Abstract
Four Ras guanine nucleotide-releasing proteins (RasGRP1 through 4) belong to the family of guanine nucleotide exchange factors (GEFs). RasGRPs catalyze the release of GDP from small GTPases Ras and Rap and facilitate their transition from an inactive GDP-bound to an active GTP-bound state. Thus, they regulate critical cellular responses via many downstream GTPase effectors. Similar to other RasGRPs, the catalytic module of RasGRP1 is composed of the Ras exchange motif (REM) and Cdc25 domain, and the EF hands and C1 domain contribute to its cellular localization and regulation. RasGRP1 can be activated by a diacylglycerol (DAG)-mediated membrane recruitment and protein kinase C (PKC)-mediated phosphorylation. RasGRP1 acts downstream of the T cell receptor (TCR), B cell receptors (BCR), and pre-TCR, and plays an important role in the thymocyte maturation and function of peripheral T cells, B cells, NK cells, mast cells, and neutrophils. The dysregulation of RasGRP1 is known to contribute to numerous disorders that range from autoimmune and inflammatory diseases and schizophrenia to neoplasia. Given its position at the crossroad of cell development, inflammation, and cancer, RASGRP1 has garnered interest from numerous disciplines. In this review, we outline the structure, function, and regulation of RasGRP1 and focus on the existing knowledge of the role of RasGRP1 in leukemia and other cancers.
1. Ras Guanine Nucleotide Exchange Factors: Introduction
Ras guanine nucleotide exchange factors (RasGEFs) are composed of three families of proteins: Ras guanine nucleotide-releasing proteins (RasGRPs), Son of Sevenless (SOS), and Ras guanine nucleotide-releasing factors (RasGRFs). The RasGRP family consists of four members, RasGRP1, RasGRP2, RasGRP3, and RasGRP4, the SOS family is composed of two members, SOS1 and SOS2, and the RasGRF family is also composed of two members, RasGRF1 and RasGRF2. The commonality is that they catalyze the removal of GDP from GTPases, such as Ras and Rap, and allow for its replacement [1] (Figure 1). While Ras itself possesses intrinsic GTPase and guanine nucleotide exchange activities, the basal activity is low. The activation of the canonical Ras pathway is characterized by the phosphorylation of Raf, Mek, and Erk. The active GTP-bound Ras has a wide range of downstream effects at the cellular level, such as a proliferation, differentiation, and apoptosis. Given these fundamental roles, numerous disease processes have been attributed to the dysregulation of Ras and RasGEFs, which range from autoimmune and inflammatory diseases to neoplasia. A broad review of all RasGEFs in various cell types is beyond the scope of this focused review of RasGRP1 in cancer; however, we direct the reader to previous reviews [2,3,4,5,6]. The structure, function, and regulation of RasGRP1 are briefly discussed, and the role of RasGRP1 in leukemia, lymphoma, squamous cell carcinoma, colorectal cancer, hepatocellular carcinoma, and breast cancer are reviewed in-depth below.
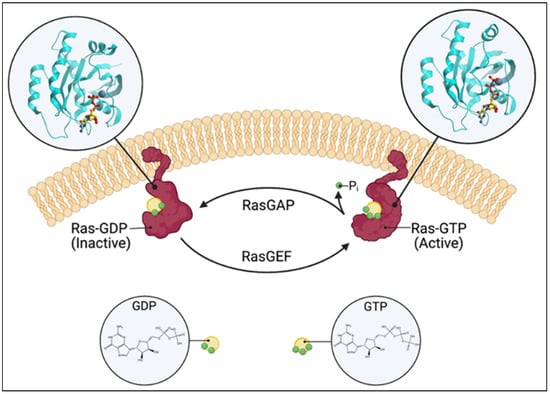
Figure 1.
Schematic of the Ras switching cycle. Ras cycles between the GTP-bound active state and the GDP-bound inactive state. RasGAP catalyzes the hydrolysis of GTP, and RasGEF facilitates guanine nucleotide exchange. Created with BioRender.com.
2. RasGRP1: Structure and Function
The catalytic module of RasGRP1 is composed of the Ras exchange motif (REM) followed by the CDC25 subunit (Figure 2). Upon the binding of the Ras to the catalytic module of RasGRP1, the helical hairpin of CDC25 removes the GDP from the GTPase. As the cellular concentration of GTP is about 10-fold higher than GDP, GTP occupies the free nucleotide-binding pocket of the enzyme. This hairpin is highly conserved in all GEFs [7]. A single amino acid change within this structure has been shown to abrogate its catalytic effect [8,9]. Despite the ability of the helical hairpin alone to convey the nucleotide dissociating ability of RasGEFs, the nucleotide exchange step is mediated by other regions of the catalytic domain [10].
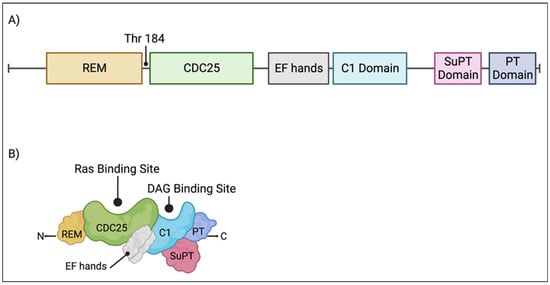
Figure 2.
RasGRP1 domains. (A) Individual protein domains and the PKC-phosphorylation site, threonine 184, are diagramed and labelled. (B) An illustration of RasGRP1 protein domains. The Ras and DAG binding sites are labelled. Created with BioRender.com.
Adjacent to the catalytic module of RasGRP1 is a pair of EF hands (Figure 2) with a calcium-binding capacity in vitro [11]. The evidence is conflicted on the importance of EF hands and calcium for the activation of RasGRP1. Some studies found the EF hands and calcium to be dispensable [12,13,14], while some found them to be necessary [15]. The current weight of evidence supports the idea that the EF hands do indeed bind calcium, induce conformational changes, and activate RasGRP1 [16,17]. The calcium source is from the endoplasmic reticulum stores, and its release is mediated by phospholipase C-γ-generated inositol-1,4,5-trisphosphate (IP3) [18].
The C1 domain of RasGRP1 binds DAG (Figure 2), generated by PLCγ, and its synthetic analog phorbol myristate acetate (PMA) and 12-O-tetradecanoylphorbol-13-acetate (TPA) [11,19,20,21]. Upon the binding of the C1 domain to the DAG, RasGRP1 becomes anchored to the plasma membrane [11,13,22,23,24,25,26] where its substrate, GDP-loaded GTPase, is present. Alternatively, RasGRP1 can also be trafficked to the endoplasmic reticulum (ER) and Golgi apparatus [23,24,27,28,29]. While the ability of the C1 domain to bind DAG is well known, it alone is insufficient for membrane targeting and requires other domains on RasGRP1, which are discussed below.
The tail region of RasGRP1 possesses an approximately 140 residue-long coiled-coil (CC), later renamed as the plasma membrane-targeting (PT) domain, and the suppressor of PT (SuPT) domain [16,23] (Figure 2). In the inactive state, the SuPT domain of RasGRP1 attenuates the plasma membrane-targeting activity of the PT domain [23]. Upon the binding of the C1 domain to DAG, it also counteracts the SuPT domain and enables the PT domain to target RasGRP1 to the plasma membrane [23,30]. At the plasma membrane, the hydrophobic residues of the PT domain bind phospholipid vesicles containing phosphoinositides. The deletion of the hydrophobic residues prevents the PI3k-dependent plasma membrane targeting of RasGRP1 [30], and the deletion of the tail region entirely leads to a T cell dysregulation [31]. The PT domain additionally facilitates the dimerization of RasGRP1 in the inactive state [16]. While the C1 domain is well-recognized for its role mediating the RasGRP1 membrane targeting capacity and activation, it is now accepted that these effects are also dependent on the tail domain of RasGRP1.
3. Ras Guanine Nucleotide-Releasing Protein 1: Regulation
The translocation and activation of the RasGRP1 membrane are reliant on the binding of the C1 domain to DAG (Figure 3). Logically, the catalyzation of DAG to phosphatidic acid (PA) by diacylglycerol kinases (DGK) should terminate RasGRP1 signaling. Indeed, DGKα is recruited to the plasma membrane after the TCR stimulation [32] and results in suppressed RasGRP1 activity and Ras signaling [33,34]. This mechanism of RasGRP1 regulation has been proposed to be a mechanism of T cell anergy [35,36]. Other DGK isoforms also regulate the RasGRP1 activity, specifically, DGKζ. Studies have found that the overexpression of kinase-dead DGKζ in Jurkat cells prolonged the Ras activation, and the overexpression of the wild-type DGKζ suppressed the ERK phosphorylation following the TCR ligation [37,38]. For in-depth reviews of the DGKs, we direct the reader to previous reviews [39,40].
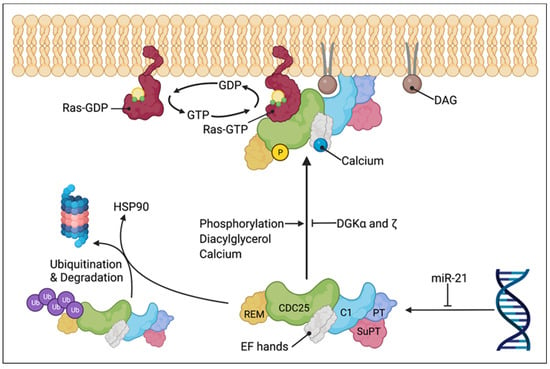
Figure 3.
Model of RasGRP1 regulation and plasma membrane translocation. RasGRP1 is sequestered in the cytoplasm in its inactive state (lower center) and translocated to the plasma membrane or endomembrane upon activation (upper center). RasGRP1 activation involves phosphorylation of threonine 184, DAG binding by C1 domain, and calcium binding by the EF hands. Termination of RasGRP1 signaling is mediated by the breakdown of DAG to PA by DGKα and DGKξ. RasGRP1 can be suppressed by miR-21 and degraded following disassociation from HSP90 and polyubiquitination. Active RasGRP1 catalyzes the release of GDP from Ras and facilitates their transition from inactive GDP-bound to active GTP-bound state. Created with BioRender.com.
The activity of RasGRP1 is regulated by multiple mechanisms in addition to endomembrane versus plasma membrane localization. RasGRP1 is also regulated by the phosphorylation (Figure 3), more specifically, of threonine 184 (T184) by protein kinase C α (PKCα) after the TCR engagement or PMA stimulation [41]. While the phosphorylation of T184 enhances the activity of RasGRP1, it is not completely required. A RasGRP1 Thr184Ala mutant did not exhibit a significant signaling defect [42]. DGKζ not only indirectly regulates the RasGRP1 activity via DAG, but it also physically associates with PKCα and inhibits the phosphorylation of RasGRP1 [43]. In unstimulated cells, RasGRP1 is believed to exist in an autoinhibited dimeric form, in which the EF domains of each monomer block DAG-binding sites on the C1 domain of the partner. It was also suggested that an invariant His 212 in RasGRP1, 2, and 3 functions as a pH sensor: lymphocyte receptor stimulation causes an increase in the intracellular pH and thus the deprotonation of His 212 [44]. The later causes the structural rearrangement of the linker between the CDC25 and EF domain and the destabilization of the autoinhibition [44]. We refer the reader to the review by Griner and Kazanietz for additional details on PKC and other DAG effectors [45].
Ding and colleagues identified RasGRP1 to be a client protein of the chaperone heat shock protein 90 (HSP90) (Figure 3). Additionally, the degradation of RasGRP1 can be mediated by HSP90 acetylation [46]. There is emerging evidence that microRNAs also play a role in the RasGRP1 expression; specifically, miR-21 was shown to suppress the expression of RasGRP1 [47,48]. Conversely, the downregulation of miR-21 increased the RasGRP1 expression in vitro [49].
4. RasGRP1: Cell Development and Function
4.1. Immature Thymocytes
The maturation of immature thymocytes undergo four double-negative (CD4−, CD8−) stages (DN1–4), an immature single positive stage (ISP; CD8+ in mice and CD4+ in humans), and a double positive (DP; CD4+, CD8+) stage (Figure 4). During this process, immature thymocytes undergo two selection checkpoints. The first of which is a process termed “β-selection”, which takes place in the DN3-DN4 transition stage, where the pre-T cell receptor (pre-TCR), composed of a somatically rearranged TCRβ chain and an invariant pre-TCRα chain, signal a first intersect with the Ras pathway. This signal is necessary for the αβ T cell precursors to eventually become mature CD4+ or CD8+ T cells. The activation of the Ras is critical in β-selection; in fact, activated Ras can replace the pre-TCR expression and generate DP thymocytes in Rag−/− mice [50]. At the β-selection step, RasGRP1 is dispensable and acts as a backup for SOS1 [51,52,53]. Immature thymocytes that pass the β-selection undergo a proliferative burst and initiate CD4 and CD8 expression to become DP thymocytes. DP thymocytes that express TCRαβ undergo subsequent checkpoints termed “positive” and “negative” selection, where the TCRαβ signal quality and strength are interrogated. RasGRP1 plays an essential role in a positive selection, and SOS1 acts as backup in a negative selection [52,53]. The knockout of RasGRP1 arrests the progression of DP thymocytes through a positive selection [51,54], whereas the double knockout of RasGRP1 and SOS1 is needed to arrest a negative selection [53].
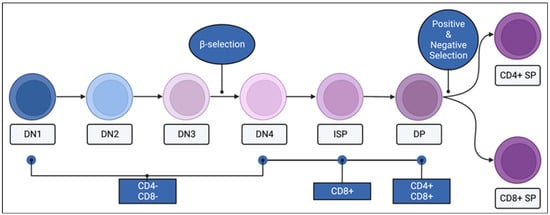
Figure 4.
CD4 and CD8 expression of thymocytes through maturation. Immature thymocytes begin the process of maturation in the thymus in the double negative (DN; CD4− CD8−) stage, followed by DN2, DN3, and DN4 stages. The process of β-selection takes place as DN3 thymocytes mature into the DN4 stage. In mice, DN4 thymocytes transition through an immature single positive (ISP, CD8+) stage to become double positive (DP; CD4+ CD8+) thymocytes. The second round of selection, positive and negative selection, takes place between the DP and the mature single positive (SP) stages. Mature SP thymocytes can be CD4+ or CD8+. Created with BioRender.com.
The compartmentalization of Ras signaling underlies the digital output (positive versus negative) seen in the pre-TCR and TCR selection checkpoints [55,56,57] (Figure 5). Negative selection signaling is molecularly characterized by the plasma membrane recruitment of the RasGRP1 and Grb2 (growth factor receptor-bound protein 2)-SOS1 complex from the cytosol, and the resultant activation of the Ras pathway [55,56,57]. The Grb2-SOS1 complex binds phosphorylated LAT at the plasma membrane and serves as another RasGEF. However, positive selecting signaling is characterized by the recruitment of RasGRP1 to the Golgi apparatus, and no involvement of the Grb2-SOS1 complex [55,56].
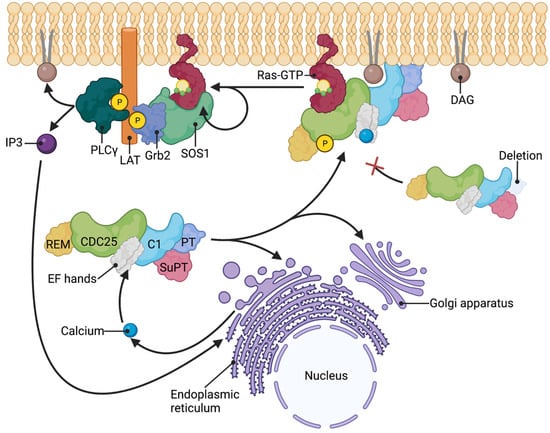
Figure 5.
Model of RasGRP1 membrane translocation. RasGRP1 activation is characterized by phosphorylation of threonine 184, binding of calcium by the EF hands, and translocation to the plasma membrane or endomembranes (Golgi apparatus and endoplasmic reticulum). Deletion of the PT domain renders RasGRP1 unable to translocate to the plasma membrane. In negative selection of αβTCR-expressing thymocytes, RasGRP1 is recruited to the plasma membrane along with SOS1-Grb2 complex. Grb2-SOS1 complex binds phospho-LAT and RasGTP at the plasma membrane and act as another RasGEF. PLCγ is also recruited to the phospho-LAT at the plasma membrane during RasGRP1 activation and generates DAG and IP3. IP3 subsequently induces the release of intracellular stores of calcium. Created with BioRender.com.
4.2. T Cells
Despite the maturation arrest of thymocytes and the loss of mature single-positive thymocytes in RasGRP1 knockout mice, this arrest is not complete. RasGRP1-deficient CD4+ and CD8+ T cells do exist [58], however, they are defective in their capacity to become activated and proliferate after an anti-CD3 and anti-CD28 antibody stimulation [58]. Interestingly, humans deficient in RasGRP1 have increased numbers of TCRγδ+ CD8+ T cells [58]. RasGRP1-deficient mice develop splenomegaly and autoantibodies as a result of T cell dysregulation, characterized by an elevated interleukin (IL)-4 secretion [31,59,60,61]. This elevation in IL-4 drives B cell proliferation and the production of autoantibodies [60]. Furthermore, one study found that the binding of RUNX1 to a putative autoimmunity-associated enhancer 1 upstream of Rasgrp1 mediates the RasGRP1 deficiency-mediated autoimmune disease [61].
4.3. B-Cells
B cells express RasGRP1 and RasGRP3. While both are involved in B cell receptor (BCR)-mediated Ras signaling, RasGRP3 plays the central role [59,62]. One study found that the BCR-mediated proliferation was suppressed more by the knockout of RasGRP3 than RasGRP1 and was absent in double knockouts [59]. The defect in the B cell proliferation due to the RasGRP1 knockout was supported by a later study [58]. Unlike T cells, the knockout of both RasGRP1 and RasGRP3 did not disrupt the development of B cells [59]. However, B cells that express a dominant negative Ras mutant have severe developmental defects at the pre–pro B cell stage [63,64].
4.4. NK Cells
NK cells exert their cytotoxic effect and produce cytokines and chemokines subsequent to the activation of various cell surface receptors [65]. Briefly, this signal cascade is dependent on RasGRP1, and the knockdown of RasGRP1 in NK cells results in a markedly decreased cytokine production and cytotoxicity [58,66]. In humans, this defect has been attributed to the protein–protein interaction between RasGRP1 and the dynein light chain (Dynll1) [58].
4.5. Granulocytes
The differentiation of myeloid progenitors into neutrophils is dependent on the transcription factor growth factor independence 1 (Gfi1) [67,68,69] and the growth factor granulocyte colony-stimulating factor (G-CSF) [70,71,72]. Gfi1 regulates G-CSFR signaling in myeloid progenitors via the upregulation of the RasGRP1 expression and Ras activation [73]. RasGRP1 has also been found to be important for a mast cell degranulation. RasGRP1−/− mice exhibit an impaired immunoglobulin E (IgE)-mediated degranulation and anaphylaxis [74].
5. RasGRP1: Role in Cancer
5.1. Lymphoma and Leukemia
While loss-of-function RasGRP1 mutants have been described in humans [75,76,77], no oncogenic mutant of RasGRP1 has been identified. These loss-of-function RasGRP1 mutants lead to the development of autoimmune lymphoproliferative syndrome (ALPS), CD4+ T cell lymphopenia, recurrent infections, hepatosplenomegaly, and lymphadenopathy [75,76,77]. It is important to note that some patients with loss-of-function RasGRP1 mutants develop Epstein–Barr virus (EBV)-induced B cell lymphoma. However, studies have found RasGRP1 to be overexpressed in nearly half of all T cell acute lymphoblastic leukemias (T-ALL) [78,79]. Retroviral insertion studies in mice have also identified wild-type RasGRP1 as a leukemogenic oncogene [80,81,82]. Furthermore, the dysregulation of RasGRP1 in mice and cell lines has been shown to lead to the development of thymic lymphomas and T cell leukemias [79,83,84]. Interestingly, cell lines with a high RasGRP1 expression required a cocktail of IL-2, -7, and -9 for proliferation [79,84]. Additionally, leukemia driven by the overexpression of RasGRP1 and K-RasG12D are mutually exclusive and represent the distinct mechanisms of leukemogenesis [79]. This is consistent with the finding from a later study that identified RasGRP1 as a negative regulator of Ras signaling in Kras−/− NrasQ61R/+-driven leukemia [85]. Various studies have shown that the dysregulation of RasGRP1 itself is insufficient for leukemogenesis [79,86]; however, it does bestow a proliferative advantage in bone marrow progenitors over wild type cells [86]. Consistent with Knudson’s “two-hit” theory that was proposed over 50 years ago [87], the dysregulation of RasGRP1 requires a second cooperating oncogene or cytokine stimulation for transformation [78,79,84]. The knockout of RasGRP1 negative regulators has also been shown to be oncogenic; specifically, DGKα−/− DGKζ−/− double knockout mice develop thymic lymphoma due to the failure to prevent the overactivation of RasGRP1 and Ras [88]. Beyond the role of RasGRP1 as an oncogene, its overexpression has been documented to be a mechanism of resistance to MEK inhibitors [89].
No RasGRP1-specific small molecule inhibitors currently exist. Since the overexpression of RasGRP1 renders T-ALL cells responsive to pro-tumorigenic cytokines [84], PI3K inhibitors have been tested as a monotherapy in mice, but with no success [90]. Others have tried to target the RasGRP1/Ras/Erk pathway in T cell lymphoblastic lymphomas (T-LBL), which are morphologically and immunophenotypically identical to T-ALL [91]. Bromodomain-containing protein 2 (BRD2) binds to the promotor region of Rasgrp1 and conveys a doxorubicin resistance in some T-LBL patients [92]. The targeting of BRD2 via a bromodomain and extra-terminal (BET) inhibitor improved the therapeutic efficacy in vitro and in a patient-derived xenograft mouse model [92]. DAG and its analogues have long been known to activate RasGRP1 in T and B cells [93,94], and the treatment of B cell lymphoma-derived cell lines with DAG analogues promoted apoptosis [94,95]. This proapoptotic pathway induced by DAG analogues is mediated by the PKC/RasGRP1/Erk pathway [94,95].
5.2. Squamous Cell Carcinoma
While studying the role of RasGRP1 in skin tumors, one group found that the overexpression of RasGRP1, driven by a K5 promotor, in keratinocytes resulted in the development of spontaneous skin tumors [96,97]. These tumors were mostly benign papillomas and there were lesser numbers of squamous cell carcinomas. Due to the observation that the incidence of tumors development was higher in co-housed animals, it was hypothesized that wounding contributed to tumor development. Indeed, when RasGRP1-K5 transgenic mice were subjected to full-thickness incision wounding, 50% of them developed skin tumors [97]. The proposed mechanism is that the act of wounding caused the release of the granulocyte colony-stimulating factor (G-CSF) by keratinocytes [96,97], and G-CSF acted in an autocrine and paracrine fashion to cooperate with RasGRP1 in the development of skin tumors [98]. When the same RasGRP1-K5 transgenic mice were subjected to multistage skin carcinogenesis protocol, 7,12-dimethylbenz(a)anthracene (DBMA) as carcinogen, and 12-O-tetradecanoylphorbol-13-acetate (TPA) as tumor promoters, it was found that the squamous cell carcinomas that developed in the transgenic mice were larger, less differentiated, and more invasive [99]. Additionally, the overexpression of RasGRP1 was found to partially replace the DMBA induction [99]. Conversely, RasGRP1 knockout mice have impaired skin tumorigenesis, evidenced by a reduced epidermal hyperplasia induced by TPA [100,101]. To study other coopering mechanisms of oncogenesis in keratinocytes, one group transduced keratinocytes derived from a Li-Fraumeni patient with RasGRP1 and found that the keratinocytes acquired morphologic changes that are associated with a transformation [102]. This result supports the idea that RasGRP1 cooperates with other genes because patients with Li-Fraumeni syndrome are deficient in p53, a well-known tumor suppressor gene.
5.3. Colorectal Cancer
Surprisingly, RasGRP1 acts as a tumor suppressor in colonic epithelium; furthermore, RasGRP1 can be used as a biomarker for predicting the efficacy of anti-epidermal growth factor receptor (EGFR) therapy for CRC (colorectal cancer) patients [103,104]. The RasGRP1 expression levels decrease with the progression of CRC and predict the poor clinical outcome of patients [104]. Mechanistically, the same group found that RasGRP1 suppresses the proliferation of the KRas mutant and negatively regulates the EGFR/SOS1/Ras signal in CRC cells [104]. This mechanism may explain its tumor suppressor activity in colorectal cancer in contrast to its oncogenic activity in most other neoplasias.
5.4. Hepatocellular Carcinoma
RasGRP1 has been found to be upregulated in hepatocellular carcinomas (HCC) [105]; furthermore, a high RasGRP1 expression is associated with the tumor size, tumor–node–metastasis (TNM) stage, and Barcelona Clinic Liver Cancer stage [105]. At the cellular level, in Huh7 and PLC cells, the downregulation of RasGRP1 inhibited cell proliferation, whereas the overexpression of RasGRP1 promoted cell proliferation [105]. Specific protein 1 (Sp1) was identified to bind the Rasgrp1 promotor and is a positive regulator [105]. For a review of the Ras pathways in HCC, we refer the reader to the work by Moon and colleagues [106].
5.5. Breast Cancer
The role of RasGRP1 in breast cancer has only recently been studied. Specifically, it was found that the upregulation of Rasgrp1 was associated with an improved overall survival in breast cancer [107], as well as overall survival and disease-free survival in the triple-negative breast cancer subtype [107,108]. The molecular mechanism that underlies these observations is unknown.
6. Conclusions
Given that approximately 46% of cancers exhibit alterations in the Ras pathway [109], it has been extensively studied over the past decades. With RasGRP1 being a RasGEF, it too has received much attention. Through this endeavor, the structure, function, regulation, and developmental role of RasGRP1 have been described at the molecular level. This has identified RasGRP1 and its regulators as promising targets in leukemia and other cancers.
Most of the domains of RasGRP1 are well characterized. The REM and CDC25 domains facilitate the Ras cycle between the GDP-bound inactive form and the GTP-bound active form. The EF hands bind calcium and induce an activation-associated conformational change [16,17]. The C1 domain binds DAG at the plasma membrane or endomembrane. The PT domain facilitates dimerization and phosphoinositide-mediated plasma membrane targeting [30]. For the regulation of RasGRP1, it is known that signal termination can be mediated by DGKα and DGKζ via the conversion of DAG to PA. For the activation, RasGRP1 can be phosphorylated at T184 by PKCα. Other less-well characterized mechanisms include HSP90- [46] and miR-21-mediated degradation [47,48].
In normal physiology, RasGRP1 plays an important role in the maturation of thymocytes. Specifically, it is necessary for a positive selection of the rearranged αβTCR [52,53]. The compartmentalization of Ras signaling to the plasma membrane or the endomembrane at the selection checkpoints adds an extra layer of complexity [55,56,57]. The dysregulation of RasGRP1 in peripheral T cells, B cells, NK cells, neutrophils, and mast cells are known to cause developmental and/or functional defects. One of the most surprising defects revealed in knockout mice is that RasGRP1 normally interacts with the dynein light chain in NK cells [58], and this indicates that RasGRP1 has additional functions besides as a RasGEF.
Given the importance of RasGRP1 in cell development, it is unsurprising that it is expressed in numerous cancers and plays a role in oncogenesis. The overexpression of RasGRP1 alone is insufficient for lymphoma- or leukemo-genesis [79,86]. The transformation of thymocytes requires the overexpression of RasGRP1 and a cooperating oncogene or knockout of a tumor suppressor. Since no Ras- or RasGRP-specific small molecule inhibitors have been identified, efforts have been made to target regulatory pathways through the use of BET inhibitors [92], DAG analogs [94,95], and HDAC inhibitors [46].
Much of the work done on RasGRP1 within the realms of immunology and cancer research in the last 5 years has focused on three areas. The first area is its role in lymphocyte homeostasis, which can be summarized by the identification of loss-of-function RasGRP1 mutants in two patients with ALPS [75], one patient with immunodeficiency, and three patients with EBV-associated lymphoproliferative disease [76,77,110]. A second area is the clinical behavior of tumors relative to the expression of RasGRP1 in various cancers, such as CRC [103], HCC [105], and breast cancer [107,108]. The third area is the mechanism by which RasGRP1 serves as a tumor suppressor in certain cancer models [85,111]. These last two emerging areas point to the idea that RasGRP1 cannot simply be described as an “oncogene” or its overexpression as a negative indicator, but rather that its role is cancer- and model-dependent. While not emphasized in this focused review, progress in RasGRP1 research is also being made in the areas of schizophrenia [112], neuro-inflammation [113], systemic lupus erythematosus [114], Parkinson’s disease [115], and angiogenesis [116]. It is evident that the relevance of RasGRP1 reaches beyond the development and function of immune cells and homeostasis and cancer.
Despite this progress, there is still much to understand about RasGRP1. First, a concise explanation for the conflicting role of calcium, or lack of, in the function of RasGRP1 has yet to be articulated. Second, since RasGRP1 is involved in the degranulation of NK cells and mast cells and the development of neutrophils, it is interesting to speculate on its potential developmental and functional role in other granulocytes. It is clear that RasGRP1 plays a role in T leukemogenesis; additionally, it is necessary for it to cooperate with other oncogenes for transformation. It is likely that the array of cooperating oncogenes has yet to be fully elucidated. Lastly, only in recent years was RasGRP1 identified as a differentially expressed gene correlated with overall and disease-free survival in breast cancer. It will be important to determine the molecular basis for this counterintuitive correlation.
Author Contributions
Conceptualization—T.C.H. and S.K.D.; writing—original draft preparation, T.C.H.; writing—review and editing, G.O.L.R., H.W., J.A.H., W.L., N.I.T. and S.K.D.; funding acquisition, S.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the intramural program of the National Cancer Institute, National Institutes of Health USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
12-O-tetradecanoylphorbol-13-acetate (TPA), 7,12-dimethylbenz(a)anthracene (DBMA), autoimmune lymphoproliferative syndrome (ALPS), B cell receptors (BCR), bromodomain and extra-terminal (BET), bromodomain-containing protein 2 (BRD2), coiled-coil (CC), colorectal cancer (CRC), diacylglycerol (DAG), diacylglycerol kinase (DGK), endoplasmic reticulum (ER), epidermal growth factor receptor (EGFR), Epstein–Barr virus (EBV), granulocyte colony-stimulating factor (G-CSF), growth factor independence 1 (Gfi1), growth factor receptor-bound protein 2 (Grb2), guanine nucleotide exchange factors (GEFs), guanosine diphosphate (GDP), guanosine triphosphate (GTP), heat shock protein 90 (HSP90), hepatocellular carcinomas (HCC), immunoglobulin E (IgE), inositol-1,4,5-trisphosphate (IP3), interleukin (IL), natural killer cells (NK cells), phorbol myristate acetate (PMA), phosphatidic acid (PA), phospholipase Cγ (PLCγ), plasma membrane-targeting (PT), pre-T cell receptor (pre-TCR), protein kinase C (PKC), Ras exchange motif (REM), Ras exchange motif (REM), Ras guanine nucleotide-releasing factors (RasGRFs), Ras guanine nucleotide-releasing protein 1 (RasGRP1), runt-related transcription factor 1 (RUNX1), Son of Sevenless (SOS), specific protein 1 (Sp1), suppressor of PT (SuPT), T cell acute lymphoblastic leukemias (T-ALL), T cell lymphoblastic lymphomas (T-LBL), and T cell receptor (TCR).
References
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Philips, M.R. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 2006, 24, 771–800. [Google Scholar] [CrossRef]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef]
- Stone, J.C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer 2011, 2, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Rubio, I.; Roose, J.P. Regulation of ras exchange factors and cellular localization of ras activation by lipid messengers in T cells. Front. Immunol. 2013, 4, 239. [Google Scholar] [CrossRef]
- Ksionda, O.; Limnander, A.; Roose, J.P. RasGRP Ras guanine nucleotide exchange factors in cancer. Front. Biol. 2013, 8, 508–532. [Google Scholar] [CrossRef]
- Quilliam, L.A.; Rebhun, J.F.; Castro, A.F. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol. 2002, 71, 391–444. [Google Scholar] [CrossRef] [PubMed]
- Vanoni, M.; Bertini, R.; Sacco, E.; Fontanella, L.; Rieppi, M.; Colombo, S.; Martegani, E.; Carrera, V.; Moroni, A.; Bizzarri, C.; et al. Characterization and properties of dominant-negative mutants of the ras-specific guanine nucleotide exchange factor CDC25(Mm). J. Biol. Chem. 1999, 274, 36656–36662. [Google Scholar] [CrossRef]
- Bossu, P.; Vanoni, M.; Wanke, V.; Cesaroni, M.P.; Tropea, F.; Melillo, G.; Asti, C.; Porzio, S.; Ruggiero, P.; Di Cioccio, V.; et al. A dominant negative RAS-specific guanine nucleotide exchange factor reverses neoplastic phenotype in K-ras transformed mouse fibroblasts. Oncogene 2000, 19, 2147–2154. [Google Scholar] [CrossRef]
- Sacco, E.; Fantinato, S.; Manzoni, R.; Metalli, D.; De Gioia, L.; Fantucci, P.; Alberghina, L.; Vanoni, M. The isolated catalytic hairpin of the Ras-specific guanine nucleotide exchange factor Cdc25Mm retains nucleotide dissociation activity but has impaired nucleotide exchange activity. FEBS Lett. 2005, 579, 6851–6858. [Google Scholar] [CrossRef]
- Ebinu, J.O.; Bottorff, D.A.; Chan, E.Y.; Stang, S.L.; Dunn, R.J.; Stone, J.C. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science 1998, 280, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.E.; Kirk, H.E.; Passmore, L.A.; Whitehead, I.P.; Der, C.J.; Kay, R.J. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol. Cell. Biol. 1998, 18, 6995–7008. [Google Scholar] [CrossRef]
- Ebinu, J.O.; Stang, S.L.; Teixeira, C.; Bottorff, D.A.; Hooton, J.; Blumberg, P.M.; Barry, M.; Bleakley, R.C.; Ostergaard, H.L.; Stone, J.C. RasGRP links T-cell receptor signaling to Ras. Blood 2000, 95, 3199–3203. [Google Scholar] [CrossRef]
- Tazmini, G.; Beaulieu, N.; Woo, A.; Zahedi, B.; Goulding, R.E.; Kay, R.J. Membrane localization of RasGRP1 is controlled by an EF-hand, and by the GEF domain. Biochim. Biophys. Acta 2009, 1793, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, B.; Kay, R.J. RasGRP1 sensitizes an immature B cell line to antigen receptor-induced apoptosis. J. Biol. Chem. 2004, 279, 19523–19530. [Google Scholar] [CrossRef] [PubMed]
- Iwig, J.S.; Vercoulen, Y.; Das, R.; Barros, T.; Limnander, A.; Che, Y.; Pelton, J.G.; Wemmer, D.E.; Roose, J.P.; Kuriyan, J. Structural analysis of autoinhibition in the Ras-specific exchange factor RasGRP1. eLife 2013, 2, e00813. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Lockyer, P.J. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 339–348. [Google Scholar] [CrossRef]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Rambaratsingh, R.A.; Stone, J.C.; Blumberg, P.M.; Lorenzo, P.S. RasGRP1 represents a novel non-protein kinase C phorbol ester signaling pathway in mouse epidermal keratinocytes. J. Biol. Chem. 2003, 278, 52792–52801. [Google Scholar] [CrossRef]
- Tuthill, M.C.; Oki, C.E.; Lorenzo, P.S. Differential effects of bryostatin 1 and 12-O-tetradecanoylphorbol-13-acetate on the regulation and activation of RasGRP1 in mouse epidermal keratinocytes. Mol. Cancer Ther. 2006, 5, 602–610. [Google Scholar] [CrossRef]
- Johnson, J.E.; Goulding, R.E.; Ding, Z.; Partovi, A.; Anthony, K.V.; Beaulieu, N.; Tazmini, G.; Cornell, R.B.; Kay, R.J. Differential membrane binding and diacylglycerol recognition by C1 domains of RasGRPs. Biochem. J. 2007, 406, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Elhalem, E.; Donadio, L.G.; Zhou, X.; Lewin, N.E.; Garcia, L.C.; Lai, C.C.; Kelley, J.A.; Peach, M.L.; Blumberg, P.M.; Comin, M.J. Exploring the influence of indololactone structure on selectivity for binding to the C1 domains of PKCalpha, PKCepsilon, and RasGRP. Bioorg. Med. Chem. 2017, 25, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, N.; Zahedi, B.; Goulding, R.E.; Tazmini, G.; Anthony, K.V.; Omeis, S.L.; de Jong, D.R.; Kay, R.J. Regulation of RasGRP1 by B cell antigen receptor requires cooperativity between three domains controlling translocation to the plasma membrane. Mol. Biol. Cell 2007, 18, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Campi, G.; Du, G.; Zheng, Y.; Foster, D.A.; Dustin, M.L.; Philips, M.R. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat. Cell Biol. 2007, 9, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Caloca, M.J.; Zugaza, J.L.; Bustelo, X.R. Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J. Biol. Chem. 2003, 278, 33465–33473. [Google Scholar] [CrossRef]
- Zugaza, J.L.; Caloca, M.J.; Bustelo, X.R. Inverted signaling hierarchy between RAS and RAC in T-lymphocytes. Oncogene 2004, 23, 5823–5833. [Google Scholar] [CrossRef]
- Bivona, T.G.; Perez De Castro, I.; Ahearn, I.M.; Grana, T.M.; Chiu, V.K.; Lockyer, P.J.; Cullen, P.J.; Pellicer, A.; Cox, A.D.; Philips, M.R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 2003, 424, 694–698. [Google Scholar] [CrossRef]
- Choy, E.; Chiu, V.K.; Silletti, J.; Feoktistov, M.; Morimoto, T.; Michaelson, D.; Ivanov, I.E.; Philips, M.R. Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell 1999, 98, 69–80. [Google Scholar] [CrossRef]
- Chiu, V.K.; Bivona, T.; Hach, A.; Sajous, J.B.; Silletti, J.; Wiener, H.; Johnson, R.L., 2nd; Cox, A.D.; Philips, M.R. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 2002, 4, 343–350. [Google Scholar] [CrossRef]
- Zahedi, B.; Goo, H.J.; Beaulieu, N.; Tazmini, G.; Kay, R.J.; Cornell, R.B. Phosphoinositide 3-kinase regulates plasma membrane targeting of the Ras-specific exchange factor RasGRP1. J. Biol. Chem. 2011, 286, 12712–12723. [Google Scholar] [CrossRef]
- Fuller, D.M.; Zhu, M.; Song, X.; Ou-Yang, C.W.; Sullivan, S.A.; Stone, J.C.; Zhang, W. Regulation of RasGRP1 function in T cell development and activation by its unique tail domain. PLoS ONE 2012, 7, e38796. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Jones, D.R.; Izquierdo, M.; Merida, I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.A.; Pradet-Balade, B.; Jones, D.R.; Martinez, A.C.; Stone, J.C.; Garcia-Sanz, J.A.; Merida, I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: A novel mechanism for Ras attenuation. J. Immunol. 2003, 170, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Sanjuan, M.A.; Stone, J.C.; Merida, I. Expression of a catalytically inactive form of diacylglycerol kinase alpha induces sustained signaling through RasGRP. FASEB J. 2002, 16, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Marks, R.; Ho, A.W.; Peterson, A.C.; Janardhan, S.; Brown, I.; Praveen, K.; Stang, S.; Stone, J.C.; Gajewski, T.F. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 2006, 7, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Olenchock, B.A.; Guo, R.; Carpenter, J.H.; Jordan, M.; Topham, M.K.; Koretzky, G.A.; Zhong, X.P. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006, 7, 1174–1181. [Google Scholar] [CrossRef]
- Topham, M.K.; Prescott, S.M. Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism. J. Cell Biol. 2001, 152, 1135–1143. [Google Scholar] [CrossRef]
- Zhong, X.P.; Hainey, E.A.; Olenchock, B.A.; Zhao, H.; Topham, M.K.; Koretzky, G.A. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J. Biol. Chem. 2002, 277, 31089–31098. [Google Scholar] [CrossRef]
- Sakane, F.; Imai, S.; Kai, M.; Yasuda, S.; Kanoh, H. Diacylglycerol kinases: Why so many of them? Biochim. Biophys. Acta 2007, 1771, 793–806. [Google Scholar] [CrossRef]
- Merida, I.; Arranz-Nicolas, J.; Torres-Ayuso, P.; Avila-Flores, A. Diacylglycerol Kinase Malfunction in Human Disease and the Search for Specific Inhibitors. Handb. Exp. Pharmacol. 2020, 259, 133–162. [Google Scholar] [CrossRef]
- Roose, J.P.; Mollenauer, M.; Gupta, V.A.; Stone, J.; Weiss, A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 2005, 25, 4426–4441. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, H.; Coughlin, J.; Zheng, J.; Li, L.; Stone, J.C. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood 2005, 105, 3648–3654. [Google Scholar] [CrossRef]
- Luo, B.; Prescott, S.M.; Topham, M.K. Association of diacylglycerol kinase zeta with protein kinase C alpha: Spatial regulation of diacylglycerol signaling. J. Cell Biol. 2003, 160, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Vercoulen, Y.; Kondo, Y.; Iwig, J.S.; Janssen, A.B.; White, K.A.; Amini, M.; Barber, D.L.; Kuriyan, J.; Roose, J.P. A Histidine pH sensor regulates activation of the Ras-specific guanine nucleotide exchange factor RasGRP1. eLife 2017, 6, e29002. [Google Scholar] [CrossRef] [PubMed]
- Griner, E.M.; Kazanietz, M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Peterson, K.L.; Correia, C.; Koh, B.; Schneider, P.A.; Nowakowski, G.S.; Kaufmann, S.H. Histone deacetylase inhibitors interrupt HSP90*RASGRP1 and HSP90*CRAF interactions to upregulate BIM and circumvent drug resistance in lymphoma cells. Leukemia 2017, 31, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Wasik, U.; Kempinska-Podhorodecka, A.; Bogdanos, D.P.; Milkiewicz, P.; Milkiewicz, M. Enhanced expression of miR-21 and miR-150 is a feature of anti-mitochondrial antibody-negative primary biliary cholangitis. Mol. Med. 2020, 26, 8. [Google Scholar] [CrossRef]
- Pan, W.; Zhu, S.; Yuan, M.; Cui, H.; Wang, L.; Luo, X.; Li, J.; Zhou, H.; Tang, Y.; Shen, N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010, 184, 6773–6781. [Google Scholar] [CrossRef]
- Wickramasinghe, N.S.; Manavalan, T.T.; Dougherty, S.M.; Riggs, K.A.; Li, Y.; Klinge, C.M. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009, 37, 2584–2595. [Google Scholar] [CrossRef]
- Swat, W.; Shinkai, Y.; Cheng, H.L.; Davidson, L.; Alt, F.W. Activated Ras signals differentiation and expansion of CD4+8+ thymocytes. Proc. Natl. Acad. Sci. USA 1996, 93, 4683–4687. [Google Scholar] [CrossRef]
- Dower, N.A.; Stang, S.L.; Bottorff, D.A.; Ebinu, J.O.; Dickie, P.; Ostergaard, H.L.; Stone, J.C. RasGRP essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 2000, 1, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kortum, R.L.; Sommers, C.L.; Alexander, C.P.; Pinski, J.M.; Li, W.; Grinberg, A.; Lee, J.; Love, P.E.; Samelson, L.E. Targeted Sos1 deletion reveals its critical role in early T-cell development. Proc. Natl. Acad. Sci. USA 2011, 108, 12407–12412. [Google Scholar] [CrossRef] [PubMed]
- Kortum, R.L.; Sommers, C.L.; Pinski, J.M.; Alexander, C.P.; Merrill, R.K.; Li, W.; Love, P.E.; Samelson, L.E. Deconstructing Ras signaling in the thymus. Mol. Cell. Biol. 2012, 32, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Priatel, J.J.; Teh, S.J.; Dower, N.A.; Stone, J.C.; Teh, H.S. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 2002, 17, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.A.; Teixeiro, E.; Gill, J.; Hausmann, B.; Roubaty, D.; Holmberg, K.; Werlen, G.; Hollander, G.A.; Gascoigne, N.R.; Palmer, E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 2006, 444, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Zikherman, J.; Das, J.; Roose, J.P.; Weiss, A.; Chakraborty, A.K. Origin of the sharp boundary that discriminates positive and negative selection of thymocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 528–533. [Google Scholar] [CrossRef]
- Das, J.; Ho, M.; Zikherman, J.; Govern, C.; Yang, M.; Weiss, A.; Chakraborty, A.K.; Roose, J.P. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell 2009, 136, 337–351. [Google Scholar] [CrossRef]
- Salzer, E.; Cagdas, D.; Hons, M.; Mace, E.M.; Garncarz, W.; Petronczki, O.Y.; Platzer, R.; Pfajfer, L.; Bilic, I.; Ban, S.A.; et al. RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics. Nat. Immunol. 2016, 17, 1352–1360. [Google Scholar] [CrossRef]
- Coughlin, J.J.; Stang, S.L.; Dower, N.A.; Stone, J.C. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J. Immunol. 2005, 175, 7179–7184. [Google Scholar] [CrossRef]
- Coughlin, J.J.; Stang, S.L.; Dower, N.A.; Stone, J.C. The role of RasGRPs in regulation of lymphocyte proliferation. Immunol. Lett. 2006, 105, 77–82. [Google Scholar] [CrossRef]
- Baars, M.J.D.; Douma, T.; Simeonov, D.R.; Myers, D.R.; Kulhanek, K.; Banerjee, S.; Zwakenberg, S.; Baltissen, M.P.; Amini, M.; de Roock, S.; et al. Dysregulated RASGRP1 expression through RUNX1 mediated transcription promotes autoimmunity. Eur. J. Immunol. 2021, 51, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Oh-hora, M.; Johmura, S.; Hashimoto, A.; Hikida, M.; Kurosaki, T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J. Exp. Med. 2003, 198, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Takahashi, Y.; Hayashi, R.; Nakamura, T.; Ishii, K.; Matsuda, J.; Ogura, A.; Shirakata, Y.; Karasuyama, H.; Sudo, T.; et al. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J. Exp. Med. 2000, 192, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Iritani, B.M.; Forbush, K.A.; Farrar, M.A.; Perlmutter, R.M. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997, 16, 7019–7031. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Nunes, J.A.; Vely, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, S.; Lee, J.; Kim, M.J.; Piao, Z.H.; Jeong, M.; Chung, J.W.; Kim, T.D.; Yoon, S.R.; Greenberg, P.D.; et al. RasGRP1 is required for human NK cell function. J. Immunol. 2009, 183, 7931–7938. [Google Scholar] [CrossRef]
- Hock, H.; Hamblen, M.J.; Rooke, H.M.; Traver, D.; Bronson, R.T.; Cameron, S.; Orkin, S.H. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 2003, 18, 109–120. [Google Scholar] [CrossRef]
- Karsunky, H.; Zeng, H.; Schmidt, T.; Zevnik, B.; Kluge, R.; Schmid, K.W.; Duhrsen, U.; Moroy, T. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat. Genet. 2002, 30, 295–300. [Google Scholar] [CrossRef]
- Person, R.E.; Li, F.Q.; Duan, Z.; Benson, K.F.; Wechsler, J.; Papadaki, H.A.; Eliopoulos, G.; Kaufman, C.; Bertolone, S.J.; Nakamoto, B.; et al. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat. Genet. 2003, 34, 308–312. [Google Scholar] [CrossRef]
- Ward, A.C.; van Aesch, Y.M.; Gits, J.; Schelen, A.M.; de Koning, J.P.; van Leeuwen, D.; Freedman, M.H.; Touw, I.P. Novel point mutation in the extracellular domain of the granulocyte colony-stimulating factor (G-CSF) receptor in a case of severe congenital neutropenia hyporesponsive to G-CSF treatment. J. Exp. Med. 1999, 190, 497–507. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.Y.; Wesselschmidt, R.; Kornaga, T.; Link, D.C. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity 1996, 5, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Grail, D.; Hodgson, G.; Metcalf, D.; Stanley, E.; Cheers, C.; Fowler, K.J.; Basu, S.; Zhan, Y.F.; Dunn, A.R. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 1994, 84, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- de la Luz Sierra, M.; Sakakibara, S.; Gasperini, P.; Salvucci, O.; Jiang, K.; McCormick, P.J.; Segarra, M.; Stone, J.; Maric, D.; Zhu, J.; et al. The transcription factor Gfi1 regulates G-CSF signaling and neutrophil development through the Ras activator RasGRP1. Blood 2010, 115, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, M.; Nishida, K.; Hirano, T.; Zhang, W. An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J. Exp. Med. 2007, 204, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Yang, W.; Latour, S.; Yang, J.; Winter, S.; Zheng, J.; Ni, K.; Lv, M.; Liu, C.; Huang, H.; et al. RASGRP1 mutation in autoimmune lymphoproliferative syndrome-like disease. J. Allergy Clin. Immunol. 2018, 142, 595–604.e516. [Google Scholar] [CrossRef]
- Somekh, I.; Marquardt, B.; Liu, Y.; Rohlfs, M.; Hollizeck, S.; Karakukcu, M.; Unal, E.; Yilmaz, E.; Patiroglu, T.; Cansever, M.; et al. Novel Mutations in RASGRP1 are Associated with Immunodeficiency, Immune Dysregulation, and EBV-Induced Lymphoma. J. Clin. Immunol. 2018, 38, 699–710. [Google Scholar] [CrossRef]
- Winter, S.; Martin, E.; Boutboul, D.; Lenoir, C.; Boudjemaa, S.; Petit, A.; Picard, C.; Fischer, A.; Leverger, G.; Latour, S. Loss of RASGRP1 in humans impairs T-cell expansion leading to Epstein-Barr virus susceptibility. EMBO Mol. Med. 2018, 10, 188–199. [Google Scholar] [CrossRef]
- Oki, T.; Kitaura, J.; Watanabe-Okochi, N.; Nishimura, K.; Maehara, A.; Uchida, T.; Komeno, Y.; Nakahara, F.; Harada, Y.; Sonoki, T.; et al. Aberrant expression of RasGRP1 cooperates with gain-of-function NOTCH1 mutations in T-cell leukemogenesis. Leukemia 2012, 26, 1038–1045. [Google Scholar] [CrossRef]
- Hartzell, C.; Ksionda, O.; Lemmens, E.; Coakley, K.; Yang, M.; Dail, M.; Harvey, R.C.; Govern, C.; Bakker, J.; Lenstra, T.L.; et al. Dysregulated RasGRP1 responds to cytokine receptor input in T cell leukemogenesis. Sci. Signal. 2013, 6, ra21. [Google Scholar] [CrossRef]
- Kim, R.; Trubetskoy, A.; Suzuki, T.; Jenkins, N.A.; Copeland, N.G.; Lenz, J. Genome-based identification of cancer genes by proviral tagging in mouse retrovirus-induced T-cell lymphomas. J. Virol. 2003, 77, 2056–2062. [Google Scholar] [CrossRef]
- Dupuy, A.J.; Morgan, K.; von Lintig, F.C.; Shen, H.; Acar, H.; Hasz, D.E.; Jenkins, N.A.; Copeland, N.G.; Boss, G.R.; Largaespada, D.A. Activation of the Rap1 guanine nucleotide exchange gene, CalDAG-GEF I, in BXH-2 murine myeloid leukemia. J. Biol. Chem. 2001, 276, 11804–11811. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shen, H.; Akagi, K.; Morse, H.C.; Malley, J.D.; Naiman, D.Q.; Jenkins, N.A.; Copeland, N.G. New genes involved in cancer identified by retroviral tagging. Nat. Genet. 2002, 32, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.B.; Guilbault, B.; Goulding, R.E.; Kay, R.J. Deregulated expression of RasGRP1 initiates thymic lymphomagenesis independently of T-cell receptors. Oncogene 2005, 24, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Ksionda, O.; Melton, A.A.; Bache, J.; Tenhagen, M.; Bakker, J.; Harvey, R.; Winter, S.S.; Rubio, I.; Roose, J.P. RasGRP1 overexpression in T-ALL increases basal nucleotide exchange on Ras rendering the Ras/PI3K/Akt pathway responsive to protumorigenic cytokines. Oncogene 2016, 35, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yun, G.; Hebert, A.; Kong, G.; Ranheim, E.A.; Finn, R.; Rajagoplan, A.; Li, S.; Zhou, Y.; Yu, M.; et al. Nras Q61R/+ and Kras-/- cooperate to downregulate Rasgrp1 and promote lympho-myeloid leukemia in early T-cell precursors. Blood 2021, 137, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Karra, L.; Romero-Moya, D.; Ksionda, O.; Krush, M.; Gu, Z.; Mues, M.; Depeille, P.; Mullighan, C.; Roose, J.P. Increased baseline RASGRP1 signals enhance stem cell fitness during native hematopoiesis. Oncogene 2020, 39, 6920–6934. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Guo, R.; Wan, C.K.; Carpenter, J.H.; Mousallem, T.; Boustany, R.M.; Kuan, C.T.; Burks, A.W.; Zhong, X.P. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc. Natl. Acad. Sci. USA 2008, 105, 11909–11914. [Google Scholar] [CrossRef]
- Lauchle, J.O.; Kim, D.; Le, D.T.; Akagi, K.; Crone, M.; Krisman, K.; Warner, K.; Bonifas, J.M.; Li, Q.; Coakley, K.M.; et al. Response and resistance to MEK inhibition in leukaemias initiated by hyperactive Ras. Nature 2009, 461, 411–414. [Google Scholar] [CrossRef]
- Ksionda, O.; Mues, M.; Wandler, A.M.; Donker, L.; Tenhagen, M.; Jun, J.; Ducker, G.S.; Matlawska-Wasowska, K.; Shannon, K.; Shokat, K.M.; et al. Comprehensive analysis of T cell leukemia signals reveals heterogeneity in the PI3 kinase-Akt pathway and limitations of PI3 kinase inhibitors as monotherapy. PLoS ONE 2018, 13, e0193849. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellstrom-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.P.; Cai, J.; Ma, S.Y.; Fang, Y.; Huang, H.Q.; Lin, T.Y.; Rao, H.L.; Li, M.; Xia, Z.J.; Kang, T.B.; et al. BRD2 induces drug resistance through activation of the RasGRP1/Ras/ERK signaling pathway in adult T-cell lymphoblastic lymphoma. Cancer Commun. 2020, 40, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.C.; Stang, S.L.; Zheng, Y.; Dower, N.A.; Brenner, S.E.; Baryza, J.L.; Wender, P.A. Synthetic bryostatin analogues activate the RasGRP1 signaling pathway. J. Med. Chem. 2004, 47, 6638–6644. [Google Scholar] [CrossRef]
- Stang, S.L.; Lopez-Campistrous, A.; Song, X.; Dower, N.A.; Blumberg, P.M.; Wender, P.A.; Stone, J.C. A proapoptotic signaling pathway involving RasGRP, Erk, and Bim in B cells. Exp. Hematol. 2009, 37, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Leo, I.R.; Aswad, L.; Stahl, M.; Kunold, E.; Post, F.; Erkers, T.; Struyf, N.; Mermelekas, G.; Joshi, R.N.; Gracia-Villacampa, E.; et al. Integrative multi-omics and drug response profiling of childhood acute lymphoblastic leukemia cell lines. Nat. Commun. 2022, 13, 1691. [Google Scholar] [CrossRef]
- Oki-Idouchi, C.E.; Lorenzo, P.S. Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res. 2007, 67, 276–280. [Google Scholar] [CrossRef]
- Diez, F.R.; Garrido, A.A.; Sharma, A.; Luke, C.T.; Stone, J.C.; Dower, N.A.; Cline, J.M.; Lorenzo, P.S. RasGRP1 transgenic mice develop cutaneous squamous cell carcinomas in response to skin wounding: Potential role of granulocyte colony-stimulating factor. Am. J. Pathol. 2009, 175, 392–399. [Google Scholar] [CrossRef]
- Obermueller, E.; Vosseler, S.; Fusenig, N.E.; Mueller, M.M. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004, 64, 7801–7812. [Google Scholar] [CrossRef]
- Luke, C.T.; Oki-Idouchi, C.E.; Cline, J.M.; Lorenzo, P.S. RasGRP1 overexpression in the epidermis of transgenic mice contributes to tumor progression during multistage skin carcinogenesis. Cancer Res. 2007, 67, 10190–10197. [Google Scholar] [CrossRef]
- Sharma, A.; Fonseca, L.L.; Rajani, C.; Yanagida, J.K.; Endo, Y.; Cline, J.M.; Stone, J.C.; Ji, J.; Ramos, J.W.; Lorenzo, P.S. Targeted deletion of RasGRP1 impairs skin tumorigenesis. Carcinogenesis 2014, 35, 1084–1091. [Google Scholar] [CrossRef]
- Sharma, A.; Luke, C.T.; Dower, N.A.; Stone, J.C.; Lorenzo, P.S. RasGRP1 is essential for ras activation by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate in epidermal keratinocytes. J. Biol. Chem. 2010, 285, 15724–15730. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.L.; Yang, W.S.; Geerts, D.; Turkson, J.; Ji, J.; Ramos, J.W. RasGRP1 induces autophagy and transformation-associated changes in primary human keratinocytes. Transl. Oncol. 2021, 14, 100880. [Google Scholar] [CrossRef] [PubMed]
- Gbenedio, O.M.; Bonnans, C.; Grun, D.; Wang, C.Y.; Hatch, A.J.; Mahoney, M.R.; Barras, D.; Matli, M.; Miao, Y.; Garcia, K.C.; et al. RasGRP1 is a potential biomarker to stratify anti-EGFR therapy response in colorectal cancer. JCI Insight 2019, 5, 127552. [Google Scholar] [CrossRef] [PubMed]
- Depeille, P.; Henricks, L.M.; van de Ven, R.A.; Lemmens, E.; Wang, C.Y.; Matli, M.; Werb, Z.; Haigis, K.M.; Donner, D.; Warren, R.; et al. RasGRP1 opposes proliferative EGFR-SOS1-Ras signals and restricts intestinal epithelial cell growth. Nat. Cell Biol. 2015, 17, 804–815. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, H.; Han, F.; Shao, X.; Liu, Y.; Ma, X.; Wang, Z.; Qiang, Z.; Li, Y. Sp1-regulated transcription of RasGRP1 promotes hepatocellular carcinoma (HCC) proliferation. Liver Int. 2018, 38, 2006–2017. [Google Scholar] [CrossRef]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef]
- Wang, S.; Beeghly-Fadiel, A.; Cai, Q.; Cai, H.; Guo, X.; Shi, L.; Wu, J.; Ye, F.; Qiu, Q.; Zheng, Y.; et al. Gene expression in triple-negative breast cancer in relation to survival. Breast Cancer Res. Treat. 2018, 171, 199–207. [Google Scholar] [CrossRef]
- Chou, C.W.; Huang, Y.M.; Chang, Y.J.; Huang, C.Y.; Hung, C.S. Identified the novel resistant biomarkers for taxane-based therapy for triple-negative breast cancer. Int. J. Med. Sci. 2021, 18, 2521–2531. [Google Scholar] [CrossRef]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e310. [Google Scholar] [CrossRef]
- Latour, S.; Fischer, A. Signaling pathways involved in the T-cell-mediated immunity against Epstein-Barr virus: Lessons from genetic diseases. Immunol. Rev. 2019, 291, 174–189. [Google Scholar] [CrossRef]
- Rchiad, Z.; Haidar, M.; Ansari, H.R.; Tajeri, S.; Mfarrej, S.; Ben Rached, F.; Kaushik, A.; Langsley, G.; Pain, A. Novel tumour suppressor roles for GZMA and RASGRP1 in Theileria annulata-transformed macrophages and human B lymphoma cells. Cell. Microbiol. 2020, 22, e13255. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Di Maio, A.; Torretta, S.; Garofalo, M.; Giorgelli, V.; Masellis, R.; Nuzzo, T.; Errico, F.; Bertolino, A.; Subramaniam, S.; et al. Abnormal RasGRP1 Expression in the Post-Mortem Brain and Blood Serum of Schizophrenia Patients. Biomolecules 2022, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Kim, S.Y.; Gil, J.E.; Byun, J.S.; Cha, D.W.; Ku, B.; Lee, W.; Kim, W.K.; Oh, K.J.; Lee, E.W.; et al. Nurr1 performs its anti-inflammatory function by regulating RasGRP1 expression in neuro-inflammation. Sci. Rep. 2020, 10, 10755. [Google Scholar] [CrossRef] [PubMed]
- Molineros, J.E.; Singh, B.; Terao, C.; Okada, Y.; Kaplan, J.; McDaniel, B.; Akizuki, S.; Sun, C.; Webb, C.F.; Looger, L.L.; et al. Mechanistic Characterization of RASGRP1 Variants Identifies an hnRNP-K-Regulated Transcriptional Enhancer Contributing to SLE Susceptibility. Front. Immunol. 2019, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, M.; Ramirez-Jarquin, U.N.; Shahani, N.; Nuzzo, T.; De Rosa, A.; Swarnkar, S.; Galli, N.; Rivera, O.; Tsaprailis, G.; Scharager-Tapia, C.; et al. RasGRP1 is a causal factor in the development of l-DOPA-induced dyskinesia in Parkinson’s disease. Sci. Adv. 2020, 6, eaaz7001. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, M.; Yu, M.; Shen, J.; Zhou, J.; Hu, J.; Zhou, Y.; Zhang, W. RasGRP1 is a target for VEGF to induce angiogenesis and involved in the endothelial-protective effects of metformin under high glucose in HUVECs. IUBMB Life 2019, 71, 1391–1400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).