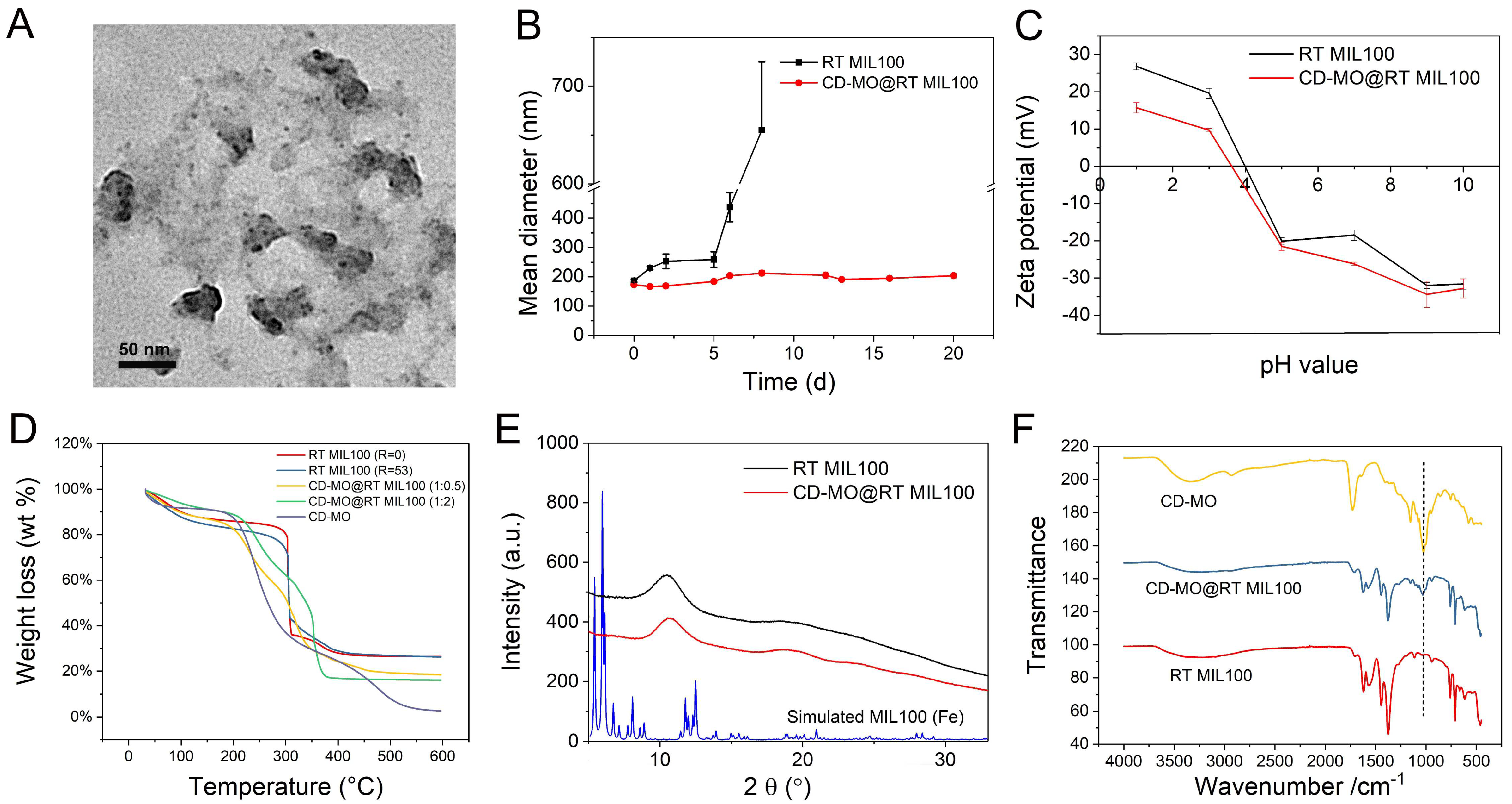
2.2. Characterization of RT MIL100
2.3. Synthesis of CD-MO
2.4. Physicochemical Characterizations of CD-MO@RT MIL100
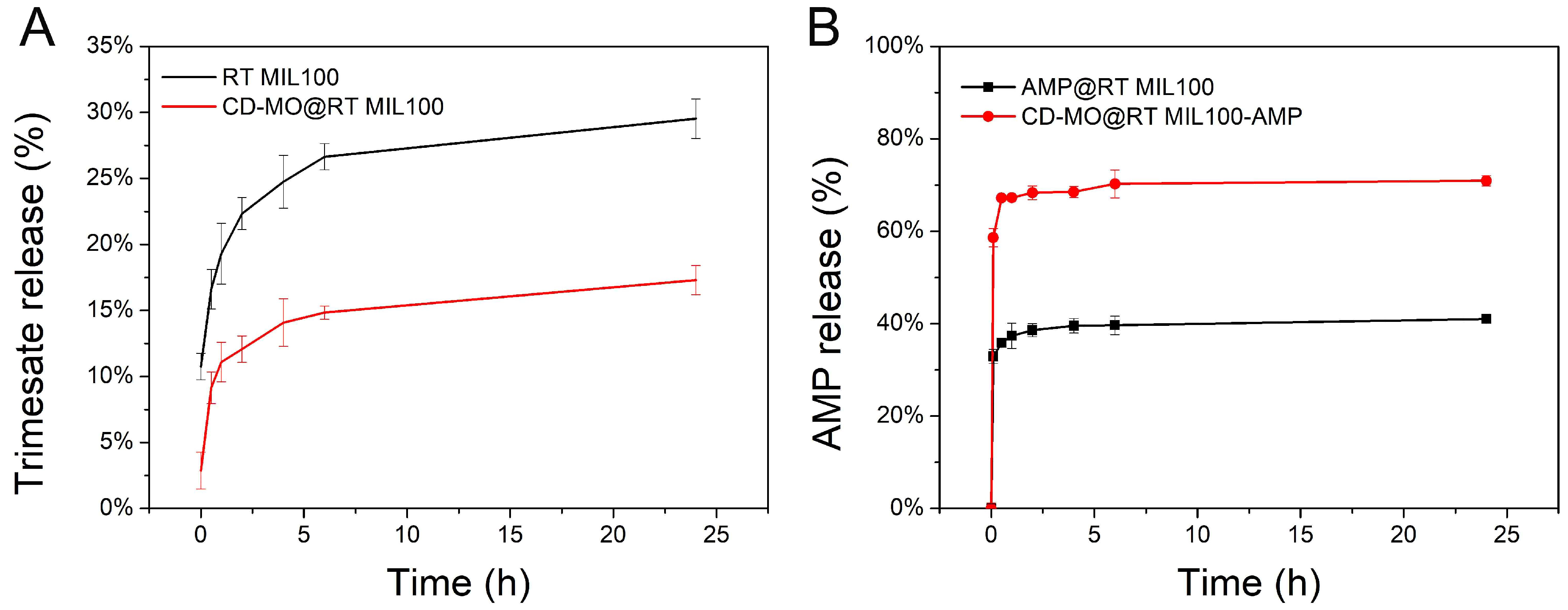
2.5. Drug Loading, Release, and RT MIL100 Degradation Study
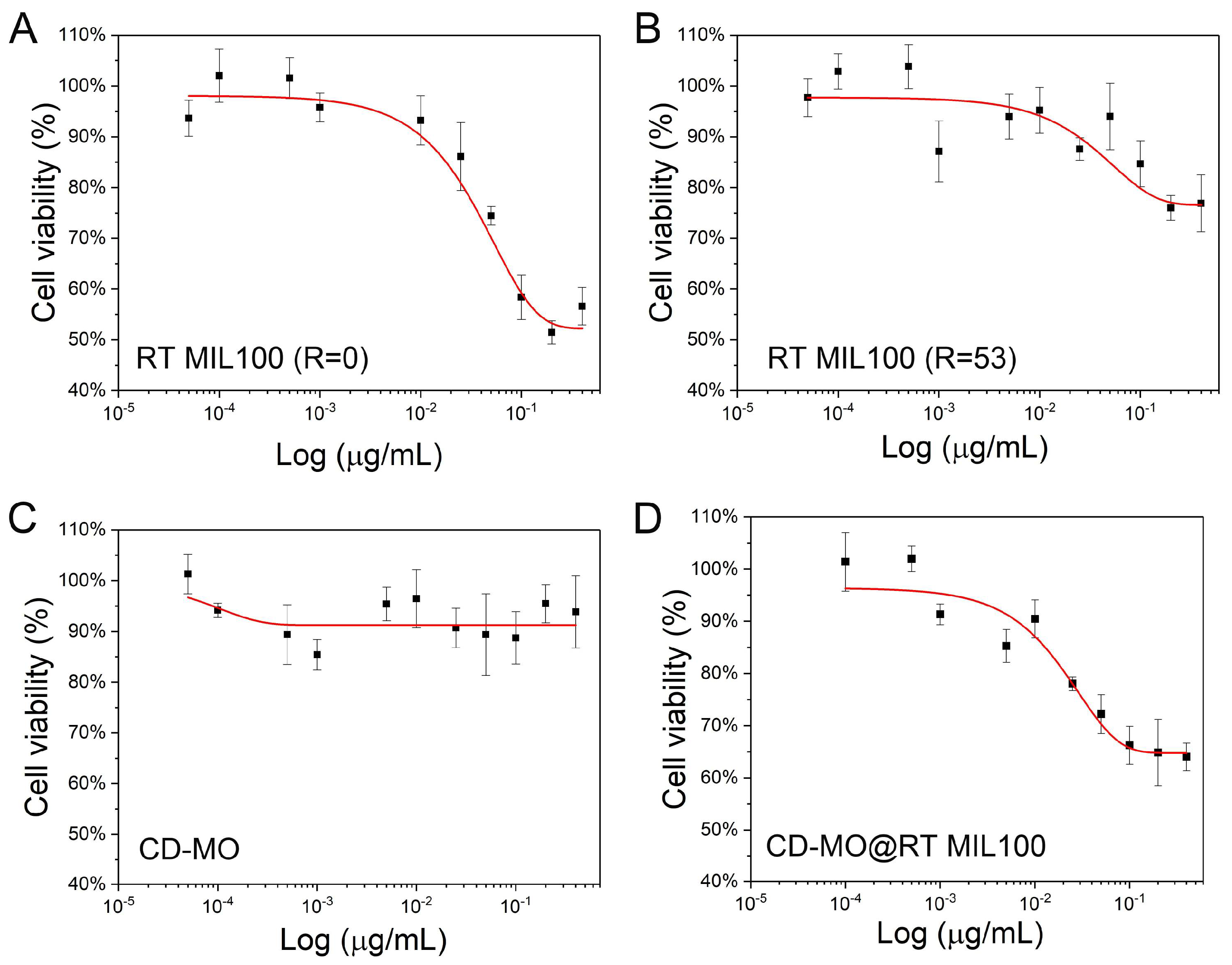
2.6. Toxicity Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization
3.2.1. Preparation of RT MIL100 with/without Modulators
3.2.2. Preparation of CD-MO
3.2.3. Preparation of CD-MO@RT MIL100
3.2.4. Preparation of AMP@RT MIL100 and CD-MO@RT MIL100-AMP
3.2.5. Characterization of RT MIL100
3.2.6. Characterization of CD-MO
3.3. Drug Encapsulation and Release
3.3.1. AMP Encapsulation and Payload Efficiency Test
3.3.2. AMP Release and RT MIL100 Degradation
3.4. Cell Toxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B. 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.H.; Richard, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Dutta, A.; Pan, Y.; Liu, J.Q.; Kumar, A. Multicomponent isoreticular metal-organic frameworks: Principles, current status and challenges. Coord. Chem. Rev. 2021, 445, 214074–214108. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098–112105. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496–114518. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. Applications of Porous Metal–Organic Framework MIL-100(M) (M = Cr, Fe, Sc, Al, V). Cryst. Growth Des. 2018, 18, 7730–7744. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, C.; Liang, Y.; Liu, T.; Tian, H.; Zhang, Y. Multifunctional drug delivery nanoparticles based on MIL-100 (Fe) for photoacoustic imaging-guided synergistic chemodynamic/chemo/photothermal breast cancer therapy. Mater. Des. 2022, 223, 111132–111141. [Google Scholar] [CrossRef]
- Ettlinger, R.; Lachelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.E.; Wuttke, S. Toxicity of metal-organic framework nanoparticles: From essential analyses to potential applications. Chem. Soc. Rev. 2022, 51, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Surble, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Greneche, J.M.; Margiolaki, I.; Ferey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 19, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, V.; Horcajada, P.; Rodriguez-Ruiz, V.; Willaime, H.; Couvreur, P.; Serre, C.; Gref, R. ‘Green’ fluorine-free mesoporous iron(III) trimesate nanoparticles for drug delivery. Green Mater. 2013, 1, 209–217. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, Á.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef]
- Oveisi, M.; Mahmoodi, N.M.; Asli, M.A. Facile and green synthesis of metal-organic framework/inorganic nanofiber using electrospinning for recyclable visible-light photocatalysis. J. Clean. Prod. 2019, 222, 669–684. [Google Scholar] [CrossRef]
- Li, W.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Room-temperature synthesis of MIL-100(Fe) and its adsorption performance for fluoride removal from water. Colloids Surf. A. Physicochem. Eng. Asp. 2021, 624, 126791–126803. [Google Scholar] [CrossRef]
- Steenhaut, T.; Hermans, S.; Filinchuk, Y. Green synthesis of a large series of bimetallic MIL-100(Fe,M) MOFs. New J. Chem. 2020, 44, 3847–3855. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, X.; Zhou, X.; Xiao, J.; Li, Z. Novel room-temperature synthesis of MIL-100(Fe) and its excellent adsorption performances for separation of light hydrocarbons. J. Chem.Eng. 2019, 355, 679–686. [Google Scholar] [CrossRef]
- Devi, Y.; Ang, I.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Angkawijaya, A.E.; Hartono, S.B.; Tran-Nguyen, P.L.; Ismadji, S.; et al. An iron–carboxylate-based metal–organic framework for Furosemide loading and release. J. Mater. Sci. 2020, 55, 13785–13798. [Google Scholar] [CrossRef]
- Barjasteh, M.; Vossoughi, M.; Bagherzadeh, M.; Pooshang Bagheri, K. Green synthesis of PEG-coated MIL-100(Fe) for controlled release of dacarbazine and its anticancer potential against human melanoma cells. Int. J. Pharm. 2022, 618, 121647–121656. [Google Scholar] [CrossRef] [PubMed]
- Monik, P.; Farid, N.; Christian, S.; Marvin, B.; Saad, S.; Nathalie, S.; Monica, G.M. Low temperature process for the synthesis of MOF carboxylate nanoparticles. U.S. Patent 20210277042A1, 9 September 2021. [Google Scholar]
- Morris, W.; Wang, S.; Cho, D.; Auyeung, E.; Li, P.; Farha, O.K.; Mirkin, C.A. Role of Modulators in Controlling the Colloidal Stability and Polydispersity of the UiO-66 Metal-Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 33413–33418. [Google Scholar] [CrossRef] [PubMed]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chemistry 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; Steenkeste, K.; Barroca-Aubry, N.; Aymes-Chodur, C.; Roger, P.; Casas-Solvas, J.M.; Vargas-Berenguel, A.; Rihouey, C.; Picton, L.; et al. Self-assembled multifunctional core-shell highly porous metal-organic framework nanoparticles. Int. J. Pharm. 2020, 581, 119281–119290. [Google Scholar] [CrossRef]
- Li, X.; Salzano, G.; Qiu, J.; Menard, M.; Berg, K.; Theodossiou, T.; Ladaviere, C.; Gref, R. Drug-Loaded Lipid-Coated Hybrid Organic-Inorganic “Stealth” Nanoparticles for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 1027–1038. [Google Scholar] [CrossRef]
- Ploetz, E.; Zimpel, A.; Cauda, V.; Bauer, D.; Lamb, D.C.; Haisch, C.; Zahler, S.; Vollmar, A.M.; Wuttke, S.; Engelke, H. Metal-Organic Framework Nanoparticles Induce Pyroptosis in Cells Controlled by the Extracellular pH. Adv. Mater. 2020, 32, 1907267–1907274. [Google Scholar] [CrossRef]
- Marshall, R.J.; Forgan, R.S. Postsynthetic Modification of Zirconium Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4310–4331. [Google Scholar] [CrossRef]
- Aykac, A.; Noiray, M.; Malanga, M.; Agostoni, V.; Casas-Solvas, J.M.; Fenyvesi, E.; Gref, R.; Vargas-Berenguel, A. A non-covalent “click chemistry” strategy to efficiently coat highly porous MOF nanoparticles with a stable polymeric shell. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1606–1616. [Google Scholar] [CrossRef]
- Cutrone, G.; Qiu, J.; Menendez-Miranda, M.; Casas-Solvas, J.M.; Aykac, A.; Li, X.; Foulkes, D.; Moreira-Alvarez, B.; Encinar, J.R.; Ladaviere, C.; et al. Comb-like dextran copolymers: A versatile strategy to coat highly porous MOF nanoparticles with a PEG shell. Carbohydr. Polym. 2019, 223, 115085–115096. [Google Scholar] [CrossRef]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. (Fe)MIL-100-Met@alginate: A hybrid polymer–MOF for enhancement of metformin’s bioavailability and pH-controlled release. New J. Chem. 2018, 42, 11137–11146. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Shen, J. Cyclodextrins Modified/Coated Metal-Organic Frameworks. Materials 2020, 13, 1273. [Google Scholar] [CrossRef]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykac, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 7925–7931. [Google Scholar] [CrossRef]
- Cutrone, G.; Li, X.; Casas-Solvas, J.M.; Menendez-Miranda, M.; Qiu, J.; Benkovics, G.; Constantin, D.; Malanga, M.; Moreira-Alvarez, B.; Costa-Fernandez, J.M.; et al. Design of Engineered Cyclodextrin Derivatives for Spontaneous Coating of Highly Porous Metal-Organic Framework Nanoparticles in Aqueous Media. Nanomaterials 2019, 9, 1103. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, M.; Marciniak, M. Synthesis and properties of cyclodextrin-malic acid copolymers. In Proceedings of the 15th International Electronic Conference on Synthetic Organic Chemistry, Krakow, Poland, 1–31 October 2011. [Google Scholar] [CrossRef]
- Lestari, W.W.; Yunita, L.; Saraswati, T.E.; Heraldy, E.; Khafidhin, M.A.; Krisnandi, Y.K.; Arrozi, U.S.F.; Kadja, G.T.M. Fabrication of composite materials MIL-100(Fe)/Indonesian activated natural zeolite as enhanced CO2 capture material. Chem. Pap. 2021, 75, 3253–3263. [Google Scholar] [CrossRef]
- Witri, W.L.; Joni, H.; Marisa, A.; Khoirina, D.N.; Candra, P.; Sentot, B.R. Electro-Synthetic Optimization of Host Material Based on MIL-100(Fe). Molekul 2016, 11, 61–70. [Google Scholar] [CrossRef]
- Li, X.; Porcel, E.; Menendez-Miranda, M.; Qiu, J.; Yang, X.; Serre, C.; Pastor, A.; Desmaele, D.; Lacombe, S.; Gref, R. Highly Porous Hybrid Metal-Organic Nanoparticles Loaded with Gemcitabine Monophosphate: A Multimodal Approach to Improve Chemo- and Radiotherapy. ChemMedChem 2020, 15, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, V.; Anand, R.; Monti, S.; Hall, S.; Maurin, G.; Horcajada, P.; Serre, C.; Bouchemal, K.; Gref, R. Impact of phosphorylation on the encapsulation of nucleoside analogues within porous iron(iii) metal-organic framework MIL-100(Fe) nanoparticles. J. Mater. Chem. B 2013, 1, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, V.; Chalati, T.; Horcajada, P.; Willaime, H.; Anand, R.; Semiramoth, N.; Baati, T.; Hall, S.; Maurin, G.; Chacun, H.; et al. Towards an improved anti-HIV activity of NRTI via metal-organic frameworks nanoparticles. Adv. Healthc. Mater. 2013, 2, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Almaraz, M.T.; Gref, R.; Agostoni, V.; Kreuz, C.; Clayette, P.; Serre, C.; Couvreur, P.; Horcajada, P. Towards improved HIV-microbicide activity through the co-encapsulation of NRTI drugs in biocompatible metal organic framework nanocarriers. J. Mater. Chem. B 2017, 5, 8563–8569. [Google Scholar] [CrossRef] [PubMed]
- Puskas, I.; Szemjonov, A.; Fenyvesi, E.; Malanga, M.; Szente, L. Aspects of determining the molecular weight of cyclodextrin polymers and oligomers by static light scattering. Carbohydr. Polym. 2013, 94, 124–128. [Google Scholar] [CrossRef] [PubMed]
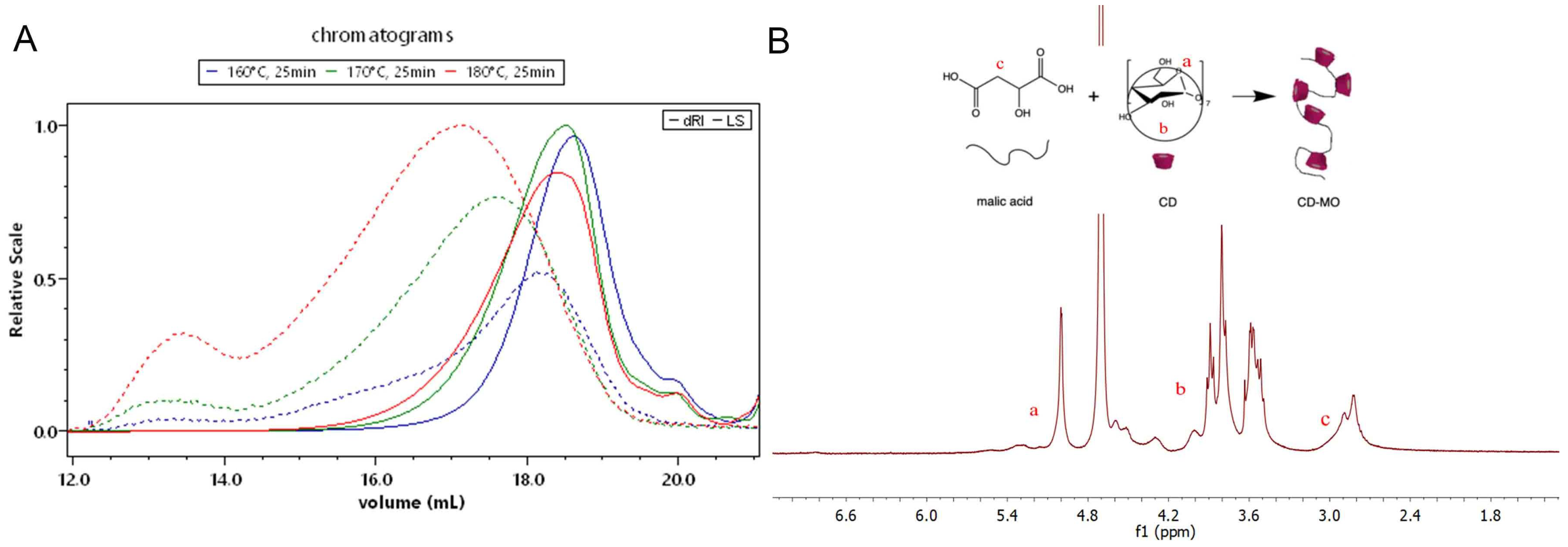
| Series | Temperature (°C) | Reaction Heating Time (Min) | Mn (g/mol) | Mw (g/mol) | Ð | Yield (%) | Molar Ratio CD: Malic |
|---|---|---|---|---|---|---|---|
| 1 | 160 | 25 | 5200 | 11,700 | 2.2 | 24% | 1:3 |
| 2 | 160 | 30 | 9200 | 26,900 | 2.9 | 27% | 1:4.8 |
| 3 | 160 | 45 | 12,300 | 37,900 | 3.1 | 30% | 1:4.5 |
| 4 | 160 | 60 | 16,700 | 54,400 | 3.3 | 37% | 1:3.8 |
| 5 | 160 | 90 | 23,000 | 90,600 | 3.9 | 38% | 1:3.6 |
| 6 | 170 | 20 | 8000 | 32,300 | 4.0 | 30% | 1.4.4 |
| 7 | 170 | 25 | 9600 | 35,000 | 3.6 | 32% | 1:4.3 |
| 8 | 170 | 30 | 9200 | 33,100 | 3.6 | 31% | 1:3.7 |
| 9 | 170 | 45 | 11,800 | 48,000 | 4.1 | 33% | 1:3.6 |
| 10 | 180 | 20 | 15,600 | 55,300 | 3.5 | 30% | 1:4 |
| 11 | 180 | 25 | 16,600 | 65,700 | 3.9 | 35% | 1:3 |
| 12 | 180 | 30 | 15,600 | 54,800 | 3.5 | 24% | 1:5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, M.; Qiu, J.; Rouzière, S.; Rihouey, C.; Picton, L.; Gref, R. Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications. Int. J. Mol. Sci. 2023, 24, 1757. https://doi.org/10.3390/ijms24021757
Ding M, Qiu J, Rouzière S, Rihouey C, Picton L, Gref R. Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications. International Journal of Molecular Sciences. 2023; 24(2):1757. https://doi.org/10.3390/ijms24021757
Chicago/Turabian StyleDing, Mengli, Jingwen Qiu, Stéphan Rouzière, Christophe Rihouey, Luc Picton, and Ruxandra Gref. 2023. "Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications" International Journal of Molecular Sciences 24, no. 2: 1757. https://doi.org/10.3390/ijms24021757
APA StyleDing, M., Qiu, J., Rouzière, S., Rihouey, C., Picton, L., & Gref, R. (2023). Acetic Acid-Modulated Room Temperature Synthesis of MIL-100 (Fe) Nanoparticles for Drug Delivery Applications. International Journal of Molecular Sciences, 24(2), 1757. https://doi.org/10.3390/ijms24021757





