Corydalis saxicola Bunting: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Clinical Applications
Abstract
1. Introduction
2. Database Search Method
3. Botanical Distribution and Description
4. Traditional Uses
5. Phytochemistry
5.1. Alkaloids
5.2. Others
6. Pharmacology
6.1. Anticancer Activity
6.1.1. Crude Extracts
6.1.2. Isolated Phytochemicals
6.2. Hepatoprotective Effects
6.2.1. Crude Extracts
6.2.2. Isolated Phytochemicals
6.3. Anti-HBV Activity
6.3.1. Crude Extract
6.3.2. Isolated Phytochemicals
6.4. Enhancement of Immune Function
Crude Extract
6.5. Antioxidant Activity
6.5.1. Crude Extract
6.5.2. Isolated Phytochemicals
6.6. Effects on the Central Nervous System
6.6.1. Crude Extract
6.6.2. Isolated Phytochemicals
6.7. Anti-Inflammatory Activity
Crude Extracts
6.8. Analgesic Effect
6.8.1. Crude Extract
6.8.2. Isolated Phytochemicals
6.9. Antibacterial Activity
6.9.1. Crude Extract
6.9.2. Isolated Phytochemicals
6.10. Choleretic Effects
Crude Extract
6.11. Other Activities
7. Clinical Applications
7.1. Icteric Hepatitis
7.2. Viral Hepatitis
7.3. Acute and Chronic Hepatitis
7.4. Liver Cancer
7.5. Hyperbilirubinemia
7.6. Others
8. Toxicity Assessment
9. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| Bcl-2 | B cell lymphoma-2 |
| BCG | bacillus calmette-guerin |
| BUN | blood urea nitrogen |
| Cdc42 | Cell division cycle 42 |
| CYP | cytochrome |
| CSB | Corydalis saxicola Bunting |
| CSBI | Corydalis saxicola Bunting injection |
| CSBTA | Corydalis saxicola Bunting total alkaloids |
| CCl4 | carbontetrachloride |
| CD86 | cluster of differentiation 86 |
| COX-2 | cyclooxygenase-2 |
| DA | dopamine |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DBiL | direct bilirubin |
| DB | direct bilirubin |
| DHBV | duck hepatitis B virus |
| DOPAC | 3,4-Dihydroxyphenylacetic acid |
| EC50 | concentration for 50% of maximal effect |
| FasL | Fas ligand |
| HT | hydroxytryptamine |
| HIAA | hydroxyindolacetic acid |
| HBV | hepatitis B virus |
| HBsAg | hepatitis B surface antigen |
| HBeAg | hepatitis B e antigen |
| hTERT | telomerase reverse transcriptase |
| HVA | homovanillic acid |
| GT | glutamyltransferase |
| i.p | intraperitoneally injected |
| iNOS | inducible nitric oxide synthase |
| IC50 | half maximal inhibitory concentration |
| IFN-γ | interferon-γ |
| IL | interleukin |
| IENF | intraepidermal nerve fiber |
| Ki | inhibition constant |
| LD50 | median lethal dose |
| LDH | lactate dehydrogenase |
| MDA | malondialdehyde |
| MAPKs | mitogen-activated protein kinases |
| MMP | matrix metal loprotease |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MIC | minimal inhibitory concentration |
| NPN | non-protein nitrogen |
| NF-κB | nuclear transcription factor-κB |
| NMR | nuclear magnetic resonance |
| NFATc1 | nuclear factor of activated T-cells c1 |
| OPG | osteoprotegerin |
| QOL | quality of life |
| RANKL | receptor activator of nuclear factor- κB ligand |
| SOD | superoxide dismutase |
| SGPT | serum glutamate pyruvate transaminase |
| SB | serum total bilirubin |
| TNF-α | tumour necrosis factor-α |
| TCA | tricarboxylic acid |
| TGF-β1 | transforming growth factor-β1 |
| TBIL | totalbilirubin |
| THP-1 | leukemia |
| TRPV1 | transient receptor potential vanilloid 1 |
| TBiL | total bilirubin |
| TACE | hepatic artery chemoembolization |
References
- Zhang, B.; Huang, R.Z.; Hua, J.; Liang, H.; Pan, Y.M.; Dai, L.M.; Liang, D.; Wang, H.S. Antitumor lignanamides from the aerial parts of Corydalis saxicola. Phytomedicine 2016, 23, 1599–1609. [Google Scholar] [CrossRef]
- He, Z.C.; Wang, D.M.; Li, G.C.; Wu, J.Y. Study on alkaloids from Corydalis saxicola and their anti-oxidative activities. Chin. Tradit. Herb. Drugs 2014, 45, 1526–1531. [Google Scholar]
- Zeng, F.L.; Xiang, Y.F.; Liang, Z.R.; Wang, X.; Huang, D.E.; Zhu, S.N.; Li, M.M.; Yang, D.P.; Wang, D.M.; Wang, Y.F. Anti-hepatitis B virus effects of dehydrocheilanthifoline from Corydalis saxicola. Am. J. Chin. Med. 2013, 41, 119–130. [Google Scholar] [CrossRef]
- Wu, J.J.; Chen, P.; Ju, L.J.; Gao, R.H.; Li, S.L.; Huang, Z.Q.; Cheng, Y.Q.; Gui, S.Q.; Qiu, Z.X.; Cheng, J.; et al. Corydalis saxicola Bunting total alkaloids ameliorate diet-induced non-alcoholic steatohepatitis by regulating hepatic PI3K/Akt and TLR4/NF-κB pathways in mice. Biomed. Pharmacother. 2022, 151, 113132. [Google Scholar] [CrossRef]
- Xie, G.Y.; Jin, S.Y.; Li, H.T.; Ai, M.K.; Han, F.; Dai, Y.Q.; Tao, W.; Zhu, Y.; Zhao, Y.C.; Qin, M.J. Chemical constituents and antioxidative, anti-inflammatory and anti-proliferative activities of wild and cultivated Corydalis saxicola. Ind. Crop. Prod. 2021, 169, 113647. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Bi, J.L.; Sun, Q.Y.; Yang, F.M.; Wang, Y.H.; Tang, G.H.; Zhao, F.W.; Wang, H.; Xu, J.J.; Kennelly, E.J.; et al. Bioactive isoquinoline alkaloids from Corydalis saxicola. Planta Med. 2012, 78, 65–70. [Google Scholar] [CrossRef]
- Qin, F.; Dai, L.M.; Zhang, B.; Huang, R.Z.; Wang, F.F.; Qin, J.K.; Liang, D.; Wang, H.S. (±)-Corysaxicolaine A: A pair of antitumor enantiomeric alkaloid dimers from Corydalis saxicola. Org. Biomol. Chem. 2022, 20, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Wang, J.Y.; Mo, B.W.; Zeng, J.R.; Yao, D. Total alkaloids of Corydalis saxicola Bunting inhibits migration of A549 cells by suppressing Cdc42 or Vav1. Oncol. Lett. 2018, 15, 475–482. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Hu, X.H.; Zhao, R.F. Study on the introduction and cultivation of Corydalis saxicola Bunting. Guihaia 2002, 22, 469–473. [Google Scholar]
- Institute of Botany, Chinese Academy of Sciences. Flora of China; Institute of Botany, Chinese Academy of Sciences: Beijing, China, 1999; Volume 32, p. 418. [Google Scholar]
- Wen, H.Q.; Xu, Z.R.; Villa-Lobos, J.; Skog, L.E. A list of threatened limestone plants in south China. Guihaia 1993, 13, 110–127. [Google Scholar]
- Wei, M.K.; Lu, J.X. High-yield cultivation techniques of Corydalis saxicola Bunting in karst mountains. Guangxi Agric. Sci. 2004, 35, 21. [Google Scholar]
- Wu, Y.; Lu, T.L.; Ji, D.; Zhou, Y.; Mao, C.Q. Isolation and structural identification of alkaloids from Corydalis saxicola. J. Nanjing Univ. Tradit. Chin. Med. 2015, 31, 81–83. [Google Scholar]
- Li, H.L.; Zhang, W.D.; Zhang, W.; Zhang, C.; Liu, R.H. A new nitro alkaloid from Corydalis saxicola Bunting. Chin. Chem. Lett. 2005, 16, 367–368. [Google Scholar]
- Li, H.L.; Han, T.; Liu, R.H.; Zhang, C.; Chen, H.S.; Zhang, W.D. Alkaloids from Corydalis saxicola and their anti-hepatitis B virus activity. Chem. Biodivers. 2008, 5, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhang, W.D.; Han, T.; Zhang, C.; Liu, R.H.; Chen, H.S. Tetrahydroprotoberberines alkaloids from Corydalis saxicola. Chem. Nat. Comp. 2007, 43, 173–175. [Google Scholar] [CrossRef]
- Ke, M.M.; Zhang, X.D.; Wu, L.Z.; Zhao, Y.; Zhu, D.Y.; Song, C.Q.; Xu, R.S. Studies on the active principles of Corydalis saxicola Bunting. Chin. Pharm. J. 1980, 15, 41. [Google Scholar]
- Wu, Y.R.; Ma, Y.B.; Zhao, Y.X.; Yao, S.Y.; Chen, J.J. Two new quaternary alkaloids and anti-hepatitis B virus active constituents from Corydalis saxicola. Planta Med. 2007, 73, 787–791. [Google Scholar] [CrossRef]
- Cheng, X.X.; Wang, D.; Jiang, L.; Yang, D.P. DNA topoisomerase I inhibitory alkaloids from Corydalis saxicola. Chem. Biodivers. 2010, 5, 1335–1344. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Liang, J.Y.; Feng, X. A new alkaloid from the herb of Corydalis saxicola. Chin. J. Nat. Medicines. 2009, 7, 0414–0416. [Google Scholar] [CrossRef]
- Ke, M.M.; Zhang, X.D.; Wu, L.Z.; Zhao, Y.; Zhu, D.Y.; Song, C.Q.; Xu, R.S. Studies on the active principles of Corydalis saxicola Bunting. Acta Bot. Sin. 1982, 24, 289. [Google Scholar]
- Wang, Q.Z.; Liang, J.Y.; Yuan, Y. Chemical constituents of Corydalis saxicola. Chin. J. Nat. Medicines. 2007, 5, 31–34. [Google Scholar]
- Mao, Y.A. Chemical Constituents and Activity of Corydalis saxicola Bunting; Guangxi Medical University: Nanning, China, 2006. [Google Scholar]
- Cheng, X.X.; Yang, D.P.; Wang, D.M.; Jiang, M. Isolation of alkaloids from Corydalis saxicola by high-speed counter-current chromatography. J. Chin. Med. Mater. 2011, 34, 1062–1064. [Google Scholar]
- Ju, J.N.; Ming, Y.Z.; Dai, Q.K.; Li, K.Z.; Huang, S.; He, J.B.; Wu, G.B.; Chen, C. Effect of Corydalis saxicola Bunting aqueous extract on proliferation and migration of hepatocellular carcinoma HepG2 cells and its possible mechanism. Chin. J. Oncol. Prev. Treat. 2018, 10, 434–438. [Google Scholar]
- Sang, Y. Research of Corydalis saxicola Bunting Total Alkaloids on Nude Mouse with Subcutaneous Transplantation Tumor and Bone Metastasis in Lung Cancer A549; Guilin Medical University: Guilin, China, 2017. [Google Scholar]
- Du, Y.J. Corydalis saxicola Bunting Extractive inhibit F-actin Polymerization in A549 Cells and Its Possible Mechanism; Guilin Medical University: Guilin, China, 2017. [Google Scholar]
- Li, J.H.; Wang, J.Y.; Zeng, J.R.; Gao, Y.; Li, M.M.; Yu, Y.Y. Effect of Corydalis Saxicola total alkaloids on human A549 cell proliferation, apoptosis and expressions of caspase surviving. Chin. J. Exp. Tradit. Med. Formul. 2015, 21, 165–169. [Google Scholar]
- An, R. Study on Mechanisms and Inhibitory Effects of Saxicola on Human A549 Cell Proliferation; Guilin Medical University: Guilin, China, 2014. [Google Scholar]
- Zhu, Y.; Liao, J.X. Effects of Yanhuanglian total alkalions on Bcl-2 activity of oral squamous carcinoma cell lines. J. Oral. Maxil. Surg. 2011, 21, 96–98. [Google Scholar]
- Yin, J.K.; Liao, J.X. Effects of Corydalis saxicola Bunting total alkaloids on Tca8113 cell proliferation and apoptosis. J. Oral. Maxil. Surg. 2010, 20, 245–248. [Google Scholar]
- Li, J.F.; Liao, J.X.; Li, H.L.; Zhang, W.D. Inhibition of Yanhuanglian total alkalions on cell proliferation and telomerase activity of Tca8113 cell lines. J. Oral. Maxil. Surg. 2007, 17, 32–35. [Google Scholar]
- Xie, P.S.; Li, A.Y.; Zhou, F.; Zhao, Y. Experimental study on anti-tumor screening of chinese herbal medicine. Shizhen J. Tradit. Chin. Med. Res. 1995, 7, 19–20. [Google Scholar]
- Lu, G.X. Experimental report on the effect of corydalis saxicola on the respiration of tumor cells. Guangxi J. Tradit. Chin. Med. 1979, 4, 11–14. [Google Scholar]
- Zhao, Y.; Li, A.Y.; Zhou, F.; Xie, P.S.; Tan, W.J.; Lu, G.X. Experimental study on antitumor effect of Corydalis saxicola bunge. Guangxi J. Tradit. Chin. Med. 1979, 5, 27. [Google Scholar]
- Tan, W.Z.; Xie, P.S.; Li, X.J.; Zhou, F.; Li, A.Y. Effect of Corydalis saxicola on tumor cells-experimental summary of methylene blue reduction and cell staining. Guangxi J. Tradit. Chin. Med. 1979, 3, 31–35. [Google Scholar]
- Tang, C.L.; Zheng, H.; Wang, J.; Song, H.; Lu, S.Y.; Cheng, B.; Wu, F.; Zhang, H.Y.; Ruan, J.X.; Liang, Y.H. Identification of the active components of Corydalis saxicola extracted from zhuang medicine rock against the inhibition of human hepatocarcinoma cell line SMMC-7721 based on group-activity relationship. Lishizhen Med. Mater. Med. Res. 2016, 27, 2372–2375. [Google Scholar]
- Xu, R.; Liao, J.X. Effects of Yanhuanglian total alkaloids and dehydroapocavidine on NF-kappa B activity of oral carcinoma cell lines. J. Oral. Maxil. Surg. 2010, 20, 241–244. [Google Scholar]
- Lei, J.; Liao, J.X. Tetradehydroscoulerine on cell proliferation and hTERT expression of Tca8113 cell lines. J. Oral. Maxil. Surg. 2008, 18, 173–177. [Google Scholar]
- Wu, F. The Study of the Hepatoprotective Effect of Total Alkaloids from Corydalis saxicola Bunting on Metabonomics and the Metabolism of Its Active Components; Guangxi Medical University: Nanning, China, 2018. [Google Scholar]
- Meng, C.; Lu, S.Y.; Liang, Y.; Mo, X.M.; Li, W.D.; Huang, Y.; Cheng, X.; Su, Z.H.; Zheng, H. Electron microscopy study on the apoptosis and autophagy of the hepatic stellate cells induced by total alkaloids. J. Guangxi Norm. Univ. (Nat. Sci. Ed.). 2018, 36, 76–79. [Google Scholar]
- Yu, J.J.; Liu, Q.; Lu, X.; Li, X.; Li, N.; Liu, B.; Fang, H.; Qiu, Z. Inhibitory and inductive effects of Corydalis saxicola Bunting total alkaloids (CSBTA) on cytochrome P450s in rats. Phytother. Res. 2018, 32, 1818–1827. [Google Scholar] [CrossRef]
- Wu, F.; Zheng, H.; Yang, Z.T.; Cheng, B.; Wu, J.X.; Liu, X.W.; Tang, C.L.; Lu, S.Y.; Chen, Z.N.; Song, F.M. Urinary metabonomics study of the hepatoprotective effects of total alkaloids from Corydalis saxicola Bunting on carbon tetrachloride-induced chronic hepatotoxicity in rats using 1H NMR analysis. J. Pharmaceut. Biomed. 2017, 140, 199–209. [Google Scholar] [CrossRef]
- Zhou, J.G. Protective effect of total alkaloids of Corydalis saxicola on chronic liver injury in rats. Shandong Med. J. 2010, 50, 3. [Google Scholar]
- Zhou, J.G. Study of hepatoprotective effect of the total alkaloids from Corydalis saxicola Bunting of the acute chemical hepatic injury model mice. China Mod. Med. 2010, 17, 29–30. [Google Scholar]
- Bi, M.G.; Zhou, J.; Xu, Y.; Sun, H.; Ji, Y.B. Improving effect of total alkaloids extract from Corydalis thalictrifolia Franch. on immune hepatic injury in mice. Chin. J. Pharmacol. Toxicol. 2009, 23, 39–44. [Google Scholar]
- Xi, G.L. Protective Effects of Total Alkaloids in Corydalis saxicola Bunting on Liver Injuries and Meta-Analysis of Telmisartan on Blood Pressure Control; The Second Military Medical University: Shanghai, China, 2009. [Google Scholar]
- Jia. J. Protective Effects and Mechanisms of Corydalis saxicola Bunting Total Alkaloids on Experimental Liver Injury; Guangxi Medical University: Nanning, China, 2009. [Google Scholar]
- Liang, Y.H.; Gu, J.; Peter, S.S.; Mao, Y.A.; Qin, C.J. Protective effect of Corydalis saxicola Alkaloids (CSA) on level of TGF-β1, MMP-9 in rats with liver fibrosis. Lishizhen Med. Mater. Med. Res. 2008, 19, 2620–2622. [Google Scholar]
- Liang, Y.H.; Gu, J.; Peter, S.S.; Mao, Y.A.; Qin, C.J. Protective effect of Corydalis saxicola alkaloids (CSA) on CCl4-induced acute liver injury in mice. Lishizhen Med. Mater. Med. Res. 2008, 19, 2922–2923. [Google Scholar]
- Liu, X.W.; Tang, C.L.; Hua, Z.; Wu, J.X.; Fang, W.; Mo, Y.Y.; Xi, L.; Zhu, H.J.; Yin, C.L.; Bang, C. Investigation of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced liver fibrosis in rats by 1H-NMR-based metabonomics and network pharmacology approaches. J. Pharmaceut. Biomed. 2018, 159, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.H.; Tang, C.L.; Lu, S.Y.; Cheng, B.; Wu, F.; Chen, Z.N.; Song, F.; Ruan, J.X.; Zhang, H.Y.; Song, H. Serum metabonomics study of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced acute hepatotoxicity in rats by 1H NMR analysis. J. Pharmaceut. Biomed. 2016, 129, 70–79. [Google Scholar] [CrossRef]
- Meng, T.X.; Xie, L.S.; Huang, M.C. Protective effect of Corydalis saxicola Buning extract on liver injury induced by CCl4 in mice. Shanghai J. Tradit. Chin. Med. 2013, 47, 89–91. [Google Scholar]
- Huang, X.Z.; Liu, X.P.; Huang, M.; Huang, Z.Q.; Li, D.; Jiang, W.Z. Study on main pharmacodynamics and acute toxicity of herba Corydalis saxicola extract. Chin. J. Hosp. Pharm. 2007, 27, 146. [Google Scholar]
- Jiang, D.P. Protective effects of Corydalis saxicola Bunting on acute liver injury in rats. Health Vocat. Educ. 2007, 25, 119–120. [Google Scholar]
- Lu, S.Y.; Zheng, H.; Cheng, B.; Fang, W.U.; Wu, J.X.; Liu, X.W.; Tang, C.L.; Liang, Y.H.; Zhang, H.Y.; Xiang, R.J. Discrimination of proliferation inhibiting ingredients in Corydalis soxicola on rat hepatic stellate cell-T6 based on composition-activity relationship. Chin. Tradit. Herb. Drugs 2017, 48, 1354–1361. [Google Scholar]
- Rafiee, F.; Nejati, V.; Heidari, R.; Ashraf, H. Protective effect of methanolic extract of Berberis integerrima Bunge root on carbon tetrachlorideinduced testicular injury in wistar rats. Iran. J. Reprod. Med. 2016, 14, 133–140. [Google Scholar]
- Wang, T.; Sun, N.L.; Zhang, W.D.; Li, H.L.; Lu, G.C.; Yuan, B.J.; Hua, J.; She, J.H.; Zhang, C. Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J. Ethnopharmacol. 2008, 117, 300–308. [Google Scholar] [CrossRef]
- Sun, N.L.; Wang, T.; Yuan, B.L.; Li, H.L.; Lu, G.C.; Zhang, W.D.; Zhang, C.; Yuan, B.J. Protective effects of dehydrocavidine on carbon tetrachloride induced acute liver injury in mice. J. Pharm. Pract. 2008, 26, 23–27. [Google Scholar]
- Wang, J.; Zhang, S.J.; Wu, D.H.; Jiang, W.Z. Inhibitory effect of extract from Corydalis saxicola Bunting on HBV in vivo. China Pharm. 2009, 18, 7–9. [Google Scholar]
- Wu, Y.R.; Ma, Y.B.; Zhao, Y.X.; Yao, S.Y.; Zhou, J.; Gong, Q.F.; Chen, J.J. Anti-hepatitis virus constituents from Corydalis saxicola. Chin. Tradit. Herb. Drugs 2012, 43, 32–37. [Google Scholar]
- Tong, K.; Wu, L.Z.; Liang, Y.Y. Effect of total alkaloids of Corydalis saxicola on immune function of mice. Immunol. J. 1995, 11, 238–241. [Google Scholar]
- Deng, W.; Zhu, L.P.; Liu, J.; Zhou, G.L.; Yao, Q.S. Effect of yanhuanglian alkaloids on serum biochemical parameters and liver tissue antioxidant capacity in long-term feeding SD rats. J. Health Toxicol. 2010, 24, 228–230. [Google Scholar]
- Wu, C.F.; Liu, W.; Li, F.L. Effect of total alkaloids of corydalis saxicola on monoamine neurotransmitters in rat brain. J. Shenyang Pharm. Univ. 1994, 000, 101–104. [Google Scholar]
- Huang, X.N.; Liu, G.X.; Zhang, Y. Stability of total alkaloids from Corydalis saxicola. Acta Pharmacol. Sin. 1981, 2, 156–159. [Google Scholar]
- Huang, X.N.; Liu, G.X.; Zhang, X.D. Analgesic, anti-inflammatory and cholagogic effects of total alkaloids from Corydalis saxicola Bunge. Acta Acad. Med. Zunyi. 1981, 4, 22–25. [Google Scholar]
- Peng, X.C. Effects of Dehydrocavidine on Behavior and Dopamine in Striatum of Rats; Zunyi Medical University: Zunyi, China, 2008; pp. 18–22. [Google Scholar]
- Chen, C.Y.; Zhao, Y. The pharmacological study of the main component dehydrocavitin in the traditional Chinese medicine Corydalis thalictrifolia. China J. Chin. Meteria Med. 1982, 7, 31–34. [Google Scholar]
- Feng, C.; Yong, X.Z.; Jiang, Q.Z.; Wu, T.T.; Jiang, L.L.; Su, Z.H.; Tao, R.C. Inhibitory effects of Corydalis saxicola Bunting total alkaloid on M1 macrophages polarization. J. Guangxi. Med. Univ. 2020, 37, 2103–2110. [Google Scholar]
- Li, L. Experimental study on anti-inflammatory effect of corydalis saxicola. Chin. J. Ethnomed. Ethnopharm. 2009, 18, 20–21. [Google Scholar]
- Xiao, P.; Lin, C.X.; Pan, B.J.; Zhu, G.M.L.; Huang, Y.; Zeng, D.Y. Pharmacodynamics of Yanhuanglian suppository for rats with chronic pelvic inflammatory diseases. Cent. South. Pharm. 2019, 17, 2052–2058. [Google Scholar]
- Zhu, G.M.L.; Jiang, W.Z.; Xiao, P.; Huang, X.Y. Experimental study on anti-inflammatory and analgesic effects of Corydalis saxicola rectal suppository. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 11–13. [Google Scholar]
- Kuai, C.P.; Lin, J.J.; Hu, P.P.; Huang, F. Corydalis saxicola alkaloids attenuate cisplatin-induced neuropathic pain by reducing loss of IENF and blocking TRPV1 activation. Am. J. Chin. Med. 2020, 48, 407–428. [Google Scholar] [CrossRef]
- Qiu, Q.Q.; Wei, Z.P.; Wu, L.M.; Liu, Y.W.; Meng, T.X. In vitro antibacterial activity of total alkaloids from Corydalis saxicola Bunting. Sci. Technol. Innov. 2020, 9, 43–44. [Google Scholar]
- Sun, T.T.; Jiang, P.; Zhong, Z.M.; Fang, Y.; Yang, Y.Q.; Dan, H.H.; Zuo, R.H. Bacteriostasis of Corydalis Saxicola combined with antibiotics on Staphylococcus Aureus in vitro. J. Jinggangshan Univ. (Nat. Sci. Ed.) 2020, 41, 111–114. [Google Scholar]
- Ye, Q.L.; Wu, L.Z.; Li, H.; Zhang, J. Antibacterial experiment of dehydrocavidine, the main component of Corydalis saxicola Bunting. Guangxi J. Tradit. Chin. Med. 1984, 7, 48–49. [Google Scholar]
- Li, H.L. Studies on Bio-Active Constituents of Corydalis saxicola Bunting and Toxic Constituents of Veratrum nigrum Linn; The Second Military Medical University: Shanghai, China, 2006. [Google Scholar]
- Liu, X.; Zheng, H.; Lu, R.; Huang, H.; Zhu, H.; Yin, C.; Mo, Y.; Wu, J.; Liu, X.; Deng, M. Intervening effects of total alkaloids of Corydalis saxicola Bunting on rats with antibiotic-induced gut microbiota dysbiosis based on 16S rRNA gene sequencing and untargeted metabolomics analyses. Front. Pharmacol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Guo, Z.M. Studies on Antihyperlipidemic and Antioxidative Activities of the Total Alkaloids Prepared from Corydalis saxicola in Rats; Sun Yat-sen University: Guangzhou, China, 2009; pp. 35–41. [Google Scholar]
- Shi, Y.J.; Xie, H.; Mao, C.Q.; Zhang, X.D.; Zheng, X.P. Intestinal transport characteristics of dehydrocavidine. Chin. Tradit. Pat. Med. 2013, 35, 256–260. [Google Scholar]
- Institue of Materia Medica Chinese Academy of Medical Science. Chinese Medicinal Materials; People’s Medical Publishing: Beijing, China, 1979; Volume 253. [Google Scholar]
- Bian, X.X.; Zhang, G.X.; Gao, X.L. Clinical observation of Corydalis thalictrifolia injection intreating chronic hepatic jaundice. J. Pract. Med. 2008, 24, 241. [Google Scholar]
- Liu, S.P.; Qu, T. Allergic reaction caused by Corydalis saxicola injection: A case report. Tradit. Chin. Drug. Res. Clin. Pharmacol. 2004, 15, 434. [Google Scholar]
- Li, J.Y.; Huang, J.J. Curative effect analysis of 464 cases of hepatitis treated with corydalis saxicola injection. Chin. Tradit. Pat. Med. 1987, 2, 19. [Google Scholar]
- Cong, C.Y. Study on the effect of Corydalis saxicola in treating jaundice hepatitis. In Proceedings of the Ninth National Conference of Infectious Diseases of Chinese Medical Association, Chengdu, Sichuan, 12–16 October 2006; p. 362. [Google Scholar]
- Wang, Y. Clinical observation of corydalis saxicola injection combined with interventional therapy for advanced liver cancer with hepatocellular jaundice. Jilin Med. J. 2010, 31, 1737–1738. [Google Scholar]
- Zhao, Y.F. Therapeutic effects of Yanhuanglian injection on acute cholestatic hepatitis. Chin. J. Clin. Ration. Drug Use 2009, 2, 20–21. [Google Scholar]
- Shao, X.J. Observation on clinical curative effect of Corydalis saxicolae injection in that treatment of icteric hepatitis b and nurse care. J. Qiqihua Med. Coll. 2006, 27, 112–113. [Google Scholar]
- Huang, W.Q.; Song, M.N.; Min, F.; Zhang, L.; Fan, R.H.; Luo, M.L.; Wu, W.B. Clinical study of Corydalis saxicola for icterohepatitis. Mod. Med. Health 2005, 21, 389. [Google Scholar]
- Zhong, H.X. Observation and nursing of triple treatment of chronic hepatitis B jaundice with chinese patent medicine. Mod. Nurs. 2005, 11, 1253–1254. [Google Scholar]
- Zhong, H.X.; Guo, X. Effect of treatment with combined traditional chinese and western medicine in chronic B hepatitis with jaundice. J. Nurs. Sci. 2005, 20, 29–30. [Google Scholar]
- Zheng, Z.X. Curative effect observation on 93 cases of chronic hepatitis B treated with Corydalis saxicola injection. Guide China Med. 2013, 5, 256–257. [Google Scholar]
- Li, S.X. Clinical Observation of corydalis saxicola in Treating Viral Hepatitis. China Foreign Med. Treat. 2010, 29, 104. [Google Scholar]
- Zhang, X.S.; Jiang, X.R. Clinical observation of Corydalis saxicola injection on acute and chronic viral hepatitis. China Healthc. Innov. 2009, 4, 10–17. [Google Scholar]
- Jiang, H.M. Analysis of therapeutic effect of Corydalis saxicola injection on 180 cases of chronic hepatitis B. World Health Dig. Med. Theory Praltice 2008, 4, 78. [Google Scholar]
- Zhao, S.H. Clinical observation on treatment of viral hepatitis with Corydalis saxicola Bunting. Sichuan Med. J. 2003, 24, 59. [Google Scholar]
- Ren, Z.X. Efficacy of injection of Corydalis saxicolae on 33 patients with viral hepatitis. Clin. Focus 2003, 18, 94–95. [Google Scholar]
- Deng, S.R.; Long, M.; Jiang, T.; Huang, Y.N. Treatment of severe jaundice of viral hepatitis with compound salvia miltiorrhiza combined with Corydalis saxicola injection. Chin. J. Primary. Med. Pharm. 2005, 12, 1630–1631. [Google Scholar]
- Wang, S.P.; Wang, X.H.; Dong, Y.Q.; Li, F. Clinical observation on treatment of 65 case of severe cholestasis of chronic hepatitis b with magnesium isoglycyrrhizinate and Corydalis saxicola Bunting. Hubei J. Tradit. Chin. Med. 2011, 33, 12–13. [Google Scholar]
- Zhang, W.W.; Yuan, X.H.; Zhu, L.; Tan, H.B.; Zhan, G.Q.; Li, R.G. Chronic severe hepatitis B in the treatment of telbivudine combined Yanhuanglian. Shaanxi. J. Tradit. Chin. Med. 2010, 31, 20–21. [Google Scholar]
- Yin, H. Effect of Corydalis saxicola injection combined with salvia miltiorrhiza injection on hepatic fibrosis in patients with chronic hepatitis B. J. Proactical Med. 2001, 17, 782–783. [Google Scholar]
- Zhou, Y.F.; Feng, Z.T.; Jiang, C.X. Effect of Corydalis saxicola decumbentis combined with radiointervention on liver function of patients with advanced liver cancer. Spec. Health 2020, 7, 112. [Google Scholar]
- Zhou, Y.F.; Feng, Z.T.; Jiang, C.X. Effect of Corydalis saxicola decumbentis combined with radiointervention on QOL score of patients with advanced liver cancer. Health Must-Read Mag. 2020, 33, 105. [Google Scholar]
- Feng, Z.T.; Jiang, C.X.; Zhou, Y.F.; Qin, P.; Qiu, A.Y. Effect of Corydalis saxicola decumbentis combined with radiointervention on levels of serum IL-2, IFN-γ, and TNF-α in patients with advanced hepatocellular carcinoma. Spec. Health 2020, 31, 74–75. [Google Scholar]
- Feng, Z.T.; Jiang, C.X.; Zhou, Y.F. Effect of Corydalis saxicola decumbentis combined with radiointervention on serum Th1/Th2 cytokines in patients with advanced liver cancer. Health Must-Read Mag. 2020, 33, 45. [Google Scholar]
- Qin, P. Efficacy of Corydalis saxicola injection in the repair of liver damage after interventional therapy for advanced hepatocellular carcinoma with hepatocellular jaundice. Health Must-Read Mag. 2020, 32, 218. [Google Scholar]
- Jiang, C.X.; Feng, Z.T.; Zhou, Y.F.; Qin, P.; Qiu, A.Y. Effect of Corydalis saxicola decumbentis combined with radiointervention on immune function of patients with moderate and advanced stage liver cancer. Spec. Health 2020, 8, 86. [Google Scholar]
- Jiang, C.X.; Feng, Z.T.; Zhou, Y.F. Effect of Corydalis saxicola bunge combined with radiointervention on adverse reactions in patients with advanced liver cancer. Spec. Health 2020, 31, 84. [Google Scholar]
- Qin, P.; Feng, Z.T.; Jiang, C.X.; Zhou, Y.F.; Qiu, A.Y. Effect of Corydalis saxicola combined with radiotherapy on improving quality of life of patients with advanced hepatocellular carcinoma. Sci. Regimen 2020, 9, 246. [Google Scholar]
- Zhang, Z.H.; Li, S.R.; Ma, G.Y.; Kong, X.D.; Chen, W.L.; Liu, Y.; Jia, L.L. Effects of Corydalis saxicola Bunting injection combined with transcatheter arterial chemoembolization on the immune function and life quality of patients with advanced liver cancer. Hebei Med. J. 2019, 41, 2902–2905. [Google Scholar]
- Sun, Z.C. Curative effect observation and nursing care of primary liver cancer treated with Corydalis saxicolae injection combined with interventional therapy. Qinghai Med. J. 2003, 33, 39–40. [Google Scholar]
- Yuan, W.P.; Hong, J.S.; Zhao, Y.N.; Chen, J.S. Treatment of liver damage after interventional chemoembolization of hepatocellular carcinoma with Corydalis saxicola Bunting. J. Guangxi Med. Univ. 2002, 19, 257. [Google Scholar]
- Qin, P.; Feng, Z.T.; Jiang, C.X.; Zhou, Y.F.; Qiu, A.Y. Efficacy and adverse reactions of octreotide combined with Corydalis saxicola injection in the treatment of advanced liver cancer. Spec. Health 2020, 23, 28. [Google Scholar]
- Zhang, X.H. 63 cases of neonatal hyperbilirubinemia treated with Corydalis saxicola Bunting. Shaanxi J. Tradit. Chin. Med. 2013, 34, 786–787. [Google Scholar]
- Gu, F.L.; Xu, S.S.; Shi, C.G. Therapeutic effects of Corydalis saxicola Bunting injection on advanced rectal cancer. China Mod. Doctor. 2020, 58, 107–109. [Google Scholar]
- Tai, Y.R.; Yin, M.S.; Pu, H.X.; Han, X.J. Observation of 60 hfrs cases cured with radix Corydalis Theaictrifoliae. Chin. J. Pract. Chin. Mod. Med. 2004, 4, 2746–2747. [Google Scholar]
- Wang, D.; Wang, Y.K. Comparison of therapeutic effects of shengmai and corydalis saxicola on liver cirrhosis. RenShen Yan Jiu 2002, 14, 28–29. [Google Scholar]
- Fang, S.X.; Dong, X.F.; Jie, S.H.; Ye, J. 40 cases of acute icteric hepatitis treated by Corydalis saxicola injection. Her. Med. 2001, 20, 567. [Google Scholar]
- Tang, Y.L. Clinical analysis of Corydalis saxicola combined with Yinzhihuang in treating icteric hepatitis. Med. Inf. 2009, 22, 2452–2453. [Google Scholar]
- Jiang, S.; You, S.G. Treatment of 33 case of icteric viral hepatitis with Corydalis saxicola Bunting and compound glycyrrhizin. Her. Med. 2008, 27, 403–404. [Google Scholar]
- Liu, L.; Zhang, G.H. Treatment of 31 cases of viral hepatitis and hyperbilirubinemia with Corydalis saxicola Bunting. Chin. J. Pharmacoepidemiol. 2001, 10, 182. [Google Scholar]
- Su, G.Q.; Wang, B.C.; Liu, S.F.; Li, J.; Ma, L.W.; Liu, L. Short-term therapeutic effect of Corydalis saxicola injection on chronic hepatitisb. Chin. J. Curr. Tradit. West. Med. 2004, 2, 117–118. [Google Scholar]
- Zhang, W.W.; Qin, M.H. 43 cases of chronic severe hepatitis treated with Corydalis saxicola Bunting and breviscapine injection. Shaanxi. J. Tradit. Chin. Med. 2006, 27, 21–23. [Google Scholar]
- Wan, S.C.; Gao, H.M. Treatment over 50 cases of chronic hepatitis B with GSH and Yanhuanglian. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 2002, 2, 13. [Google Scholar]
- Xiong, L.G.; Li, L.L. Clinical observation of Octreodide combined with Corydalis in the treatment of advanced primary liver cancer. Sichuan J. Cancer Control 2005, 18, 232–234. [Google Scholar]
- Li, X.Y.; Tian, L.T. Treatment of 54 cases of hyperbilirubinemia with corydalis saxicola injection. Mod. Tradit. Chin. Med. 2003, 5, 30. [Google Scholar]


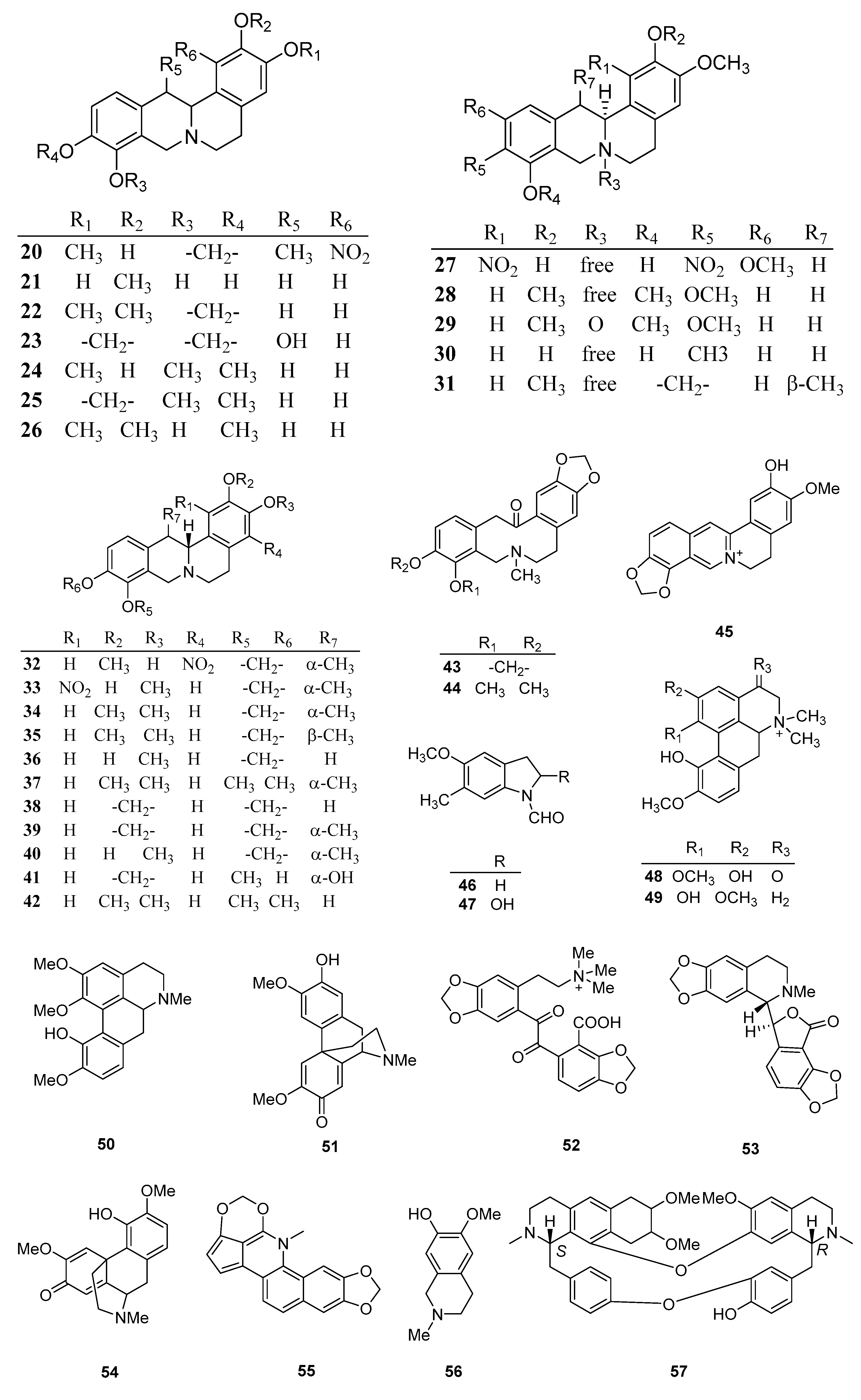


| NO. | Name | Molecular Formula | Molecular Weight | Plant Parts | References |
|---|---|---|---|---|---|
| 1 | dehydrocavidine | C21H20NO4 | 350.14 | Whole plant | [17] |
| 2 | dehydroapocavidine | C20H18NO4 | 336.12 | Root | [18] |
| 3 | dehydroisoapocavidine | C20H18NO4 | 336.12 | Whole plant | [15] |
| 4 | berberine | C20H18NO4 | 336.12 | Whole plant | [17] |
| 5 | dehydroisocorypalmine | C20H20NO4 | 338.14 | Whole plant | [15] |
| 6 | coptisine | C19H14NO4 | 320.09 | Root | [18] |
| 7 | tetradehydroscoulerine | C19H18NO4 | 324.12 | Whole plant | [15] |
| 8 | dehydrocheilanthifoline | C19H16NO4 | 322.11 | Whole plant | [19] |
| 9 | palmatine | C21H22NO4 | 352.15 | Root | [18] |
| 10 | dehydrodiscretamine | C19H18NO4 | 324.12 | Whole plant | [19] |
| 11 | thalifaurine | C19H16NO4 | 322.11 | Root | [18] |
| 12 | jatrorrhizine | C20H20NO4 | 338.14 | Whole plant | [13] |
| 13 | berberrubine | C19H16NO4 | 322.11 | Whole plant | [20] |
| 14 | sanguinarine | C20H14NO4 | 332.09 | Whole plant | [6] |
| 15 | chelerythrine | C21H18NO4 | 348.12 | Whole plant | [17] |
| 16 | 6-acetonyl-5,6-dihydrosanguinarine | C23H19NO5 | 389.13 | Root | [18] |
| 17 | dihydrosanguinarine | C20H15NO4 | 333.10 | Root | [18] |
| 18 | dihydrochelerythrine | C21H19NO4 | 349.13 | Root | [18] |
| 19 | 8-acetonyldihydrochelerythrine | C24H23NO5 | 405.16 | Whole plant | [6] |
| 20 | 1-nitro-apocavidine | C20H20N2O6 | 384.13 | Whole plant | [14] |
| 21 | 2, 9, 10-thrihydroxy-3-methoxytetrahydroprotoberberine | C18H19NO4 | 313.13 | Whole plant | [16] |
| 22 | sinactine | C20H21NO4 | 339.15 | Whole plant | [16] |
| 23 | (−)-13β-hydroxystylopine | C19H17NO5 | 339.34 | Whole plant | [17] |
| 24 | tetrahydrocolumbamine | C20H23NO4 | 341.16 | Whole plant | [17] |
| 25 | canadine | C20H21NO4 | 339.15 | Whole plant | [2] |
| 26 | tetrahydropalmatrubine | C20H23NO4 | 341.16 | Whole plant | [16] |
| 27 | (−)-2,9-dihydroxyl-3,11-dimethoxy-1,10-dinitrotetrahydroprotoberberine | C19H19N3O8 | 417.12 | Whole plant | [6] |
| 28 | (+)-tetrahydropalmatine | C21H25NO4 | 355.15 | Whole plant | [17] |
| 29 | (−)-corynoxidine | C21H25NO5 | 371.17 | Whole plant | [6] |
| 30 | (−)-scoulerine | C19H21NO4 | 327.15 | Whole plant | [17] |
| 31 | (−)-cavidine | C21H23NO4 | 353.16 | Root | [18] |
| 32 | (+)-4-nitroisoapocavidine | C20H20N2O6 | 384.13 | Whole plant | [6] |
| 33 | (+)-1-nitroapocavidine | C20H20N2O6 | 384.13 | Whole plant | [6] |
| 34 | (±)-cavidine | C21H23NO4 | 353.16 | Whole plant | [17] |
| 35 | (+)-thalictrifoline | C21H23NO4 | 353.16 | Whole plant | [21] |
| 36 | (+)-cheilanthifoline | C19H19NO4 | 325.13 | Whole plant | [16] |
| 37 | corydaline | C22H27NO4 | 369.19 | Root | [18] |
| 38 | stylopine | C19H17NO4 | 323.12 | Root | [18] |
| 39 | mesotetrahydrocorysamine | C20H19NO4 | 337.13 | Whole plant | [16] |
| 40 | apocavidine | C20H21NO4 | 339.15 | Whole plant | [16] |
| 41 | 13-β-hydroxystylopine | C19H17NO5 | 339.11 | Whole plant | [16] |
| 42 | (−)-tetrahydropalmatine | C21H25NO4 | 355.18 | Whole plant | [19] |
| 43 | protopine | C20H19NO5 | 353.13 | Whole plant | [17] |
| 44 | allocryptopine | C21H23NO5 | 369.16 | Whole plant | [20] |
| 45 | berbinium | C19H16NO4+ | 322.11 | Whole plant | [15] |
| 46 | 1-formyl-5-methoxy-6-methylindoline | C11H13NO2 | 191.09 | Whole plant | [15] |
| 47 | 1-formyl-2-hydroxy-5-methoxy-6-methylindoline | C11H13NO3 | 207.09 | Whole plant | [15] |
| 48 | saxicolaline A | C20H22NO5 | 356.15 | Root | [18] |
| 49 | (+)-magnoflorine | C20H24NO4 | 342.17 | Root | [18] |
| 50 | (+)-isocorydine | C20H23NO4 | 341.16 | Whole plant | [19] |
| 51 | (−)-pallidine | C19H21NO4 | 327.15 | Whole plant | [19] |
| 52 | N-methylnarceimicine | C22H22NO8 | 428.13 | Root | [18] |
| 53 | adlumidine | C20H17NO6 | 367.11 | Root | [18] |
| 54 | (−)-salutaridine | C19H21NO4 | 327.15 | Root | [18] |
| 55 | cavidilinine | C19H13NO4 | 319.08 | Whole plant | [20] |
| 56 | corypalline | C11H15NO2 | 193.11 | Whole plant | [19] |
| 57 | oxyacanthine | C37H40N2O6 | 608.29 | Whole plant | [22] |
| 58 | corydalisin A | C37H38N2O9 | 654.26 | aerial parts | [1] |
| 59 | corydalisin B | C38H40N2O10 | 684.27 | aerial parts | [1] |
| 60 | cannabisin F | C36H36N2O8 | 624.25 | aerial parts | [1] |
| 61 | corydalisin C | C38H40N2O10 | 684.27 | aerial parts | [1] |
| 62 | cannabisin D | C36H36N2O8 | 624.25 | aerial parts | [1] |
| 63 | 1,2-dihydro-6,8-dimethoxy-7-hydroxyl-1-(3,5-dimethoxy-4-hydroxyphenyl)-N1, N2-bis[2-(4-hydroxyphenyl)ethyl]-2,3-naphthalene dicarboxamide | C38H40N2O10 | 684.27 | aerial parts | [1] |
| 64 | cannabisin E | C36H38N2O9 | 642.26 | aerial parts | [1] |
| 65 | grossamide | C36H36N2O8 | 624.25 | aerial parts | [1] |
| 66 | feruloylagmatine | C15H22N4O3 | 306.17 | Whole plant | [19] |
| 67 | corysaxicolaine A | C39H30N2O8 | 654.20 | aerial parts | [7] |
| 68 | β-sitosterol | C29H50O | 414.39 | Whole plant | [22] |
| 69 | daucosterol | C35H60O6 | 576.44 | Whole plant | [22] |
| 70 | betulin | C30H50O2 | 484.43 | Whole plant | [22] |
| 71 | betulinic acid | C30H48O3 | 498.41 | Whole plant | [22] |
| 72 | β-amyrin | C30H50O | 426.39 | Whole plant | [20] |
| 73 | β-amyrin acetate | C32H52O2 | 468.40 | Whole plant | [22] |
| 74 | (+)-oleanolic acid | C30H48O3 | 454.38 | Whole plant | [22] |
| 75 | cholesterol | C27H46O | 386.35 | Whole plant | [23] |
| 76 | cycloeucalenol | C30H50O | 426.39 | Whole plant | [22] |
| 77 | 5-hydroxy-3′, 4′, 6, 7-tetramethoxyflavone | C19H18O7 | 358.11 | Whole plant | [20] |
| 78 | quercetin-3-O-β-D-galactoside | C21H20O12 | 461.10 | Whole plant | [20] |
| 79 | tetra-amino-27 alkane | C27H57N | 395.45 | Whole plant | [23] |
| 80 | tetra-amino-28 alkane | C28H59N | 409.46 | Whole plant | [23] |
| 81 | uracil | C4H4N2O2 | 112.03 | Whole plant | [20] |
| Tested Substance | Assay, Organism, or Cell Line | Biological Results | References | |
|---|---|---|---|---|
| Anticancer activity | CSBTA | Walker 256 induced bone pain and osteoporosis in rats, Breast Cancer Cells, RAW 264.7 macrophage cells, Walker 256 cells | Improved bone pain and osteoporosis in rats, suppressed expression of Rankl, down regulated the ratio of RANKL/OPG, inhibited pathways of NF-κB and c-Fos/NFATc1 to suppressed osteoclast formation | [25] |
| CSBTA | A549 cells | Inhibition migration of A549 cells by suppressing Cdc42 or Vav1 | [8] | |
| CSBTA | A549 cells | Inhibited tumor growth by down-regulating Survivin, reduced the degree of bone destruction | [26] | |
| CSBTA | A549 cells | Inhibited the migration ability of A549 cells, decreased the expression of Cdc42 protein | [27] | |
| CSBTA | A549 cells | Inhibition proliferation, induced apoptosis and up regulation of caspase and down of survivin | [28] | |
| CSBTA and cis-platinum | A549 cells | Inhibition proliferation | [29] | |
| CSBTA | Tca8113 cells | Induced apoptosis and suppression of Bcl-2 | [30] | |
| CSBTA | Tca8113 cells | Inhibition proliferation, induced apoptosis | [31] | |
| CSBTA | Tca8113 cells | Inhibition proliferation and telomerase activity | [32] | |
| CSBTA | CNE-1 | 112.41 µg/mL (IC50) | [23] | |
| CSBTA | CNE-2 | 123.46 µg/mL (IC50) | [23] | |
| CSBTA | A2780 | 148.40 µg/mL (IC50) | [23] | |
| CSBTA | SKOV3 | 128.51 µg/mL (IC50) | [23] | |
| CSBTA | PM2 | 166.66 µg/mL (IC50) | [23] | |
| CSB injection | mouse sarcoma S180 | The average tumor inhibition rate was more than 30% | [33] | |
| CSB injection | ehrlich ascites tumor | The average tumor inhibition rate was more than 30% | [33] | |
| CSB injection and aristolochic acid | mouse sarcoma S180 | The average tumor inhibition rate was more than 50% | [33] | |
| CSB injection and aristolochic acid | ehrlich ascites tumor | The average tumor inhibition rate was more than 50% | [33] | |
| CSB injection | mouse sarcoma S180 | Some inhibition and inhibited respiration | [17] | |
| CSB injection | ehrlich ascites tumor | Some inhibition and inhibited respiration | [17] | |
| CSB injection | liver cancer | Some inhibition and inhibited respiration | [17] | |
| CSB injection | ascites cancer | Some inhibition and inhibited respiration | [17] | |
| CSB injection | rat sarcoma 256 | Some inhibition and inhibited respiration | [17] | |
| CSB injection | mouse peritoneal macrophages | Significantly enhanced phagocytosis | [17] | |
| CSB injection | S180 | Inhibition of respiratory metabolism | [34] | |
| CSB injection | HAC | Inhibition of respiratory metabolism | [34] | |
| CSB injection | EAC | Inhibition of respiratory metabolism | [34] | |
| CSB injection | S180, HAC, EAC, W256, rat | Significantly inhibition | [35] | |
| CSB injection | S180, HAC, EAC, W256 | Killing effect | [36] | |
| aqueous extract | HepG2 | Inhibition proliferation and migration | [25] | |
| Corydalisin A | MGC-803 | 83.56 ±1.89 μM (IC50) | [1] | |
| Corydalisin A | HepG2 | > 100 μM (IC50) | [1] | |
| Corydalisin A | T24 | > 100 μM (IC50) | [1] | |
| Corydalisin A | NCI-H460 | > 100 μM (IC50) | [1] | |
| Corydalisin A | Spca-2 | > 100 μM (IC50) | [1] | |
| Corydalisin B | MGC-803 | > 100 μM (IC50) | [1] | |
| Corydalisin B | HepG2 | > 100 μM (IC50) | [1] | |
| Corydalisin B | T24 | > 100 μM (IC50) | [1] | |
| Corydalisin B | NCI-H460 | > 100 μM (IC50) | [1] | |
| Corydalisin B | Spca-2 | > 100 μM (IC50) | [1] | |
| Corydalisin C | MGC-803 | 8.81 ±2.05 μM (IC50) | [1] | |
| Corydalisin C | HepG2 | 22.23 ±1.85 μM (IC50) | [1] | |
| Corydalisin C | T24 | 9.62 ±1.46 μM (IC50) | [1] | |
| Corydalisin C | NCI-H460 | 25.79 ±1.04 μM (IC50) | [1] | |
| Corydalisin C | Spca-2 | 17.28 ±1.29 μM (IC50) | [1] | |
| Cannabisin F | MGC-803 | 10.10 ±1.15 μM (IC50) | [1] | |
| Cannabisin F | HepG2 | 38.93 ±1.07 μM (IC50) | [1] | |
| Cannabisin F | T24 | 11.54 ±1.49 μM (IC50) | [1] | |
| Cannabisin F | NCI-H460 | 30.96 ±1.27 μM (IC50) | [1] | |
| Cannabisin F | Spca-2 | 22.23 ±1.44 μM (IC50) | [1] | |
| Cannabisin E | MGC-803 | > 100 μM (IC50) | [1] | |
| Cannabisin E | HepG2 | > 100 μM (IC50) | [1] | |
| Cannabisin E | T24 | 46.54 ±1.62 μM (IC50) | [1] | |
| Cannabisin E | NCI-H460 | > 100 μM (IC50) | [1] | |
| Cannabisin E | Spca-2 | > 100 μM (IC50) | [1] | |
| Cannabisin D | MGC-803 | > 100 μM (IC50) | [1] | |
| Cannabisin D | HepG2 | > 100 μM (IC50) | [1] | |
| Cannabisin D | T24 | > 100 μM (IC50) | [1] | |
| Cannabisin D | NCI-H460 | > 100 μM (IC50) | [1] | |
| Cannabisin D | Spca-2 | > 100 μM (IC50) | [1] | |
| 1,2-dihydro-6,8-dimethoxy-7-hydroxy-1-(3,5-dimethoxy-4- hydroxyphenyl)-N 1, N 2 -bis [2-(4-hydroxyphenyl) ethyl]-2,3-naphthalene dicarboxamide | MGC-803; HepG2;T24; NCI-H460; Spca-2 | 55.16 ±0.78 μM (IC50); > 100 μM (IC50); 48.15 ±1.09 μM (IC50); > 100 μM (IC50); 43.89 ±1.57 μM (IC50) | [1] | |
| grossamide | MGC-803 | 26.95 ±1.24 μM (IC50) | [1] | |
| grossamide | HepG2 | 40.75 ±0.88 μM (IC50) | [1] | |
| grossamide | T24 | 21.19 ±1.53 μM (IC50) | [1] | |
| grossamide | NCI-H460 | 36.38 ±1.39 μM (IC50) | [1] | |
| grossamide | Spca-2 | 27.22 ±1.72 μM (IC50) | [1] | |
| Dehydrocavidine | SMMC-7721 | Significantly inhibition | [37] | |
| palmatine | SMMC-7721 | Significantly inhibition | [37] | |
| Dehydrocavidine | Tca8113 | Significantly inhibition, suppression of NF-kappa B, P50 and P60 | [38] | |
| CSBTA | Tca8113 | Significantly inhibition, suppression of NF-kappa B, P50 and P60 | [38] | |
| Dehydrocavidine | Tca8113 | Inhibition proliferation, telomerase activity and the expression of hTERT | [39] | |
| Pallidine | DNA topoisomerase I | Strong inhibitory effect on human DNA topoisomerase I | [19] | |
| scoulerine | DNA topoisomerase I | Strong inhibitory effect on human DNA topoisomerase I | [19] | |
| chelerythrine | unknown | Have certain anticancer effect | [21] | |
| (−)-13β-hydroxystylopine | unknown | Have certain anticancer effect | [21] | |
| Corysaxicolaine A | T24 | 7.63 μM (IC50) | [7] | |
| Corysaxicolaine A | A549 | 13.32 μM (IC50) | [7] | |
| Corysaxicolaine A | HepG2 | 12.39 μM (IC50) | [7] | |
| Corysaxicolaine A | MGC-803 | 9.98 μM (IC50) | [7] | |
| Corysaxicolaine A | SKOV3 | 12.36 μM (IC50) | [7] | |
| Hepatoprotective effects | CSBTA | rats | Interventional treatment of chronic liver injury | [40] |
| CSBTA | HSC-T6 | Induced apoptosis and autophagy | [41] | |
| CSBTA | CYP450s in rats | CYP1A2 (IC50, 38.08 μg/mL; Ki, 14.3 μg/mL), CYP2D1 (IC50, 20.89 μg/mL; Ki, 9.34 μg/mL), CYP2C6/11 (IC50 for diclofenac and S-mephenytoin, 56.98 and 31.59 μg/mL; Ki, 39.0 and 23.8 μg/mL), CYP2B1 (IC50, 48.49 μg/mL; )Ki, 36.3 μg/mL) | [42] | |
| CSBTA | chronic hepatotoxicity in rats | Restored the levels of 2-oxoglutarate, citrate, hippurateand taurine | [43] | |
| CSBTA | acute hepatic injury rats | Significantly reduced the content of AST, ALT | [44] | |
| chronic hepatic injury rats | Significantly increased the level of serum TP, reduced the content of AST, ALT, AKP, LN and HA | [45] | ||
| CSBTA | immune hepatic injury rat | Reduced serum GOT activity, IL-4, increased the rate of IFN-γ/IL-4 | [46] | |
| CSBTA | rats | Have certain preventive and therapeutic effect on acute liver injury and on chronic liver fibrosis | [47] | |
| CSBTA | rats | Obvious protective effect on acute liver injury, inhibited the formation of chronic liver fibrosis | [48] | |
| CSBTA | hepatic fibrosis rats | Inhibited the expression of TGF-β1 and MMP-9 | [49] | |
| CSBTA | acute hepatic injury rats | Increased the content of AST, ALT and SOD, reduced MDA | [50] | |
| aqueous extract | liver fibrosis in rats | Regulated the level of some amino acids, identified 157 potential targets of CS and265 targets of liver fibrosis | [51] | |
| aqueous extract | acute hepatic injury rats | Improved deviations of metabolites (soleucine, alanine, glutamine, phosphocholine and glycerol) | [52] | |
| aqueous extract | acute hepatic injury rats | Increased the content of AST, ALT and SOD, reduced MDA | [53] | |
| aqueous extract | acute hepatic injury rats | Reduced the contents of AST and ALTpromote the production of mouse hemolysin antibody LD50 = 298.5 mg·kg-1 | [54] | |
| aqueous extract | acute hepatic injury rats | Reduced the content of AST and ALT | [55] | |
| Dehydrocavidine | HSC-T6 | Inhibition proliferation, induced apoptosis | [56] | |
| palmatine | HSC-T6 | Inhibition proliferation, induced apoptosis | [56] | |
| berberine | HSC-T6 | Inhibition proliferation, induced apoptosis | [56] | |
| Dehydrocavidine | hepatic fibrosis rats | Reduced hepatic hydroxyproline, increases urinary hydroxyproline | [57] | |
| Dehydrocavidine | liver injury in rats | Down regulated EPHX2, HYOU1, GSTM3, Sult1a2 and P450, reduce free radical, lose weight, MDA, ALT, AST, ALP and TBIL | [58] | |
| Dehydrocavidine | liver injury in rats | Increased ALT, AST and TBIL, Reduces the inflammatory cell infiltration of cell degeneration and necrosis and damages the ultrastructure of liver cells | [59] | |
| Anti-HBV activity | extract | Duck hepatitis B virus | Reduced DHBV-DNA | [60] |
| total extract of root | HBsAg | 0.17 mg/mL (IC50) | [18] | |
| total extract of root | HBeAg | <0.04 mg/mL (IC50) | [18] | |
| Saxicolalines A | HBsAg | 2.19 μM (IC50) | [18] | |
| Saxicolalines A | HBeAg | >2.81μM (IC50) | [18] | |
| N-methylnarceimicine | HBsAg | 1.22 μM (IC50) | [18] | |
| N-methylnarceimicine | HBeAg | 1.84 μM (IC50) | [18] | |
| 6-acetonyl-5,6-dihydrosanguinarine | HBsAg | 6.55 μM (IC50) | [18] | |
| 6-acetonyl-5,6-dihydrosanguinarine | HBeAg | >2.54 μM (IC50) | [18] | |
| dihydrochelerythrine | HBsAg | <0.05 μM (IC50) | [18] | |
| dihydrochelerythrine | HBeAg | <0.05 μM (IC50) | [18] | |
| adlumidine | HBsAg | 1.35 μM (IC50) | [18] | |
| adlumidine | HBeAg | >2.73 μM (IC50) | [18] | |
| (−)-salutaridine | HBsAg | 0.26 μM (IC50) | [18] | |
| (−)-salutaridine | HBeAg | 0.43 μM (IC50) | [18] | |
| palmatine | HBsAg | >4.26 μM (IC50) | [18] | |
| palmatine | HBeAg | >4.26 μM (IC50) | [18] | |
| protopine | HBsAg | 2.61 μM (IC50) | [18] | |
| protopine | HBeAg | >4.25 μM (IC50) | [18] | |
| coptisine | HBsAg | 2.74 μM (IC50) | [18] | |
| coptisine | HBeAg | 3.19 μM (IC50) | [18] | |
| (+)-magnoflorine | HBsAg | >4.39 μM (IC50) | [18] | |
| (+)-magnoflorine | HBeAg | >4.39 μM (IC50) | [18] | |
| dehydrocheilanthifoline | HepG2.2.15 | 115.95 μM (CC50) | [3] | |
| dehydrocheilanthifoline | HBsAg | 15.84 ± 0.36 μM (IC50) | [3] | |
| dehydrocheilanthifoline | HBeAg | 17.12 ± 0.45 μM (IC50) | [3] | |
| dehydrocheilanthifoline | Extracellular DNA | 15.08 ± 0.66 μM (IC50) | [3] | |
| dehydrocheilanthifoline | Intracellular DNA | 7.62 ± 0.24 μM (IC50) | [3] | |
| dehydrocheilanthifoline | Intracellular cccDNA | 8.25 ± 0.43 μM (IC50) | [3] | |
| Crude extract | HBsAg | 0.16 mg/mL (IC50) | [61] | |
| Crude extract | HBeAg | < 0.04 mg/mL (IC50) | [61] | |
| 6-acetonyl-5, 6-dihydrosanguinarine | HBsAg | 0.65 mg/mL (IC50) | [61] | |
| 6-acetonyl-5, 6-dihydrosanguinarine | HBeAg | >1.00 mg/mL (IC50) | [61] | |
| dihydrochelerythrine | HBsAg | <0.02 mg/mL (IC50) | [61] | |
| dihydrochelerythrine | HBeAg | <0.02 mg/mL (IC50) | [61] | |
| adlumidine | HBsAg | 0.50 mg/mL (IC50) | [61] | |
| adlumidine | HBeAg | >1.00 mg/mL (IC50) | [61] | |
| (−)-salutaridine | HBsAg | 0.09 mg/mL (IC50) | [61] | |
| (−)-salutaridine | HBeAg | 0.15 mg/mL (IC50) | [61] | |
| palmatine | HBsAg | >1.50 mg/mL (IC50) | [61] | |
| palmatine | HBeAg | >1.50 mg/mL (IC50) | [61] | |
| protopine | HBsAg | 0.92 mg/mL (IC50) | [61] | |
| protopine | HBeAg | >1.50 mg/mL (IC50) | [61] | |
| coptisine | HBsAg | 0.88 mg/mL (IC50) | [61] | |
| coptisine | HBeAg | >1.02 mg/mL (IC50) | [61] | |
| (+)-magnoflorine | HBsAg | >1.50 mg/mL (IC50) | [61] | |
| (+)-magnoflorine | HBeAg | 1.50 mg/mL (IC50) | [61] | |
| dehydrocavidine | HBsAg | 33% inhibition [62.5 μg/mL] | [15] | |
| dehydrocavidine | HBeAg | 22% inhibition [62.5 μg/mL] | [15] | |
| dehydroapocavidine | HBsAg | 39% inhibition [62.5 μg/mL] | [15] | |
| dehydroapocavidine | HBeAg | 24% inhibition [62.5 μg/mL] | [15] | |
| dehydroisoapocavidine | HBsAg | 29% inhibition [62.5 μg/mL] | [15] | |
| dehydroisoapocavidine | HBeAg | 23% inhibition [62.5 μg/mL] | [15] | |
| berberine | HBsAg | 8% inhibition [62.5 μg/mL] | [15] | |
| berberine | HBeAg | 7% inhibition [62.5 μg/mL] | [15] | |
| dehydroisocorypalmine | HBsAg | 6% inhibition [62.5 μg/mL] | [15] | |
| dehydroisocorypalmine | HBeAg | 6% inhibition [62.5 μg/mL] | [15] | |
| coptisine | HBsAg | 6% inhibition [62.5 μg/mL] | [15] | |
| coptisine | HBeAg | 9% inhibition [62.5 μg/mL] | [15] | |
| tetradehydroscoulerine | HBsAg | 7% inhibition [62.5 μg/mL] | [15] | |
| tetradehydroscoulerine | HBeAg | 6% inhibition [62.5 μg/mL] | [15] | |
| berbinium | HBsAg | 9% inhibition [62.5 μg/mL] | [15] | |
| berbinium | HBeAg | 6% inhibition [62.5 μg/mL] | [15] | |
| 1-formyl-5-methoxy-6-methylindoline | HBsAg | 2% inhibition [62.5 μg/mL] | [15] | |
| 1-formyl-5-methoxy-6-methylindoline | HBeAg | 7% inhibition [62.5 μg/mL] | [15] | |
| 1-formyl-2-hydroxy-5-methoxy-6-methylindoline | HBsAg | 5% inhibition [62.5 μg/mL] | [15] | |
| 1-formyl-2-hydroxy-5-methoxy-6-methylindoline | HBeAg | 3% inhibition [62.5 μg/mL] | [15] | |
| Enhancement of immune function | CSBTA | rats | CSBTA (40 μg/mL) began to enhance, enhanced the levels of T cell production of IFN-γ and IL-2 | [62] |
| Antioxidant activity | CSBTA | rats | Reduced the of content MDA and increase SOD activity, enhance the antioxidant capacity of rat liver | [63] |
| cavidine | DPPH assay | 6.85 mg/mL (IC50) | [2] | |
| cheilanthifoline | DPPH assay | 0.25 mg/mL (IC50) | [2] | |
| tetrahydropalmatine | DPPH assay | 3.79 mg/mL (IC50) | [2] | |
| stylopine | DPPH assay | 2.56 mg/mL (IC50) | [2] | |
| canadine | DPPH assay | 2.18 mg/mL (IC50) | [2] | |
| dehydrocavidine | DPPH assay | 16.51 mg/mL (IC50) | [2] | |
| dehydrocheilanthifoline | DPPH assay | 1.63 mg/mL (IC50) | [2] | |
| berberine | DPPH assay | 7.40 mg/mL (IC50) | [2] | |
| pallidine | DPPH assay | 1.00 mg/mL (IC50) | [2] | |
| Effects on the central nervous system | CSBTA | rats | Reduced the content of DOPAC, HVA, 5-HT and 5-HIAA, the level of DA has no effect (50, 100 mg/kg CSBTA) | [64] |
| CSBTA | rats | Reduced activity (25 mg/kg CSBTA) | [65] | |
| CSBTA | monkey | Reduced activity (12 mg/kg CSBTA) | [65] | |
| CSBTA | cats | Reduced activity (10-15 mg/kg CSBTA) | [65] | |
| CSBTA | rats | Reduced irritated response (50 mg/kg CSBTA) | [65] | |
| CSBTA | rats | 77% suppressed conditional emission (50 mg/kg CSBTA) | [65] | |
| CSBTA | rats | Increased the hypnotic time of pentobarbital sodium by more than 2 to 4 times (25 mg/kg CSBTA) | [65] | |
| CSBTA | rabbit | Activity slow down (20-30 mg/kg CSBTA) | [65] | |
| CSBTA | rats | LD50 = 223 mg/kg | [65] | |
| CSBTA | rats | Reduced the arthritis (50 mg/kg CSBTA) | [66] | |
| Dehydrocavidine | rats | Reduced the content of DA and HVA | [67] | |
| Dohydrocyaidine | rats | Reduced spontaneous activity | [68] | |
| Dohydrocyaidine | rats | Synergistic effect with barbiturates | [68] | |
| Anti-inflammatory activity | CSBTA | M1 macrophages | Obvious toxic effect on the activity of M1-Mφ, significantly reduced the mRNA level of IL-6, TNF-α, CD86, IL-1β | [69] |
| CSBTA | rats | Significantly inhibited the addition of capillary permeability, and suppressed exudation, edema and connective tissue hyperplasia | [70] | |
| Corydalis saxicola suppository | chronic pelvic inflammatory disease model rats | Significantly inhibited the uterine swelling, significantly reduced the spleen index, hemameba, neutrophil, TNF-α, IL-6 and MDA, improved thymus index, ovary index, lgG, lgM and SOD | [71] | |
| Corydalis saxicola rectal suppository | rats | Obvious inhibited ear swelling, the addition of capillary permeability, and writhing reaction in rat | [72] | |
| Analgesic effect | CSBTA | rats | Reduced the level of proinflammatory cytokines, such as TNF-α, IL-1β and PGE2. inhibited the overexpression level of DRG, TG, p-p38 and TRPV1 | [73] |
| CSBTA | rats | Inhibited the "writhing reaction" in rat (50 mg/kg CSBTA), improve the "pain closure" of rats to heat stimulation (100 mg/kg CSBTA) | [66] | |
| Dohydrocyaidine | rats | The effects of sedative, analgesic, and spasmolysis, LD50 = 71.6±2.92 mg/kg | [68] | |
| deheydrocavidine | unknown | Have certain sedative and analgesic effects | [21] | |
| Antibacterial activity | CSBTA | staphylococcus aureus | 17.8 mg/mL (MIC) | [74] |
| CSBTA | staphylococcus aureus | 70.0 mg/mL (MBC) | [74] | |
| CSBTA | streptococcus pyogenes | 20.5 mg/mL (MIC) | [74] | |
| CSBTA | streptococcus pyogenes | 70.0 mg/mL (MBC) | [74] | |
| CSBTA | streptococcus faecalis | 17.8 mg/mL (MIC) | [74] | |
| CSBTA | 70.0 mg/mL (MBC) | [74] | ||
| CSBTA | escherichia coli | 35.5 mg/mL (MIC) | [74] | |
| CSBTA | 70.0 mg/mL (MBC) | [74] | ||
| CSBTA | pseudomonas aeruginosa | 70.0 mg/mL (MIC) | [74] | |
| CSBTA | >300 mg/mL (MBC) | [74] | ||
| CSBTA | shigella flexneri | 16.8 mg/mL (MIC) | [74] | |
| CSBTA | 35.5 mg/mL (MBC) | [74] | ||
| CSBTA | salmonella typhi | 35.5 mg/mL (MIC) | [74] | |
| CSBTA | 70.0 mg/mL (MBC) | [74] | ||
| CSBTA | salmonella enteritidis | 16.8 mg/mL (MIC) | [74] | |
| CSBTA | 70.0 mg/mL (MBC) | [74] | ||
| CSBTA | klebsiella pneumoniae | 70.0 mg/mL (MIC) | [74] | |
| CSBTA | 130.0 mg/mL (MBC) | [74] | ||
| CSBTA | proteus | 70.0 mg/mL (MIC) | [74] | |
| CSBTA | 130.0 mg/mL (MBC) | [74] | ||
| CSBTA | candida albicans | 130.0 mg/mL (MIC) | [74] | |
| CSBTA | > 300 mg/mL (MBC) | [74] | ||
| extract | staphylococcus aureus | 20.0 mg/mL (MIC) | [75] | |
| extract +Cefradine | staphylococcus aureus | 0.375 (FIC) synergistic effect | [75] | |
| extract + Levofloxacin | staphylococcus aureus | 0.5 (FIC) synergistic effect | [75] | |
| extract + Fosfomycin | staphylococcus aureus | 1.5 (FIC) irrelevant effect | [75] | |
| extract + Penicillin | staphylococcus aureus | 0.375 (FIC) synergistic effect | [75] | |
| dehydrocarvidine | gram-positive strains; gram-negative bacterium | Have certain inhibitory effect on gram-positive strains, minimum concentration is 0.078 mg/mL, and has no inhibitory effect on gram-negative bacteria | [76] | |
| deheydrocavidine | staphylococcus aureus; beta hemolytic streptococcus; corynebacterium diphtheriae; penicillin-resistant white staphylococcus aureus | Have an inhibiting effect | [21] | |
| dohydrocyaidine | rats | Antibacterial effect | [68] | |
| ChOleretic effects | CSBTA | guinea pig | Bile secretion is temporarily reduced | [66] |
| extract | rats | Increased the amount of bile excretion | [77] | |
| Other activities | CSBTA | rats | Intervention of host co-metabolism and intestinal flora in rats with intestinal flora imbalance | [78] |
| CSBTA | rats | Significantly decreased the levels of plasma total cholesterol and low-density lipoprotein cholesterol in rats, regulated blood lipid levels in high-fat diet rats | [79] | |
| Dehydrocarvidine | rats | The transport of dehydrocavidine was carried out in vitro at different intestine segments | [80] | |
| Dehydrocarvidine | Caco-2 cells | The bi-directional transport of dehydrocavidine in Caco-2 monolayer model showed significant difference | [80] |
| Class | Drugs | Cases | Result | Adverse Reaction | References |
|---|---|---|---|---|---|
| Icteric hepatitis | CSBI | unknow | The total effective rate of the treatment group was 87.5%, the control group was 62.5% (p < 0.01) | [85] | |
| CSBI | 42 | Significantly decreased contents of ALT, AST, γ-GT and TBIL (p < 0.05). | [86] | ||
| CSBI | 60 | The clinical symptoms and liver function in the treatment group were better than those in the control group (p < 0.05) | [87] | ||
| CSBI | 82 | Significantly improved symptoms of fatigue, abdominal distension, hepatalgia and poor appetite; obvious decrease of transaminase and bilirubin | [82] | ||
| CSBI | 98 | Improvement rate of poor appetite, hepatalgia, fatigue and abdominal distension was 85.7%, 84.4%, 76.8% and 87.8%, respectively, summary improvement is 83.4%. Significantly decreased contents of ALT, TBIL | [88] | ||
| CSBI | 29 | The total effective rate of the treatment group was 93.1%, the control group was 71.0% (p < 0.05) | [89] | ||
| CSBI | 1 | Caused allergic reactions | Itching, palpitating, chills, fever | [83] | |
| Salvia miltiorrhiza Bge & Yinzhihuang & CSB | 90 | The total effective rate of the treatment group was 96.0%, the control group was 82.5% (p < 0.05) | [90] | ||
| Salvia miltiorrhiza Bge & Yinzhihuang & CSB | 90 | The total effective rate of the treatment group was 96.0%, the control group was 82.5% (p < 0.05) | Precardiac discomfort, urticaria, skin itching, pain at the injection site | [91] | |
| Viral hepatitis | CSBI | 93 | The total effective rate of the treatment group was 91.7%, the control group was 68.9% (p < 0.03) | There were no adverse reactions | [92] |
| CSBI | 50 | Significantly decreased ALT and TBIL (p < 0.05), the total effective rate of the treatment group was 94.0% | [93] | ||
| CSBI | 208 | TBil of treatment group dropped by 71.22%, the control group was 44.30% (p < 0.01); Dbil of treatment group dropped by 67.53% (p < 0.01) | low-grade fever | [94] | |
| CSBI | 360 | Significant difference in improvement rate of hepatalgia and poor appetite (p < 0.01), decreased the levels of T-BILI, D-BILI and ALT | [95] | ||
| CSBI | 60 | The total effective rate of the treatment group was 88.23%, the control group was 76.92% (p < 0.05) | Local vascular pain | [96] | |
| CSBI | 33 | Decreased the contents of ALT and AST in a short time, improved protein metabolism | [97] | ||
| Compound Danshen & CSB | 100 | The total effective rate of the treatment group was 96.14%, the control group was 64.58% (p < 0.01) | Mild rash | [98] | |
| Acute and chronic hepatitis | CSBI | 93 | The total effective rate of the treatment group was 91.7%, the control group was 68.9% (p < 0.03) | There were no adverse reactions | [92] |
| Magnesium isoglycyrrhizinate & CSB | 65 | The total effective rate of the treatment group was 89.23%, the control group was 70.14% (p < 0.05) | [99] | ||
| CSB & Telbivudine | 80 | The total effective rate of the treatment group was 72.5%, the control group was 50% (p < 0.05) | [100] | ||
| CSB & Danshen injection | 70 | Significantly decreased ALT and TBIL, increased the ratio of A/G | [101] | ||
| Liver cancer | CSBI | 96 | The total effective rate of the treatment group was 83.3%, the control group was 72.9% (p < 0.05), Significantly decreased ALT and AST | [102] | |
| CSBI | 96 | The total effective rate of the treatment group was 83.3%, the control group was 72.9% (p < 0.05), the QOL of the treatment group (68.6±7.2) more than the control group (60.5±6.1) after treatment (p < 0.05) | [103] | ||
| CSBI | 96 | The total effective rate of the treatment group was 79.2%, the control group was 50.0% (p < 0.05), effectively improve the levels of serum IL-2, IFN-γ and TNF-α, better than the control group | [104] | ||
| CSBI | 96 | The total effective rate of the treatment group was 81.3%, the control group was 70.8% (p < 0.05), after treatment, the INF-γ, IL-4 and the ratio of INF-γ/IL-4 in the observation group were significantly better than those in the control group (p < 0.05) | [105] | ||
| CSBI | 120 | After treatment, the liver function recovery effect of the injection group was better than that of the control group (p < 0.05) | fever, skin itch; the injection group (6.7%), the control group (8.3%) | [106] | |
| CSBI | 96 | The total effective rate of the treatment group was 72.9%, the control group was 52.1% (p < 0.05) | [107] | ||
| CSBI | 96 | The total effective rate of the treatment group was 83.3%, the control group was 64.4% (p < 0.05) | The adverse effects rate of the treatment group was 20.8%, the control group was 39.6% (p < 0.05) | [108] | |
| CSBI | 96 | The total effective rate of the treatment group was 97.92%, the control group was 87.50% (p < 0.05) | The adverse effects rate of the treatment group was 10.42%, the control group was 39.6% (p < 0.05) | [109] | |
| CSBI | 110 | Quality of life improvement rate in the treatment group was 81.82%, the control group was 52.73% (p < 0.05) | [110] | ||
| CSBI | 42 | Significantly decreased the levels of ALT, AST, TBIL and γ-GT | Fever, shiver, skin itch | [86] | |
| CSBI | 60 | Significantly decreased the level of AFP, the white blood cell count goes up | [111] | ||
| CSBI | 46 | Significantly increased the level of ALT and AST (p < 0.05), decreased TBIL (p > 0.05) | [112] | ||
| CSBI & Octreotide | 116 | The total effective rate of the treatment group was 96.4%, the control group was 92.9% (p < 0.05) | The adverse effects rate of the treatment group was 5.2%, the control group was 17.2% (p < 0.05) | [113] | |
| Hyperbilirubinemia | CSBI | 126 | The total effective rate of the treatment group was 95.3%, the control group was 93.6% (p > 0.05) | Diarrhea, rash | [114] |
| Others | |||||
| Rectal cancer | CSBI | 68 | The total effective rate of the treatment group was 38.24%, the control group was 5.88% (p < 0.05) | [115] | |
| Hemorrhagic fever with renal syndrome | CSBI & Ribavirin | 60 | Significantly decreased the duration of fever period and oliguria period, obvious the recovery of AST, ALT, LDH and BUN than the control group (p < 0.05) | [116] | |
| Liver cirrhosis | CSBI | 60 | Significantly effect in relieving symptoms, protecting liver and gallbladder | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Chen, Y.; Wang, F.-F.; Tang, S.-Q.; Fang, Y.-L. Corydalis saxicola Bunting: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Clinical Applications. Int. J. Mol. Sci. 2023, 24, 1626. https://doi.org/10.3390/ijms24021626
Qin F, Chen Y, Wang F-F, Tang S-Q, Fang Y-L. Corydalis saxicola Bunting: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Clinical Applications. International Journal of Molecular Sciences. 2023; 24(2):1626. https://doi.org/10.3390/ijms24021626
Chicago/Turabian StyleQin, Feng, Yao Chen, Fan-Fan Wang, Shao-Qing Tang, and Yi-Lin Fang. 2023. "Corydalis saxicola Bunting: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Clinical Applications" International Journal of Molecular Sciences 24, no. 2: 1626. https://doi.org/10.3390/ijms24021626
APA StyleQin, F., Chen, Y., Wang, F.-F., Tang, S.-Q., & Fang, Y.-L. (2023). Corydalis saxicola Bunting: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Clinical Applications. International Journal of Molecular Sciences, 24(2), 1626. https://doi.org/10.3390/ijms24021626





