The Pyramiding of Elite Allelic Genes Related to Grain Number Increases Grain Number per Panicle Using the Recombinant Lines Derived from Indica–japonica Cross in Rice
Abstract
1. Introduction
2. Results
2.1. GNPP Distribution of RILs Populations Cross from Indica LH9 and Japonica RPY
2.2. Significant Genetic Background Differences between Parents
2.3. Principal Component Analysis Reveals Superior Genotype Combinations
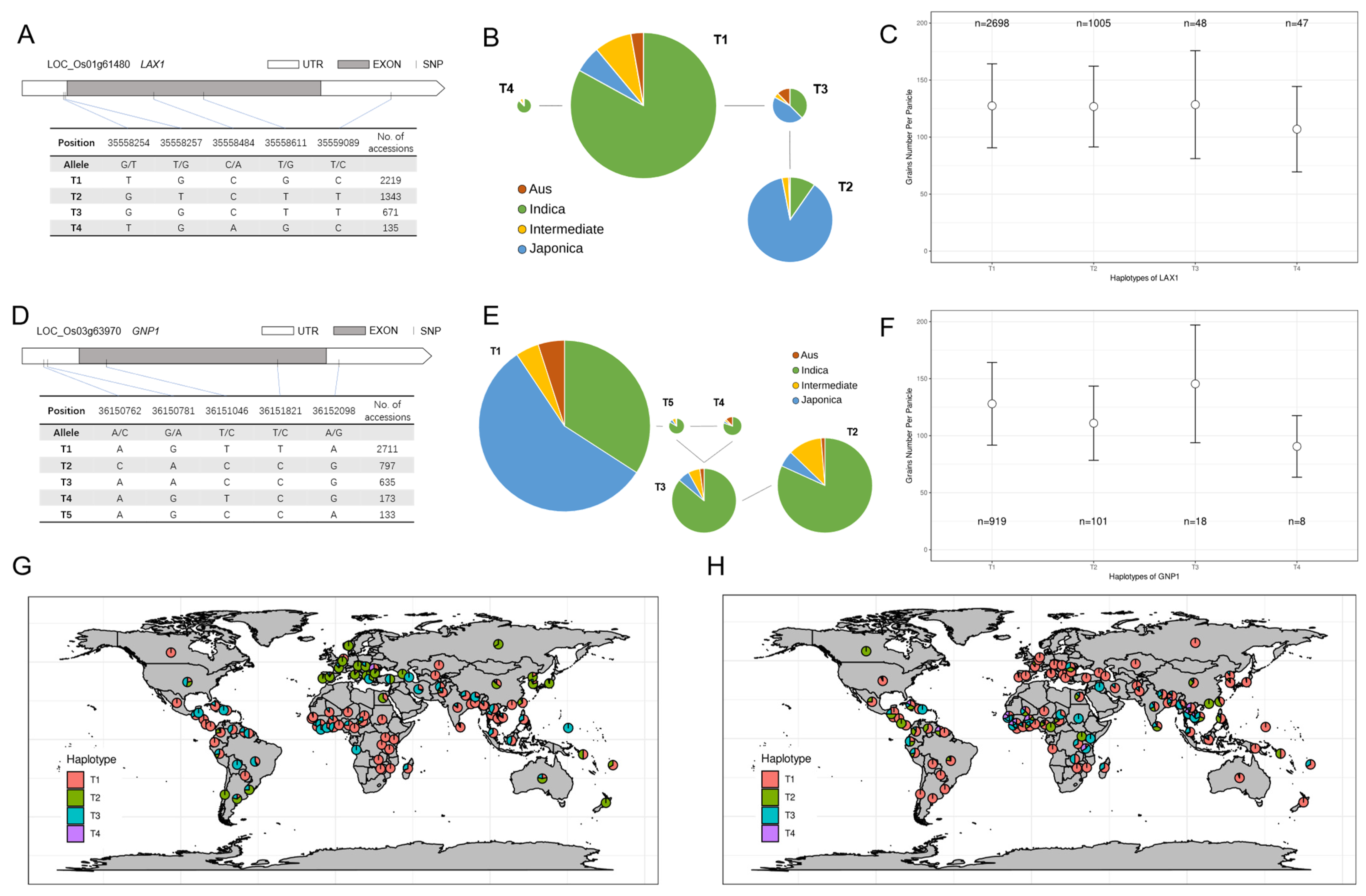
2.4. Haplotype Analysis of Target Genes and Their Geographic Origin
2.5. Specific Combinations of Indica–japonica Alleles Increase the GNPP
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. SNP Variation and Effect Prediction
4.3. Haplotype Variation in Genes Related to Grains Number per Panicle
4.4. Identify Superior Haplotypes of the Target Genes
4.5. Hierarchical Clustering and Principal Component Analysis
4.6. Statistical Analysis and Visualization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Demont, M.; Stein, A.J. Global Value of GM Rice: A Review of Expected Agronomic and Consumer Benefits. New Biotechnol. 2013, 30, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhu, Y.; Li, X.; Lin, Y. Molecular and Genetic Aspects of Grain Number Determination in Rice (Oryza Sativa L.). Int. J. Mol. Sci. 2021, 22, 728. [Google Scholar] [CrossRef] [PubMed]
- Crowell, S.; Korniliev, P.; Falcão, A.; Ismail, A.; Gregorio, G.; Mezey, J.; McCouch, S. Genome-Wide Association and High-Resolution Phenotyping Link Oryza Sativa Panicle Traits to Numerous Trait-Specific QTL Clusters. Nat. Commun. 2016, 7, 10527. [Google Scholar] [CrossRef]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The Rice Genome Revolution: From an Ancient Grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Q. Genetic and Molecular Bases of Rice Yield. Annu. Rev. Plant Biol. 2010, 61, 421–442. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, C.; Zhou, H.; Sun, W.; Wang, P.; Wang, D.; Qiu, X.; Ali, J.; Yu, S. Identification of Heterotic Loci with Desirable Allelic Interaction to Increase Yield in Rice. Rice 2021, 14, 97. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Chen, L.; Huang, X.; Cheng, Z.; Han, B.; Zhang, Q.; Wu, C. Rice Functional Genomics Research: Past Decade and Future. Mol. Plant 2018, 11, 359–380. [Google Scholar] [CrossRef]
- Chen, L.; Bian, J.; Shi, S.; Yu, J.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Luo, X.; Tong, S.; Yang, X.; et al. Genetic Analysis for the Grain Number Heterosis of a Super-Hybrid Rice WFYT025 Combination Using RNA-Seq. Rice 2018, 11, 37. [Google Scholar] [CrossRef]
- Kong, W.; Deng, X.; Yang, J.; Zhang, C.; Sun, T.; Ji, W.; Zhong, H.; Fu, X.; Li, Y. High-resolution Bin-based Linkage Mapping Uncovers the Genetic Architecture and Heterosis-related Loci of Plant Height in Indica–Japonica Derived Populations. Plant J. 2022, 110, 814–827. [Google Scholar] [CrossRef]
- Bevan, M.W.; Uauy, C.; Wulff, B.B.H.; Zhou, J.; Krasileva, K.; Clark, M.D. Genomic Innovation for Crop Improvement. Nature 2017, 543, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A Map of Rice Genome Variation Reveals the Origin of Cultivated Rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Nayak, S.N.; May, G.D.; Jackson, S.A. Next-Generation Sequencing Technologies and Their Implications for Crop Genetics and Breeding. Trends Biotechnol. 2009, 27, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, K.; Chen, Z.; Cao, Y.; Gao, Q.; Li, Y.; Li, X.; Lu, H.; Du, H.; Lu, M.; et al. MBKbase for Rice: An Integrated Omics Knowledgebase for Molecular Breeding in Rice. Nucleic Acids Res. 2019, gkz921. [Google Scholar] [CrossRef]
- Li, X.; Wu, L.; Wang, J.; Sun, J.; Xia, X.; Geng, X.; Wang, X.; Xu, Z.; Xu, Q. Genome Sequencing of Rice Subspecies and Genetic Analysis of Recombinant Lines Reveals Regional Yield- and Quality-Associated Loci. BMC Biol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Deveshwar, P.; Prusty, A.; Sharma, S.; Tyagi, A.K. Phytohormone-Mediated Molecular Mechanisms Involving Multiple Genes and QTL Govern Grain Number in Rice. Front. Genet. 2020, 11, 586462. [Google Scholar] [CrossRef]
- Kong, W.; Deng, X.; Liao, Z.; Wang, Y.; Zhou, M.; Wang, Z.; Li, Y. De Novo Assembly of Two Chromosome-Level Rice Genomes and Bin-Based QTL Mapping Reveal Genetic Diversity of Grain Weight Trait in Rice. Front. Plant Sci. 2022, 13, 995634. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Zhao, S.-C.; He, W.-M.; Guo, L.-B.; Peng, Y.-L.; Wang, J.-J.; Guo, X.-S.; Zhang, X.-M.; Rao, Y.-C.; Zhang, C.; et al. Dissecting Yield-Associated Loci in Super Hybrid Rice by Resequencing Recombinant Inbred Lines and Improving Parental Genome Sequences. Proc. Natl. Acad. Sci. USA 2013, 110, 14492–14497. [Google Scholar] [CrossRef]
- Zhao, K.; Tung, C.-W.; Eizenga, G.C.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-Wide Association Mapping Reveals a Rich Genetic Architecture of Complex Traits in Oryza Sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic Architecture of Heterosis for Yield Traits in Rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Wei, X.; Qiu, J.; Yong, K.; Fan, J.; Zhang, Q.; Hua, H.; Liu, J.; Wang, Q.; Olsen, K.M.; Han, B.; et al. A Quantitative Genomics Map of Rice Provides Genetic Insights and Guides Breeding. Nat. Genet. 2021, 53, 243–253. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN Web Server: A Tool to Predict the Functional Effect of Amino Acid Substitutions and Indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Deshmukh, R.; Singh, A.; Jain, N.; Anand, S.; Gacche, R.; Singh, A.; Gaikwad, K.; Sharma, T.; Mohapatra, T.; Singh, N. Identification of Candidate Genes for Grain Number in Rice (Oryza sativa L.). Funct. Integr. Genom. 2010, 10, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huang, W.; Gao, J.-P.; Yang, J.; Shi, M.; Zhu, M.-Z.; Luo, D.; Lin, H.-X. Genetic Control of Rice Plant Architecture under Domestication. Nat. Genet. 2008, 40, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Hao, Y.; Xia, X.; Khan, A.; Xu, Y.; Varshney, R.K.; He, Z. Crop Breeding Chips and Genotyping Platforms: Progress, Challenges, and Perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef]
- Oikawa, T.; Kyozuka, J. Two-Step Regulation of LAX PANICLE1 Protein Accumulation in Axillary Meristem Formation in Rice. The Plant Cell 2009, 21, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Mi, X.-F.; Shan, J.-X.; Li, X.-M.; Xu, J.-L.; Lin, H.-X. The QTL GNP1 Encodes GA20ox1, Which Increases Grain Number and Yield by Increasing Cytokinin Activity in Rice Panicle Meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Yang, L.; Qin, G.; Xia, C.; Xu, X.; Su, Y.; Liu, Y.; Ming, L.; Chen, L.-L.; et al. An Inferred Functional Impact Map of Genetic Variants in Rice. Mol. Plant 2021, 14, 1584–1599. [Google Scholar] [CrossRef]
- Zhao, H.; Yao, W.; Ouyang, Y.; Yang, W.; Wang, G.; Lian, X.; Xing, Y.; Chen, L.; Xie, W. RiceVarMap: A Comprehensive Database of Rice Genomic Variations. Nucleic Acids Res. 2015, 43, D1018–D1022. [Google Scholar] [CrossRef]



| Gene | RGAP Locus ID | GNPP | PRB | SRB | Number of Mutations | Functional Impact of Mutations |
|---|---|---|---|---|---|---|
| Gn1a | LOC_Os01g10110 | - | - | - | 4 | N535K, H116R, G54A, A79_A80del |
| NOG1 | LOC_Os01g54860 | + | 1 | E346del * | ||
| PYL1 | LOC_Os01g61210 | - | - | - | 1 | F49C |
| LAX1 | LOC_Os01g61480 | + | + | + | 2 | D74E *, S117A |
| LP | LOC_Os02g15950 | - | - | - | 2 | L3fs *, S32fs * |
| PYL4 | LOC_Os03g18600 | - | - | - | 1 | A86P |
| OSH1 | LOC_Os03g51690 | + | + | + | 1 | Q23_H24dup |
| DST | LOC_Os03g57240 | - | - | - | 2 | T201dup, A124_V125insAAAAAV |
| GNP1 | LOC_Os03g63970 | + | + | 1 | V41A | |
| An-1 | LOC_Os04g28280 | - | - | - | 1 | Q87fs * |
| LAX2 | LOC_Os04g32510 | + | + | 8 | H65_H66dup, T131_P138del, L177P, A180T, P210A, R225M *, A237del, A237V | |
| APO1 | LOC_Os06g45460 | + | + | + | 3 | G292_G294del, R204G, I17V |
| DTH7 | LOC_Os07g49460 | + | + | + | 8 | D68E |
| DTH8 | LOC_Os08g07740 | + | + | + | 8 | N295S |
| PAY1 | LOC_Os08g31470 | + | + | 2 | W2R, P150T | |
| GAD1 | LOC_Os08g37890 | + | 1 | R101fs * | ||
| IPA1 | LOC_Os08g39890 | + | + | + | 1 | L292I |
| DEP1 | LOC_Os09g26999 | + | + | + | 3 | L228H, Q283fs *, C324S |
| TAW1 | LOC_Os10g33780 | + | + | + | 1 | A33_A34insSASA |
| SP1 | LOC_Os11g12740 | + | + | + | 5 | A550_G551del, D475_G476del *, A401G, V328A, H301_A306del |
| Gene | Genotype | HN19 | EZ18 | EZ17 | LS17 | EZ16 | Score |
|---|---|---|---|---|---|---|---|
| Gn1a | F | 182.6497 | 173.4107 | 167.1413 | 152.6666 | 212.2488 | 0 |
| M | 213.6251 | 209.3672 | 201.3538 | 194.33 | 266.876 | 5 | |
| NOG1 | F | 216.5889 | 213.6555 | 209.3696 | 191.0993 | 261.7751 | 3 |
| M | 209.5623 | 204.0087 | 194.7091 | 192.5892 | 266.6108 | 2 | |
| PYL1 | F | 214.8368 | 212.1481 | 208.8955 | 197.772 | 267.9565 | 5 |
| M | 211.2413 | 204.1541 | 192.9816 | 188.7112 | 262.2566 | 0 | |
| LAX1 | F | 217.2252 | 214.2353 | 210.1405 | 199.207 | 270.9662 | 5 |
| M | 209.5813 | 202.1089 | 192.1287 | 187.8076 | 261.0606 | 0 | |
| LP | F | 222.9414 | 202.7627 | 200.4978 | 204.8714 | 260.9552 | 2 |
| M | 213.5856 | 211.2246 | 202.819 | 192.9035 | 268.4735 | 3 | |
| PYL4 | F | 198.6192 | 185.3971 | 171.8941 | 160.8207 | 219.5586 | 0 |
| M | 210.525 | 209.27 | 200.3168 | 192.4851 | 265.109 | 5 | |
| OSH1 | F | 212.5358 | 222.7958 | 191.7582 | 199.8374 | 289.2342 | 4 |
| M | 211.9244 | 203.0429 | 200.4007 | 189.2127 | 255.0527 | 1 | |
| DST | F | 201.8318 | 200.084 | 187.2243 | 187.9039 | 234.3329 | 0 |
| M | 219.2916 | 211.2264 | 207.4977 | 196.3835 | 275.7076 | 5 | |
| GNP1 | F | 201.8206 | 199.0928 | 191.335 | 192.5 | 259.8899 | 0 |
| M | 217.3728 | 211.268 | 204.5793 | 192.7047 | 266.2327 | 5 | |
| An-1 | F | 209.6562 | 215.0945 | 202.7716 | 188.6553 | 260.9996 | 2 |
| M | 213.6572 | 200.3582 | 199.252 | 198.3246 | 268.3011 | 3 | |
| LAX2 | F | 212.1621 | 210.6081 | 200.55 | 189.7581 | 251.7476 | 3 |
| M | 211.6038 | 206.166 | 199.4247 | 193.7761 | 274.4033 | 2 | |
| APO1 | F | 208.4567 | 200.882 | 198.3117 | 195.7946 | 246.0994 | 1 |
| M | 213.6812 | 208.9489 | 200.5811 | 191.586 | 268.7792 | 4 | |
| DTH7 | F | 216.7544 | 203.4692 | 219.8124 | 200.7108 | 274.3199 | 4 |
| M | 211.679 | 207.5063 | 198.7814 | 191.6447 | 263.6816 | 1 | |
| DTH8 | F | 219.7284 | 210.7304 | 206.7825 | 196.22 | 271.8351 | 5 |
| M | 209.1165 | 206.4734 | 196.9023 | 190.8444 | 262.2673 | 0 | |
| PAY1 | F | 218.0417 | 214.4899 | 214.4749 | 192.9009 | 278.6728 | 4 |
| M | 212.2218 | 207.3872 | 196.8358 | 193.6027 | 263.799 | 1 | |
| GAD1 | F | 213.9167 | 210.7948 | 204.68 | 192.4931 | 261.6666 | 3 |
| M | 211.1683 | 207.3715 | 196.0528 | 194.2355 | 262.2458 | 2 | |
| DEP1 | F | 211.652 | 220.5395 | 205.0621 | 226.2993 | 266.4615 | 4 |
| M | 212.3448 | 206.3899 | 199.034 | 188.2502 | 263.9062 | 1 | |
| TAW1 | F | 212.9674 | 192.356 | 187.9528 | 198.0376 | 253.4211 | 2 |
| M | 212.3691 | 210.8745 | 203.1138 | 191.4399 | 267.4437 | 3 | |
| SP1 | F | 217.9557 | 219.8128 | 206.507 | 199.3304 | 278.6635 | 5 |
| M | 209.8068 | 203.1178 | 194.7337 | 188.4645 | 257.744 | 0 |
| Gene | ID | Genotype | GNPP | FERT/% | EPN | TGW/g | GYPM/kg | Aus | Indica | Japonica | Intermediate | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PYL1 | LOC_Os01g61210 | LH9 | T1 | 127.2 ± 47.22 | 80.16 ± 9.94 | 9.02 ± 3.54 | 25.1 ± 3.87 | 396.14 ± 102.47 | 52 | 1779 | 103 | 56 | 1990 |
| RPY | T3 | 131.42 ± 33.65 | 81.13 ± 9.98 | 10.45 ± 2.73 | 26.3 ± 4.88 | 518.73 ± 105.69 | 12 | 98 | 751 | 75 | 936 | ||
| LAX1 | LOC_Os01g61480 | LH9 | T4 | 112.06 ± 39.1 | 74.87 ± 16.54 | 26.06 ± 3.42 | 367.73 ± 71.71 | 0 | 134 | 4 | 0 | 138 | |
| RPY | T2 | 128.06 ± 35.34 | 81.22 ± 9,42 | 9.56 ± 2.82 | 25.48 ± 2.53 | 549.23 ± 90.84 | 0 | 65 | 1304 | 23 | 1392 | ||
| OSH1 | LOC_Os03g51690 | LH9 | T15 | 24.25 ± 2.98 | 400 | 0 | 26 | 0 | 0 | 26 | |||
| RPY | T3 | 127.47 ± 31.73 | 80.64 ± 10.26 | 10.39 ± 2.76 | 26.16 ± 4.65 | 523.32 ± 96.86 | 0 | 9 | 261 | 6 | 276 | ||
| SP1 | LOC_Os11g12740 | LH9 | T2 | 128.1 ± 35.29 | 81.15 ± 9.51 | 9.56 ± 2.82 | 25.5 ± 2.92 | 545.93 ± 91.16 | 8 | 551 | 54 | 12 | 625 |
| RPY | T3 | 126.8 ± 31.39 | 80.47 ± 10.18 | 10.41 ± 2.76 | 26.01 ± 4.27 | 535.14 ± 99.06 | 2 | 6 | 224 | 1 | 233 | ||
| DTH8 | LOC_Os08g07740 | LH9 | T3 | 126.64 ± 31.35 | 80.40 ± 10.10 | 10.43 ± 2.76 | 25.97 ± 4.26 | 534.86 ± 98.71 | 2 | 231 | 40 | 4 | 277 |
| RPY | T1 | 127.79 ± 37.03 | 81.11 ± 9.98 | 9.49 ± 2.70 | 25.32 ± 3.48 | 521.64 ± 108.78 | 0 | 121 | 1471 | 40 | 1632 | ||
| DST | LOC_Os03g57240 | LH9 | T2 | 127.97 ± 35.63 | 80.87 ± 9.94 | 9.55 ± 2.82 | 25.4 ± 3.21 | 533.27 ± 98.41 | 15 | 843 | 41 | 29 | 928 |
| RPY | T1 | 126.99 ± 35.56 | 81.26 ± 9.97 | 9.55 ± 2.70 | 25.4 ± 3.27 | 531.51 ± 102.87 | 7 | 58 | 1108 | 27 | 1200 | ||
| PYL4 | LOC_Os03g18600 | LH9 | T6 | 135.87 ± 56.44 | 78.08 ± 7.17 | 23.58 ± 3.67 | 337.22 ± 89.13 | 8 | 194 | 9 | 1 | 212 | |
| RPY | T1 | 126.58 ± 35.19 | 81.25 ± 9.94 | 9.57 +2.72 | 25.44 ± 3.17 | 533.95 ± 100.24 | 0 | 92 | 1443 | 28 | 1563 | ||
| GNP1 | LOC_Os03g63970 | LH9 | T3 | 126.84 ± 31.59 | 80.46 ± 10.02 | 10.43 ± 2.77 | 25.86 ± 4.25 | 526.83 ± 105.58 | 8 | 581 | 36 | 22 | 647 |
| RPY | T1 | 126.56 ± 35.41 | 81.21 ± 9.88 | 9.52 ± 2.74 | 25.44 ± 3.27 | 535.59 ± 101.01 | 130 | 618 | 1941 | 150 | 2839 | ||
| Name | Group | Origin | LAX1 LOC_Os01g61480 | GNP1 LOC_Os03g63970 |
|---|---|---|---|---|
| ZhongHan502 | Japonica | China | T2 | T6 |
| Bg90-2 | Intermediate(hybrid) | Sri Lanka | T6 | T1 |
| NingGeng28Hao | Japonica | China | T2 | T1 |
| YanGeng7Hao | Japonica | China | T5 | T1 |
| XiangQing | Japonica | China | T2 | T1 |
| C9083 | Japonica | China | T2 | T1 |
| FUNAKIOMACHI | Japonica | Japan | T2 | T1 |
| HOUMANSHINDENINE | Japonica | Japan | T2 | T1 |
| KABASHIKO | Japonica | Japan | T2 | T1 |
| KAMEJI | Japonica | Japan | T2 | T1 |
| KAMENOO | Japonica | Japan | T2 | T1 |
| NingGeng24Hao | Japonica | China | T2 | T1 |
| RAIDEN | Japonica | Japan | T2 | T1 |
| WATARIBUNE1681 | Japonica | Japan | T2 | T1 |
| CP231 | Japonica | United States | T2 | T1 |
| Basmati370 | Indica | India | T1 | T5 |
| Zhongchao 123 | Japonica | China | T2 | T21 |
| ChangShu-6-85 | Japonica | China | T2 | T1 |
| LianGeng11Hao | Japonica | China | T2 | T1 |
| PuTe6Hao | Japonica | China | T2 | T1 |
| SongGeng15 | Japonica | China | T2 | T1 |
| TASENSHO | Japonica | Japan | T2 | T1 |
| R162 | Japonica | China | T2 | T1 |
| NingGeng35Hao | Japonica | China | T13 | T1 |
| SHINYAMADABO1 | Japonica | Japan | T2 | T1 |
| GORIKI | Japonica | Japan | T2 | T1 |
| MANGOKU | Japonica | Japan | T2 | T1 |
| JC1 | Indica | India | T3 | T1 |
| SEKIYAMA | Japonica | Japan | T2 | T1 |
| AMBARIKORI | Indica | Africa | T1 | T3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Deng, X.; Kong, W.; Sun, T.; Li, Y. The Pyramiding of Elite Allelic Genes Related to Grain Number Increases Grain Number per Panicle Using the Recombinant Lines Derived from Indica–japonica Cross in Rice. Int. J. Mol. Sci. 2023, 24, 1653. https://doi.org/10.3390/ijms24021653
Liu X, Deng X, Kong W, Sun T, Li Y. The Pyramiding of Elite Allelic Genes Related to Grain Number Increases Grain Number per Panicle Using the Recombinant Lines Derived from Indica–japonica Cross in Rice. International Journal of Molecular Sciences. 2023; 24(2):1653. https://doi.org/10.3390/ijms24021653
Chicago/Turabian StyleLiu, Xuhui, Xiaoxiao Deng, Weilong Kong, Tong Sun, and Yangsheng Li. 2023. "The Pyramiding of Elite Allelic Genes Related to Grain Number Increases Grain Number per Panicle Using the Recombinant Lines Derived from Indica–japonica Cross in Rice" International Journal of Molecular Sciences 24, no. 2: 1653. https://doi.org/10.3390/ijms24021653
APA StyleLiu, X., Deng, X., Kong, W., Sun, T., & Li, Y. (2023). The Pyramiding of Elite Allelic Genes Related to Grain Number Increases Grain Number per Panicle Using the Recombinant Lines Derived from Indica–japonica Cross in Rice. International Journal of Molecular Sciences, 24(2), 1653. https://doi.org/10.3390/ijms24021653







