Abstract
The coronavirus disease (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). COVID-19 has had a catastrophic effect globally causing millions of deaths worldwide and causing long-lasting health complications in COVID-19 survivors. Recent studies including ours have highlighted that adipose tissue can act as a reservoir where SARS-CoV-2 can persist and cause long-term health problems. Here, we evaluated the effect of SARS-CoV-2 infection on adipose tissue physiology and the pathogenesis of fat loss in a murine COVID-19 model using humanized angiotensin-converting enzyme 2 (hACE2) mice. Since epidemiological studies reported a higher mortality rate of COVID-19 in males than in females, we examined hACE2 mice of both sexes and performed a comparative analysis. Our study revealed for the first time that: (a) viral loads in adipose tissue and the lungs differ between males and females in hACE2 mice; (b) an inverse relationship exists between the viral loads in the lungs and adipose tissue, and it differs between males and females; and (c) CoV-2 infection alters immune signaling and cell death signaling differently in SARS-CoV-2 infected male and female mice. Overall, our data suggest that adipose tissue and loss of fat cells could play important roles in determining susceptibility to CoV-2 infection in a sex-dependent manner.
1. Introduction
COVID-19 is a viral respiratory illness, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. It causes debilitating disease manifestations in many infected people and increases mortality in people with comorbidities, including metabolic disorders and heart diseases [2,3,4,5,6,7,8]. The causes of death in COVID-19 patients include cardiomyopathy, stroke, cardiac arrest, sepsis, and organ failure [9,10,11,12,13,14]. At least 50% of COVID-19 survivors are known to face lingering health issues, which include a racing heartbeat, shortness of breath, achy joints, and damage to the heart, lungs, kidney, and brain [15,16]. A recent meta-analysis review that included 47,910 patients (age 17–87 years) estimated the prevalence of 55 long-term post-COVID-19 effects, where 58% of patients suffer from fatigue, 12% of which is due to significant weight loss [17]. Other clinical reports suggest that the post-COVID-19 stage is associated with acute (30%) and chronic weight loss (56%) and malnutrition [18]. Loss of body weight is linked with body fat mass and the pathophysiology of fat cells. Adipocytes, also known as fat cells, regulate inflammatory signaling and immune response [19,20,21,22,23,24]. It is well known that body fat levels and distribution patterns differ between the sexes and races [25,26,27], which may influence the susceptibility to SARS-CoV-2 infection, COVID-19-associated symptoms, and side effects. Importantly, recent studies have shown that SARS-CoV-2 infects adipose tissue [28,29].
In the present pilot study, we investigated the effect of SARS-CoV-2 infection on adipose tissue physiology and the pathogenesis of fat loss in a murine model of COVID-19 using humanized angiotensin-converting enzyme 2 (hACE2) mice. We used both male and female hACE2 mice intra-nasally infected with SARS-CoV-2. We demonstrated that CoV-2 infects adipose tissue and persists around the lipid droplets in white adipose tissue (WAT) of CoV-2-infected mice 10 days post infection (DPI). Our studies revealed that in male and female mice, CoV-2 infection differently affects adipose tissue and regulates immune signaling. Thus, the alterations in adipose tissue metabolic and immunologic functions may affect the whole-body immune and metabolic homeostasis differently in males and females during acute COVID-19 illness and the post-COVID-19 phase. These data may help explain the higher COVID-19 susceptibility in males compared to females.
2. Results
Earlier, we demonstrated that SARS-CoV-2 infection alters pulmonary pathology in hACE2 mice differently in males and females [30]. In particular, we showed a significantly increased viral load and infiltration of immune cells in the lungs of infected male mice compared to female mice at 10 DPI [30]. However, both male and female mice showed decreased body weight compared to control mice. Our earlier studies suggest that decreased body weight is likely caused by a loss of body fat mass [30]. Therefore, we investigated the role of adipose tissue in CoV-2 infection using white adipose tissue (WAT) of infected and uninfected control mice (10 DPI) as detailed in the Materials and Methods section. To investigate the pathological effects of SARS-CoV-2 infection in the WAT of hACE2 mice, we performed histological and biochemical analyses of WAT samples at 10 DPI. Age and sex-matched uninfected mice served as controls. We used n = 8 mice/group (4 uninfected and 4 CoV-2 infected) for both sexes. We observed no mortality during CoV-2 infection in mice up to and including 10 DPI. However, the histological analysis of WAT samples has revealed a significant difference in their pathology between the sexes. Therefore, we analyzed all data separately for males and females as presented below.
2.1. SARS-CoV-2 Infection Alters Adipose Tissue Morphology Differently in Male and Female hACE2 Mice
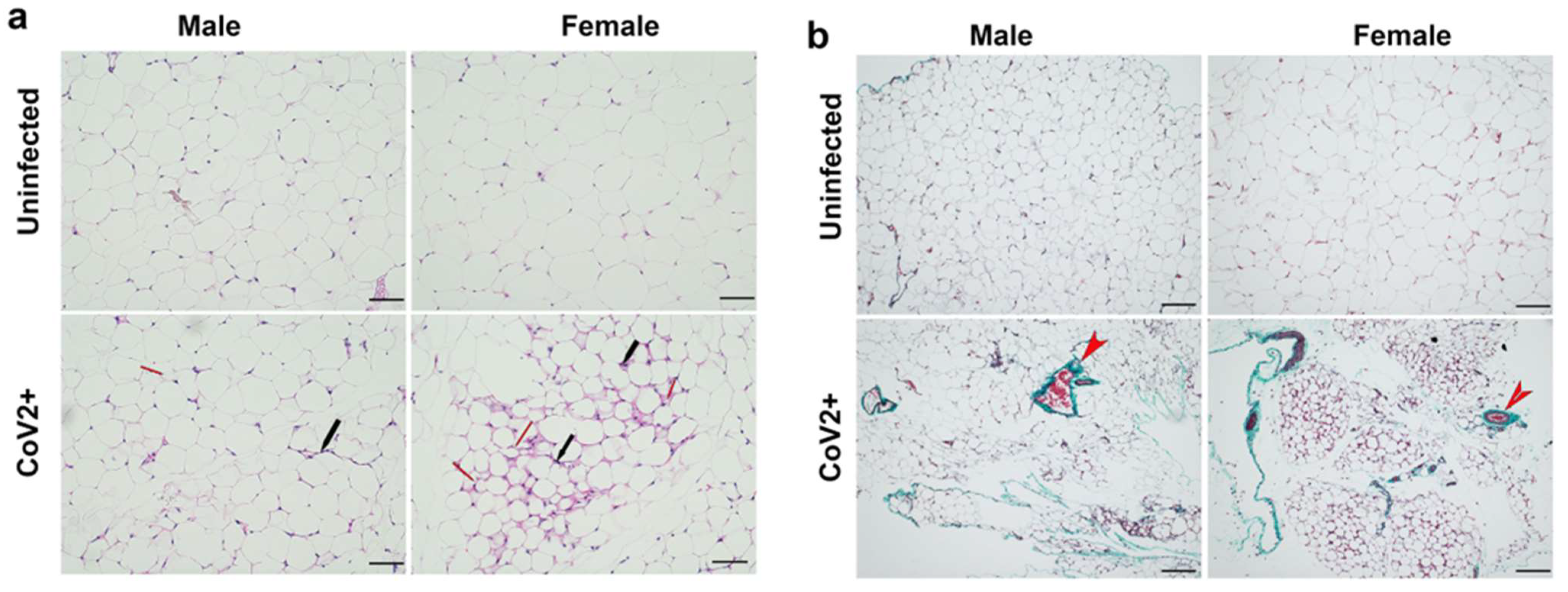
Histological analysis of WAT was performed using H&E (Figure 1a) and Masson-trichrome (Figure 1b) stained sections as described in Materials and Methods. Microscopic analysis of the histological sections of WAT demonstrated significantly increased levels of infiltrating immune cells, loss of lipid droplets, and evidence of increased fibrosis in CoV-2 infected hACE2 mice compared to uninfected mice (Figure 1). Between uninfected male and female mice, the size of adipocytes was relatively larger in females (Figure 1a). However, female mice lost a significant amount of body fat compared to males during CoV-2 infection (Figure 1a) [31,32]. We observed increased fibrosis in adipose tissue in infected mice compared to uninfected mice (Supplemental Figure S2). These data suggest that adipose tissue undergoes significant morphological changes, including increased immune cell infiltration and loss of lipid droplets, which can alter the local and systemic immune and metabolic homeostasis during CoV-2 infection.
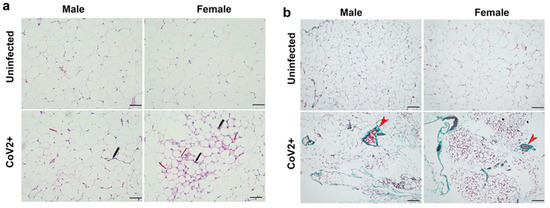
Figure 1.
SARS-CoV-2 infection alters adipose tissue morphology at 10 DPI in hACE2 mice (n = 4 mice/sex). (a) H&E-stained sections of WAT of both males and females showing infiltrated immune cells (black arrow) and loss in lipid droplets (red arrow, smaller adipocytes) with CoV-2 infection compared to uninfected mice (20× magnification, scale bar—50 µm); (b) Masson-trichrome staining of WAT sections showing fibrosis and collagen (green color, red arrowhead) in CoV-2 infected mice (10× magnification, scale bar—100 µm).
2.2. Sex Differences in the Tissue CoV-2 Tropism in the Lungs and Visceral Fat Pads
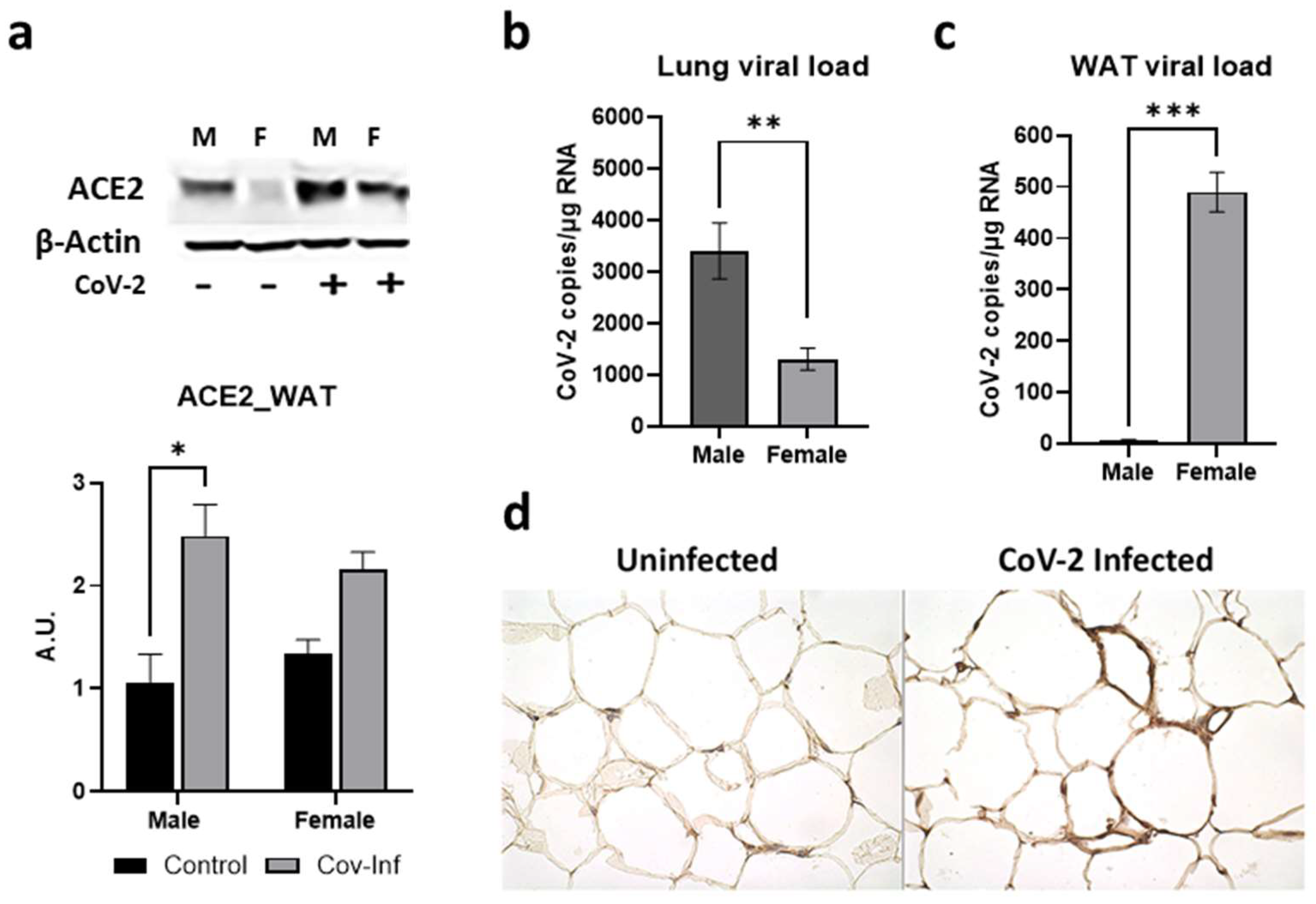
ACE2 protein is a well-recognized receptor for CoV-2 entry into the host cell [33,34]. Earlier we showed by Western blotting that CoV-2 infection increases the expression levels of ACE2 protein in the lungs of hACE2 mice [30]. The levels of ACE2 were significantly higher in the lungs of both male (p ≤ 0.0001) and female (p ≤ 0.01) mice infected with SARS-CoV-2 compared to sex-matched uninfected (control) mice [30]. Here, we analyzed whether CoV-2 infection also alters the levels of ACE2 in WAT. In WAT, the levels of ACE2 were significantly higher in male (p < 0.05) CoV-2 infected mice compared to sex-matched control mice (Figure 2a). We analyzed the viral loads in the lung and WAT by qPCR analysis. Lung viral loads were significantly greater in male CoV-2-infected mice compared to female CoV-2-infected mice (Figure 2b), which may be due to increased ACE2 levels in male mice [30]. However, qPCR analysis demonstrated significantly higher levels of viral load in the WAT of female CoV-2 infected mice (64-fold, p ≤ 0.005) compared to male CoV-2 infected mice, although the levels of ACE2 were not significantly increased (Figure 2c). We also performed immunohistochemistry (IHC) analysis of SARS-CoV-2 using a monoclonal antibody against the SARS-CoV-2 nucleocapsid protein, which demonstrated the presence of SARS-CoV-2 nucleocapsid protein in adipose tissue around the lipid droplets in infected mice (Figure 2d). These data demonstrate that: (i) CoV-2 infection alters ACE2 levels and viral loads differently in male and female mice; (ii) SARS-CoV-2 infects and persists in adipose tissue; (iii) adipose tissue in females may act as a sink/reservoir for CoV-2; and (iv) an inverse relationship exists between the viral loads in the lungs and adipose tissue.
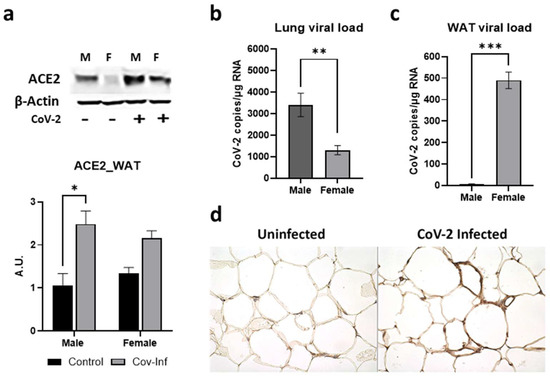
Figure 2.
Alterations in ACE2 levels and SARS-CoV-2 viral load in the WAT of male and female CoV-2 infected mice. (a) Immunoblot analysis of ACE2 in WAT (top). β-Actin was used as loading control. Fold changes in the protein levels of ACE2 were normalized to β-Actin expression and is shown as a bar graph (bottom). The error bars represent standard error of the mean. * p < 0.05 compared to uninfected sex-matched mice (n = 4/sex/group) (M, male; F, female); Real-Time PCR analysis showing the number of CoV-2 viral copies/µg of RNA in the (b) lungs and (c) WATs of male and female CoV-2 infected mice. The error bars represent standard error of the mean (** p ≤ 0.01 and *** p ≤ 0.001); (d) IHC staining of anti-SARS-CoV-2 nucleocapsid protein showing the presence of CoV-2 in adipose tissue around the lipid droplets of infected female hACE2 mice (n = 4 mice, a minimum number of five images/section were analyzed).
2.3. SARS-CoV-2 Infection Alters Immune Signaling in the Adipose Tissue Differently in Male and Female hACE2 Mice
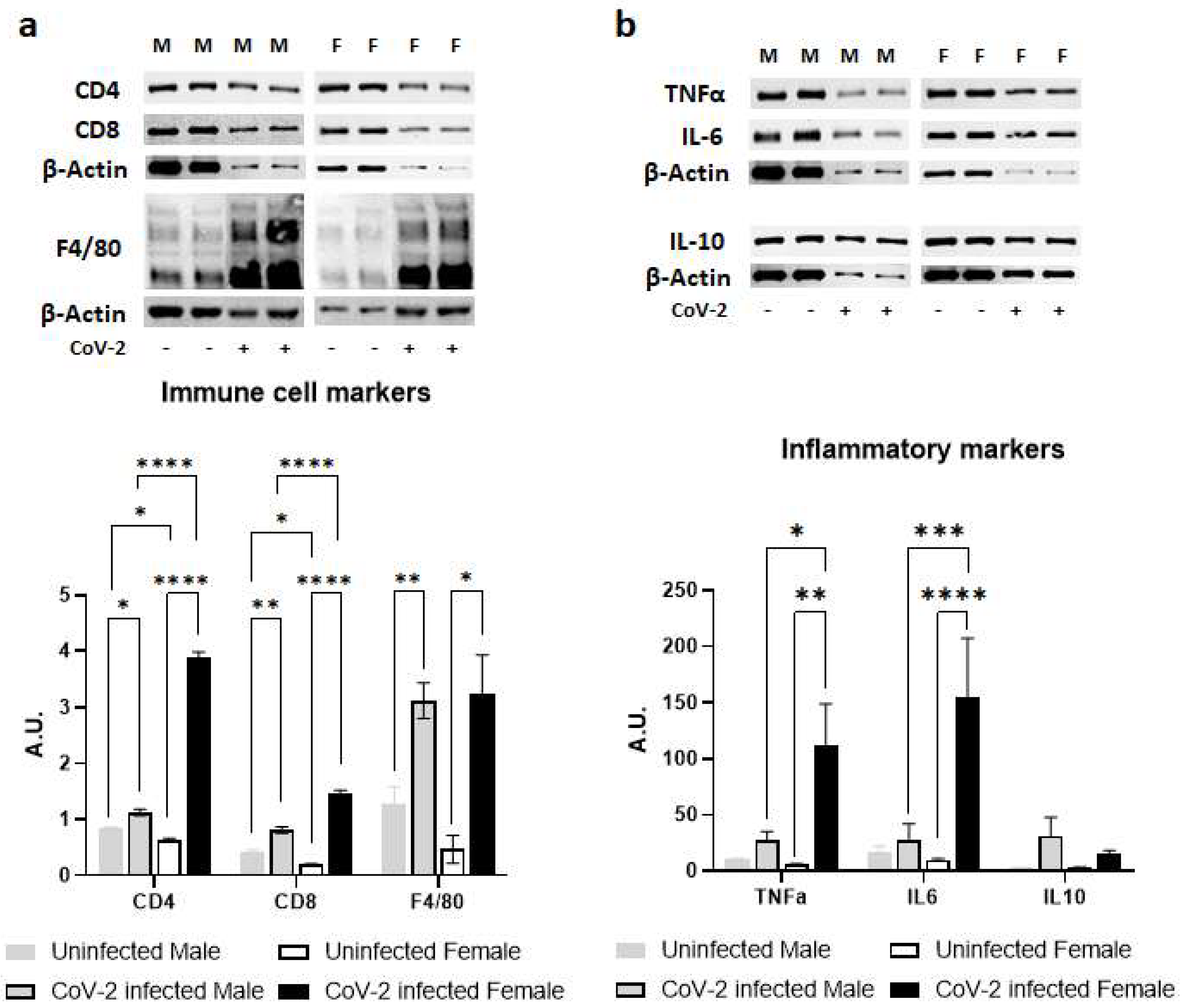
Immunoblot analysis of WAT lysates demonstrated significant differences in the protein levels of immune cell markers indicating altered levels of CD4+ cells CD8+ cells and F4/80+ cells; and inflammatory cytokines such as TNFα, IL-6, and IL-10 between the sexes during CoV-2 infection (Figure 3). Uninfected female mice showed significantly lower levels of resident CD4+ cells (p < 0.05) and CD8+ cells (p < 0.05) compared to uninfected male mice (Figure 3a). CoV-2 infection significantly increased the infiltration of CD4+ and CD8+ cells in WAT in both males (p < 0.05 and p < 0.005, respectively) and females (p < 0.0001 and p < 0.0001, respectively) compared to their respective sex-matched uninfected mice. The levels of F4/80+ cells in WAT were significantly elevated (p < 0.01) in the WAT of CoV-2 infected mice compared to uninfected mice, irrespective of their sex (Figure 3a). Overall, the levels of CD4+ cells and CD8+ cells) were significantly increased (p < 0.0001) in the WAT of female CoV-2 mice compared to male CoV-2 mice.
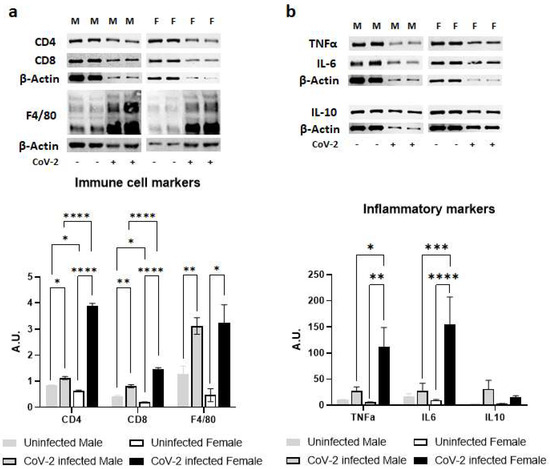
Figure 3.
Immune signaling in the WAT during CoV-2 infection is altered differently between the sexes. Immunoblot images of (a) immune cell markers (CD4, CD8, F4/80) and (b) inflammatory markers (TNFα, IL-6, IL-10). β-actin was used as loading control. Fold changes in the protein levels were normalized to β-actin expression and are shown as bar graphs. The error bars represent standard error of the mean. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared to uninfected sex-matched mice and between the infected groups (n = 4/sex/group) (M, male; F, female).
There was no significant difference between the levels of proinflammatory TNFα in the WAT of male and female control mice (Figure 3b). However, CoV-2 infection significantly increased the levels of TNFα in female mice compared to their sex-matched controls (p < 0.01) and infected male counterparts (p < 0.05). Similarly, the levels of IL-6 were significantly elevated in CoV-2-infected female mice compared to their male counterparts (p < 0.001) and sex-matched controls (p < 0.0001). No significant change in the levels of IL-10 was observed in either male or female CoV-2-infected mice compared to the corresponding sex-matched control groups. These data demonstrated that CoV-2 infection induces stronger proinflammatory signaling in the WAT of female mice compared to male mice.
2.4. SARS-CoV-2 Infection Alters Immune Signaling in the Adipose Tissue Differently in Male and Female hACE2 Mice
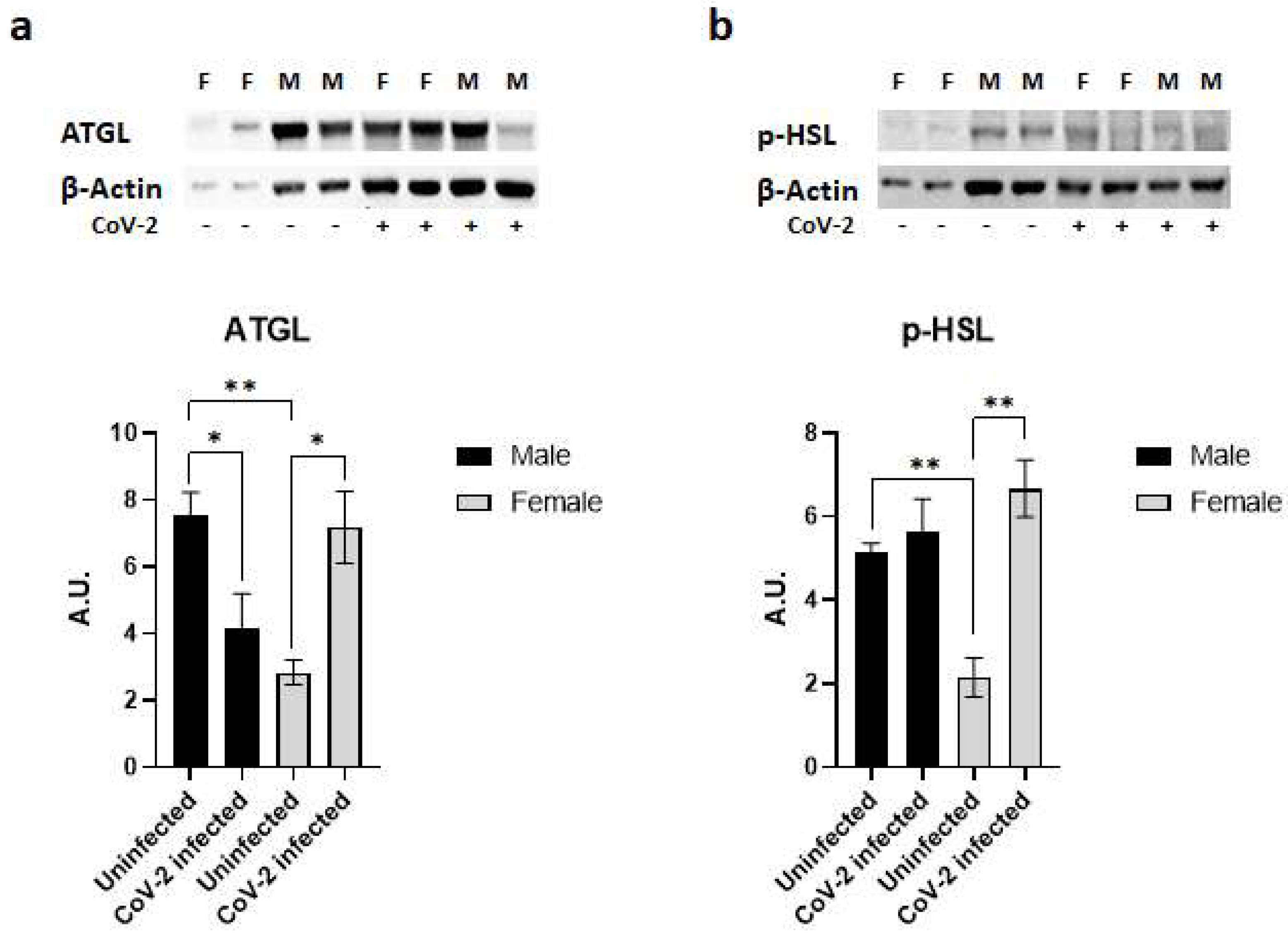
Immunoblot analysis of WAT lysates demonstrated significant differences in the protein levels of lipases (ATGL and p-HSL) between the sexes in hACE2 mice and during CoV-2 infection (Figure 4). Uninfected female mice showed significantly lower levels of ATGL (p < 0.01) and p-HSL (p < 0.01) expression compared to uninfected male mice (Figure 4). Furthermore, CoV-2 infection significantly increased the levels of ATGL (p < 0.05) and p-HSL (p < 0.01) in females compared to female uninfected mice. However, the levels of ATGL significantly decreased (p < 0.05), and the levels of p-HSL were not altered in infected male mice compared to uninfected male mice. These data indicate that CoV-2 infection differently activates lipases in WAT between male and female hACE2 mice.
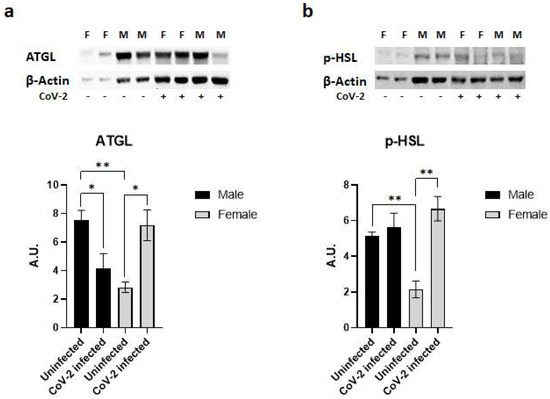
Figure 4.
SARS-CoV-2 infection activates lipases in the adipose tissue of female hACE2 mice. Western blot images of (a) ATGL and (b) p-HSL. β-actin was used as loading control. Fold changes in the protein levels were normalized to β-actin expression and are shown as bar graphs. The error bars represent standard error of the mean. * p < 0.05 and ** p < 0.01 compared to uninfected mice (n = 4/sex/group) (M, male; F, female).
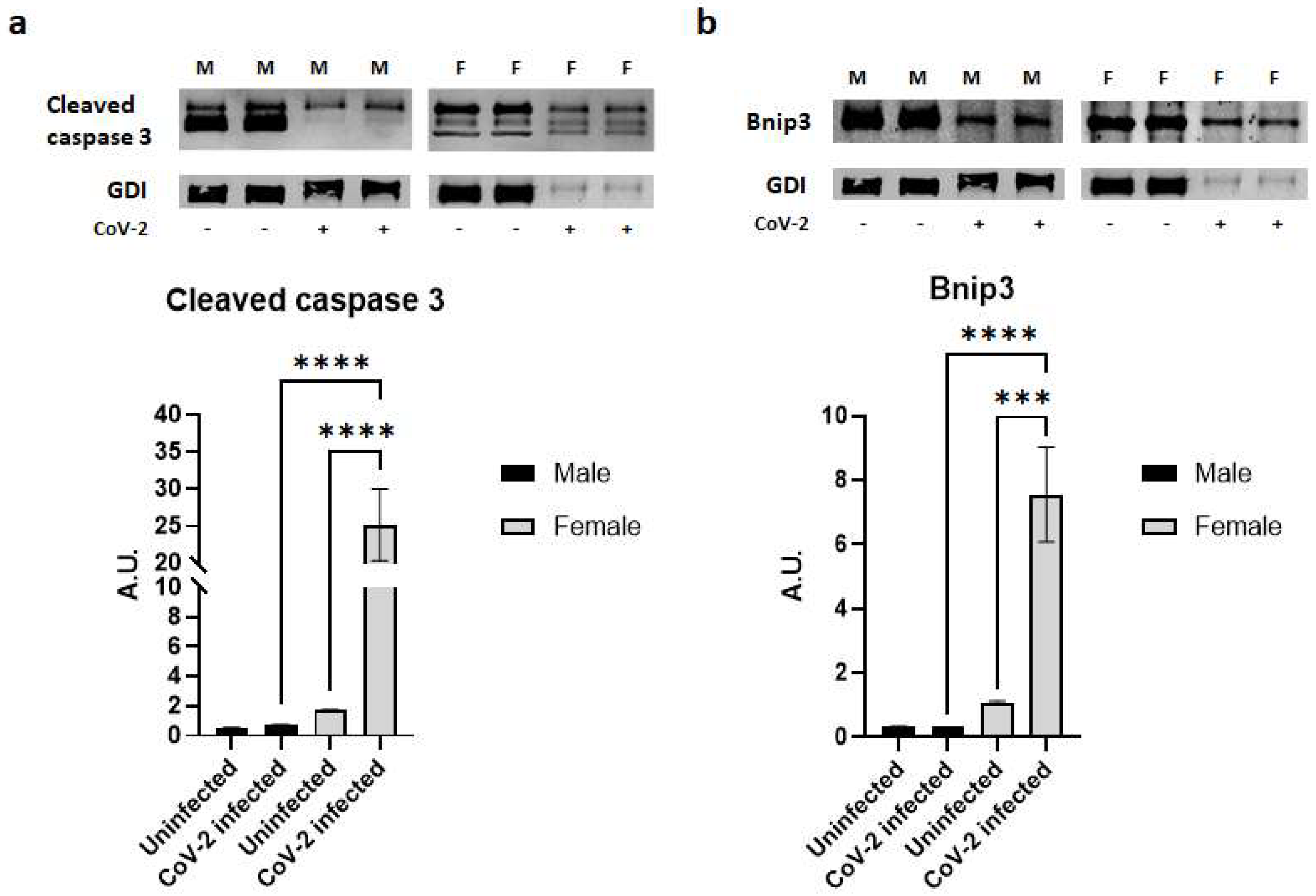
2.5. SARS-CoV-2 Infection Causes a Loss of Fat Cells in hACE2 Mice
Histological analysis demonstrated a significant loss of lipid droplets and adipocytes in CoV-2 infected mice compared to their control groups (Figure 1). We analyzed whether the cause for the loss of adipocytes was due to apoptosis or necrosis by quantitating the protein levels of cleaved caspase 3 and Bnip3, respectively, in the WAT (Figure 5). In the WAT of uninfected female mice, the levels of cleaved caspase 3 and Bnip3 were slightly elevated compared to uninfected male mice; however, this difference was not statistically significant. In contrast, CoV-2 infection significantly increased the levels of cleaved caspase 3 (p < 0.0001) (Figure 5a) and Bnip3 (p < 0.001) (Figure 5b) in females compared to uninfected controls. Interestingly, the levels of cleaved caspase 3 in the WAT of CoV-2 females were significantly higher (p < 0.0001) compared to their male counterparts, suggesting that cell death in the WAT of female mice may be predominantly driven by apoptosis (Figure 5a). In addition, the levels of necrotic cell death markers in the WAT of CoV-2 infected mice were also significantly elevated (p < 0.0001) in females compared to males (Figure 5b). These data indicate that during CoV-2 infection adipose tissue is lost via both apoptotic and necrotic cell death in females more so than in males.
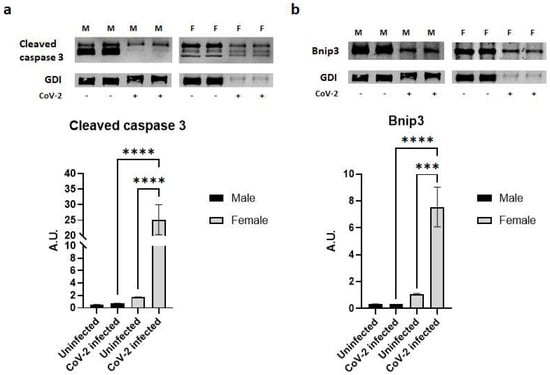
Figure 5.
SARS-CoV-2 infection induces severe cell death in adipose tissue in female hACE2 mice. Western blot images of (a) Cleaved caspase 3 (apoptosis marker) and (b) Bnip3 (necrosis marker). GDI was used as loading control. Fold changes in the protein levels were normalized to GDI expression and are shown as bar graphs. The error bars represent standard error of the mean. *** p < 0.001 and **** p < 0.0001 compared to uninfected sex-matched mice (n = 4/sex/group) (M, male; F, female).
3. Discussion
The epidemiological findings reported globally indicate higher morbidity and mortality in males than in females with SARS-CoV-2 infection [35,36,37]. Although women also get infected with CoV-2, many clinical studies indicate that males are more susceptible to developing severe COVID-19 compared to females, and many researchers have attributed this difference to sex-specific hormones [38,39,40,41]. A few reports have also suggested a difference in immune responses between the sexes [42,43,44]. However, how exactly the immune response may change between males and females during CoV-2 infection is not well understood. In our previous studies, we have demonstrated that pathogens such as parasites, such as Trypanosoma cruzi, and bacteria, such as Mycobacterium tuberculosis, can infect and persist in adipose tissue [30,45,46]. Recently, we and others have shown that CoV-2 can also infect adipose tissue [28,30,47,48]. In particular, biopsies have demonstrated the presence of CoV-2 in subcutaneous thoracic fat [28] and abdominal fat [29] in COVID-19 patients. These studies have shown that adipose tissue can be a significant reservoir for SARS-CoV-2 and an important source of inflammatory mediator IFN-γ [29]. The present study investigated sex differences in: (i) SARS-CoV-2 viral loads in adipose tissue; and (ii) immune signaling due to the presence of CoV-2 in adipose tissue. Moreover, this study assessed whether the relationship between lung and adipose tissue viral loads differs between male and female infected hACE2 mice. Our study revealed for the first time that: (a) viral loads in adipose tissue and the lungs differ between males and females; (b) an inverse relationship exists between the viral loads in the lungs and adipose tissue, and it differs between the males and females in hACE2 mice; and (c) CoV-2 infection alters immune signaling, lipolysis, and cell death signaling differently in the adipose tissue of SARS-CoV-2 infected male and female mice.
Earlier we showed that the viral loads in the lungs of female CoV-2 infected mice were significantly lower compared to male CoV-2 infected mice, which is reminiscent of the observations made in COVID-19 patients [30,49]. The increased lung pathology observed in male mice is likely due to increased viral loads and infiltrated immune cells in the lungs. Interestingly, in female mice, CoV-2 levels were significantly reduced in the lungs but significantly increased in the WAT compared to infected male mice. It has been shown that estrogen reduces the levels of ACE2 [50]. Thus, although ACE2 is more highly expressed in adipose tissue than in the lungs [51], females may have reduced levels of ACE2 in WAT because of their higher levels of estrogen. However, SARS-CoV-2 can also infect and invade cells via other receptors and cellular mechanisms [34,52]. For example, SARS-CoV-2 can infect cells through the cholesterol-rich lipid rafts [53,54,55], which may be the case in adipose tissue in female mice. Our data suggested that in female mice adipose tissue may act as a sink/reservoir for SARS-CoV-2 and thus spares the lungs from a greater viral load, preventing pulmonary damage due to infiltrated immune cells and activated pro-inflammatory cytokines. The reduced viral load in the lungs of female mice may also be attributed to an increased pro-inflammatory environment in female mice caused by increased IL-6 and TNF-a levels in adipose tissue, which increases the levels of circulating pro-inflammatory cytokines.
We observed an increased average size of adipocytes in uninfected females compared to uninfected males in the adipose tissue of hACE2 mice, which may be attributed to the lower levels of lipases in female mice. However, the increased levels of lipases in the adipose tissue of infected female mice compared to infected male mice may cause a loss of lipid droplets. CoV-2 infection causes a loss of lipid droplets and promotes cell death in adipose tissue. Our histological and Western blotting analysis demonstrated that the loss of lipid droplets and increased cell death due to lipolysis, necrosis, and apoptosis were significantly higher in the WAT of infected female mice compared to infected male mice. Like other viruses and parasites, SARS-CoV-2 utilizes host lipids for its biosynthetic needs [56]. It has been shown that lipid droplets increase the replication of SARS-CoV-2 [56]. Isolated monocytes from COVID-19 patients showed an increased accumulation of intracellular lipid droplets compared to SARS-CoV-2 negative donors [56], suggesting that CoV-2 manipulates cellular metabolism to acquire lipid resources from the host. Thus, adipocytes, which are rich in lipid droplets, provide the necessary fuel for viral replication. In female hACE2 mice, the presence of CoV-2 in adipose tissue increased the loss of lipid droplets and caused cell death, which likely resulted in the infiltration of immune cells and the elevation of cytokines such as IL-6 and TNF-α. The difference in viral load and immune cell activation can be attributed to lipid droplets. The loss of lipid droplets due to deregulated lipolysis has been linked to the infiltration of immune cells, immune cell activation, and cell death (apoptotic or necrotic) [57,58,59,60]. The process of cell death initiates the infiltration of immune cells and the release of TNFα, which in turn further elevates lipolysis [61]. In general, male mice have more fat compared to female mice and male mice are more susceptible to developing obesity [62,63]. However, the levels of body fat in hACE2 mice were not measured. These basic metabolic differences in fat tissue between males and females likely contribute to the greater levels of CoV-2 in females, leading to higher levels of pro-inflammatory cytokines in adipose tissue. The cytokines released in adipose tissue contribute to the elevated cytokine levels in circulation [64]; thus, in infected female mice, TNF-α and IL-6 released from the adipose tissue could activate immune cells and contribute to the observed reduction of viral load in the lungs [65,66].
4. Materials and Methods
4.1. Biosafety
All aspects of this study were approved by the Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee of Center for Discovery and Innovation (CDI)—Hackensack University Medical Center and adhered to the National Research Council guidelines.
4.2. Animal Model and Experimental Design
The transgenic mice expressing human angiotensin-converting enzyme 2 (hACE2) were purchased from Jackson Laboratories, Bar Harbor, ME and bred at CDI animal research facility. Both male and female mice (n = 8) were intra-nasally infected with 1 × 104 pfu SARS-CoV-2 (NR-52281, Isolate USA-WA1/2020 CoV-2 virus, NIH-BEI Resources, Manassas, VA, USA). After 10 days post infection (DPI), the animals (n = 4/sex) were euthanized, and samples such as blood, lungs, spleen, and mesenteric white adipose tissue (WAT) were collected. Age and sex-matched uninfected hACE2 mice served as controls (Supplementary Figure S1). The lungs and WAT samples alone were used in the present study.
4.3. Histological Analysis of Adipose Tissue
Freshly isolated WAT were fixed with 10% neutral-buffered formalin for a minimum of 48 h and then embedded in paraffin wax (n = 4/sex). Hematoxylin and eosin (H&E) and Masson’s trichrome staining were performed, and the images were captured as previously published [46]. Four to six sections of each WAT were analyzed in this study. For each WAT section, the histological evidence of adipose tissue pathology was classified in terms of the presence of infiltrating immune cells, loss of lipid droplets, and fibrosis [46,67].
4.4. Determination of SARS-CoV-2 Load in the Tissue
Total RNA was isolated from the lungs and WAT of SARS-CoV-2 infected hACE2 mice using TRIzol reagent. The number of SARS-CoV-2 copies was quantified using a 2019-nCoV_N2 primer/probe mix and One-Step PrimeScript RT-PCR kit (Takara Bio Inc., San Jose, CA, USA). All assays were performed on Agilent AriaMx Real-time PCR System according to the following cycling conditions: 15 min at 42 °C (1 cycle, reverse transcription), followed by 10 sec at 95 °C (1 cycle, hot start) and continuing with 5 sec at 95 °C, and 30 sec at 55 °C (40 cycles, PCR amplification).
4.5. Immunohistochemistry Analysis of SARS-CoV-2
Freshly isolated WAT tissues were fixed with 10% neutral-buffered formalin for a minimum of 48 h and then embedded in paraffin wax (n = 4/sex) and sectioned for immunohistochemistry analysis (IHC). IHC was performed using a rabbit monoclonal anti-SARS-CoV-2 nucleocapsid protein (#NR-53791, Sino Biological, Wayne, PA, USA) with a dilution of 1:1000 followed by biotinylated secondary antibody using VECTASTAIN Elite ABC-HRP kit (#PK-6101, Vector Laboratories, Newark, CA, USA). The sections were then washed and incubated with peroxidase substrate and counterstained with hematoxylin.
4.6. Immunoblot Analysis
Tissue lysates were prepared as previously described [30]. The protein concentration quantitation was performed using a Pierce BCA protein assay kit (#23225, ThermoFisher Scientific, Waltham, MA, USA). Then, 30 µg total protein from each sample was loaded and resolved on 8% or 15% SDS-PAGE as appropriate and transferred onto nitrocellulose membrane for immunoblot analysis. Primary antibodies against CD4 (#NBP1-19371, Novus Biologicals, Centennial, CO, USA), CD8 (#NBP2-29475, Novus Biologicals, Centennial, CO, USA), F4/80 (#NB 600-404, Novus Biologicals, Centennial, CO, USA), TNFα (#ab6671, Abcam, Waltham, MA, USA), IL-6 (#66146-1-lg, Proteintech, Rosemont, IL, USA), IL-10 (#20850-1-AP, Proteintech, Rosemont, IL, USA), ATGL (#2439, Cell Signaling Technology, Danvers, MA, USA), HSL (#4139, Cell Signaling Technology, Danvers, MA, USA), caspase 3 (#9662, Cell Signaling Technology, Danvers, MA, USA), and BNIP3 (#44060, Cell Signaling Technology, Danvers, MA, USA) were used to detect the expression of corresponding proteins. Horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (#7076, Cell Signaling Technology, Danvers, MA, USA), HRP-conjugated anti-rabbit immunoglobulin (#7074, Cell Signaling Technology, Danvers, MA, USA), and HRP-conjugated anti-rat immunoglobulin (#112-035-003, Jackson ImmunoResearch, West Grove, PA, USA) were used as appropriate secondary antibodies to detect chemiluminescent signal using Invitrogen iBright Imaging Systems. Β-Actin (#4967, Cell Signaling Technology, Danvers, MA, USA) and Guanosine nucleotide dissociation inhibitor (GDI) (#71-0300, Invitrogen, Waltham, MA, USA) were used as appropriate control to assess equal protein loading.
4.7. Statistical Analysis
Data represent means ± S.E. Data were pooled, and statistical analysis was performed on GraphPad Prism software version 9.4.1 using two-way ANOVA and Student’s t-test as appropriate and significant differences were determined as p values between <0.0001 and <0.05 as appropriate.
5. Conclusions
In conclusion, our studies suggest that CoV-2 infection affects adipose tissue physiology which could alter systemic metabolic and immune homeostasis during COVID-19. It will be of great importance to further investigate the link between adipose tissue pathophysiology and pulmonary viral load and COVID-19 severity in COVID-19 research. Thus, further mechanistic studies are warranted to understand the role of the pathophysiology of adipose tissue in the pathogenesis of CoV-2 infection and COVID-19 outcomes. Further studies may determine the mechanistic roles of various fat tissues in regulating immune and metabolic signaling in male and female COVID-19 patients.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ijms24021314/s1.
Author Contributions
Conceptualization, J.F.N.; Data curation, J.F.N.; Formal analysis, H.T., D.D., K.L., and J.F.N.; Funding acquisition, J.F.N.; Investigation, H.T., D.D., and K.L.; Methodology, K.L., N.O., and E.D.; Project administration, J.F.N.; Supervision, J.F.N.; Validation, D.S.P. and J.F.N.; Writing—original draft, H.T. and D.D.; Writing—review and editing, J.F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, grant #AI150765-01 to Jyothi F. Nagajyothi.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of Hackensack Meridian Health—Center for Discover and Innovation (protocol #282, approved on 2 August 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Erika Shor at the Center for Discovery and Innovation, HMH for the critical reading of the manuscript. We also thank Steven Park at CDI for the managerial support in executing the BSL3 work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 14 September 2022).
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.; Santos-Burgoa, C. Obesity and its Implications for COVID-19 Mortality. Obesity 2020, 28, 1005. [Google Scholar] [CrossRef] [PubMed]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Kamyari, N.; Soltanian, A.R.; Mahjub, H.; Moghimbeigi, A. Diet, Nutrition, Obesity, and Their Implications for COVID-19 Mortality: Development of a Marginalized Two-Part Model for Semicontinuous Data. JMIR Public Health Surveill. 2021, 7, e22717. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Pellicori, P.; Doolub, G.; Wong, C.M.; Lee, K.S.; Mangion, K.; Ahmad, M.; Berry, C.; Squire, I.; Lambiase, P.D.; Lyon, A.; et al. COVID-19 and its cardiovascular effects: A systematic review of prevalence studies. Cochrane Database Syst. Rev. 2021, 3, CD013879. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems during the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Moody, W.E.; Mahmoud-Elsayed, H.M.; Senior, J.; Gul, U.; Khan-Kheil, A.M.; Horne, S.; Banerjee, A.; Bradlow, W.M.; Huggett, R.; Hothi, S.S.; et al. Impact of Right Ventricular Dysfunction on Mortality in Patients Hospitalized With COVID-19, According to Race. CJC Open 2021, 3, 91–100. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, X.; Hu, Z.; Yang, C.; Lei, P. Risk Factors for Mortality of COVID-19 Patient Based on Clinical Course: A Single Center Retrospective Case-Control Study. Front. Immunol. 2021, 12, 581469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, W.; Wang, Y.; Wang, X.; Liu, Y.; Zhao, S.; Long, D.; Chen, L.; Yu, L. Clinical Course and Mortality of Stroke Patients With Coronavirus Disease 2019 in Wuhan, China. Stroke 2020, 51, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, P.; Yang, D.X.; Xiong, W.N.; Zhou, M.; Qu, J.M.; Feng, Y.; Guo, Y. Development of multiple organ dysfunction syndrome in patients with Coronavirus Disease 2019: Clinical characteristics and risk factors. Zhonghua Jie He He Hu Xi Za Zhi 2021, 44, 435–442. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Chippa, V.; Aleem, A.; Anjum, F. Post Acute Coronavirus (COVID-19) Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Winer, D.A. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 2012, 90, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef]
- Exley, M.A.; Hand, L.; O’Shea, D.; Lynch, L. Interplay between the immune system and adipose tissue in obesity. J. Endocrinol. 2014, 223, R41–R48. [Google Scholar] [CrossRef]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef]
- Lopez-Ortega, O.; Moreno-Corona, N.C.; Cruz-Holguin, V.J.; Garcia-Gonzalez, L.D.; Helguera-Repetto, A.C.; Romero-Valdovinos, M.; Arevalo-Romero, H.; Cedillo-Barron, L.; Leon-Juarez, M. The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity. Int. J. Mol. Sci. 2022, 23, 6154. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Van Wijk, K.; Nakajima, O. Crosstalk between Metabolic Disorders and Immune Cells. Int. J. Mol. Sci. 2021, 22, 10017. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.; Faith, M.S.; Pietrobelli, A.; Heymsfield, S.B. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am. J. Clin. Nutr. 2012, 95, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Arora, A.; Feng, J.; Steiner, H.E.; Sulieman, L.; Boerwinkle, E.; Clark, C.; Cicek, M.; Cohn, E.; Gebo, K.; et al. Racial, ethnic, and gender differences in obesity and body fat distribution: An All of Us Research Program demonstration project. PLoS ONE 2021, 16, e0255583. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Mousovich-Neto, F.; Ludwig, R.G.; Carregari, V.C.; Dos Anjos Souza, A.B.; Dos Passos, A.S.C.; Martini, M.C.; Barbosa, P.P.; de Souza, G.F.; Muraro, S.P.; et al. SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner. Nat. Commun. 2022, 13, 5722. [Google Scholar] [CrossRef]
- Basolo, A.; Poma, A.M.; Bonuccelli, D.; Proietti, A.; Macerola, E.; Ugolini, C.; Torregrossa, L.; Giannini, R.; Vignali, P.; Basolo, F.; et al. Adipose tissue in COVID-19: Detection of SARS-CoV-2 in adipocytes and activation of the interferon-alpha response. J. Endocrinol. Invest. 2022, 45, 1021–1029. [Google Scholar] [CrossRef]
- Dhanyalayam, D.; Thangavel, H.; Lizardo, K.; Oswal, N.; Dolgov, E.; Perlin, D.S.; Nagajyothi, J.F. Sex Differences in Cardiac Pathology of SARS-CoV2 Infected and Trypanosoma cruzi Co-infected Mice. Front. Cardiovasc. Med. 2022, 9, 783974. [Google Scholar] [CrossRef]
- Golden, J.W.; Cline, C.R.; Zeng, X.; Garrison, A.R.; Carey, B.D.; Mucker, E.M.; White, L.E.; Shamblin, J.D.; Brocato, R.L.; Liu, J.; et al. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight. 2020, 5, e142032. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife 2020, 9, e61390. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Bwire, G.M. Coronavirus: Why Men are More Vulnerable to Covid-19 Than Women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef]
- Pradhan, A.; Olsson, P.E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020, 11, 53. [Google Scholar] [CrossRef]
- Raza, H.A.; Sen, P.; Bhatti, O.A.; Gupta, L. Sex hormones, autoimmunity and gender disparity in COVID-19. Rheumatol. Int. 2021, 41, 1375–1386. [Google Scholar] [CrossRef]
- Brandi, M.L. Are sex hormones promising candidates to explain sex disparities in the COVID-19 pandemic? Rev. Endocr. Metab. Disord. 2022, 23, 171–183. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses. Science 2021, 371, 347–348. [Google Scholar] [CrossRef]
- Qi, S.; Ngwa, C.; Morales Scheihing, D.A.; Al Mamun, A.; Ahnstedt, H.W.; Finger, C.E.; Colpo, G.D.; Sharmeen, R.; Kim, Y.; Choi, H.A.; et al. Sex differences in the immune response to acute COVID-19 respiratory tract infection. Biol. Sex Differ. 2021, 12, 66. [Google Scholar] [CrossRef]
- Ayyappan, J.P.; Vinnard, C.; Subbian, S.; Nagajyothi, J.F. Effect of Mycobacterium tuberculosis infection on adipocyte physiology. Microbes Infect. 2018, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, J.P.; Ganapathi, U.; Lizardo, K.; Vinnard, C.; Subbian, S.; Perlin, D.S.; Nagajyothi, J.F. Adipose Tissue Regulates Pulmonary Pathology during TB Infection. mBio 2019, 10, e02771-18. [Google Scholar] [CrossRef]
- Martinez-Colon, G.J.; Ratnasiri, K.; Chen, H.; Jiang, S.; Zanley, E.; Rustagi, A.; Verma, R.; Chen, H.; Andrews, J.R.; Mertz, K.D.; et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci. Transl. Med. 2022, 14, eabm9151. [Google Scholar] [CrossRef]
- Olivo, A.; Marlin, R.; Lazure, T.; Maisonnasse, P.; Bossevot, L.; Mouanga, C.; Lemaitre, J.; Pourcher, G.; Benoist, S.; Le Grand, R.; et al. Detection of SARS-CoV-2 in subcutaneous fat but not visceral fat, and the disruption of fat lymphocyte homeostasis in both fat tissues in the macaque. Commun. Biol. 2022, 5, 542. [Google Scholar] [CrossRef]
- Gomez, J.M.D.; Du-Fay-de-Lavallaz, J.M.; Fugar, S.; Sarau, A.; Simmons, J.A.; Clark, B.; Sanghani, R.M.; Aggarwal, N.T.; Williams, K.A.; Doukky, R.; et al. Sex Differences in COVID-19 Hospitalization and Mortality. J. Womens Health 2021, 30, 646–653. [Google Scholar] [CrossRef]
- Aguilar-Pineda, J.A.; Albaghdadi, M.; Jiang, W.; Vera-Lopez, K.J.; Nieto-Montesinos, R.; Alvarez, K.L.F.; Davila Del-Carpio, G.; Gomez, B.; Lindsay, M.E.; Malhotra, R.; et al. Structural and Functional Analysis of Female Sex Hormones against SARS-CoV-2 Cell Entry. Int. J. Mol. Sci. 2021, 22, 11508. [Google Scholar] [CrossRef]
- Al-Benna, S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef]
- Nassar, A.; Ibrahim, I.M.; Amin, F.G.; Magdy, M.; Elgharib, A.M.; Azzam, E.B.; Nasser, F.; Yousry, K.; Shamkh, I.M.; Mahdy, S.M.; et al. A Review of Human Coronaviruses’ Receptors: The Host-Cell Targets for the Crown Bearing Viruses. Molecules 2021, 26, 6455. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef]
- Fecchi, K.; Anticoli, S.; Peruzzu, D.; Iessi, E.; Gagliardi, M.C.; Matarrese, P.; Ruggieri, A. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front. Microbiol. 2020, 11, 1821. [Google Scholar] [CrossRef]
- Palacios-Rapalo, S.N.; De Jesus-Gonzalez, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martinez-Mier, G.; Quistian-Galvan, J.; Munoz-Perez, A.; Bernal-Dolores, V.; Del Angel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.G.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; Nunes da Silva, M.A.; Barreto, E.; Mattos, M.; et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020, 16, e1009127. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- van Dierendonck, X.; Vrieling, F.; Smeehuijzen, L.; Deng, L.; Boogaard, J.P.; Croes, C.A.; Temmerman, L.; Wetzels, S.; Biessen, E.; Kersten, S.; et al. Triglyceride breakdown from lipid droplets regulates the inflammatory response in macrophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2114739119. [Google Scholar] [CrossRef]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 2010, 120, 3466–3479. [Google Scholar] [CrossRef]
- Varghese, M.; Griffin, C.; McKernan, K.; Eter, L.; Lanzetta, N.; Agarwal, D.; Abrishami, S.; Singer, K. Sex Differences in Inflammatory Responses to Adipose Tissue Lipolysis in Diet-Induced Obesity. Endocrinology 2019, 160, 293–312. [Google Scholar] [CrossRef]
- Green, A.; Dobias, S.B.; Walters, D.J.; Brasier, A.R. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology 1994, 134, 2581–2588. [Google Scholar] [CrossRef]
- Reed, D.R.; Bachmanov, A.A.; Tordoff, M.G. Forty mouse strain survey of body composition. Physiol. Behav. 2007, 91, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.E.; Dukay, B.; Peter, M.; Balogh, G.; Szucs, G.; Zvara, A.; Szebeni, G.J.; Hajdu, P.; Sarkozy, M.; Puskas, L.G.; et al. Male and Female Animals Respond Differently to High-Fat Diet and Regular Exercise Training in a Mouse Model of Hyperlipidemia. Int. J. Mol. Sci. 2021, 22, 4198. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006, 74, 443–477. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, F.; Weiss, L.M.; Zhao, D.; Koba, W.; Jelicks, L.A.; Cui, M.H.; Factor, S.M.; Scherer, P.E.; Tanowitz, H.B. High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl. Trop. Dis. 2014, 8, e3118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).