Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes
Abstract
1. Introduction
1.1. Plant Materials and Treatments
1.2. Potassium Content in Grape Fruits and Soil
1.3. Berry Skin Color Measurement
1.4. Fruit Quality Parameters
1.5. Glucose and Fructose Content
1.6. Tartaric Acid and Malic Acid Content
1.7. RNA-Seq Data Analysis
1.8. Functional Classification Enrichment Analyses
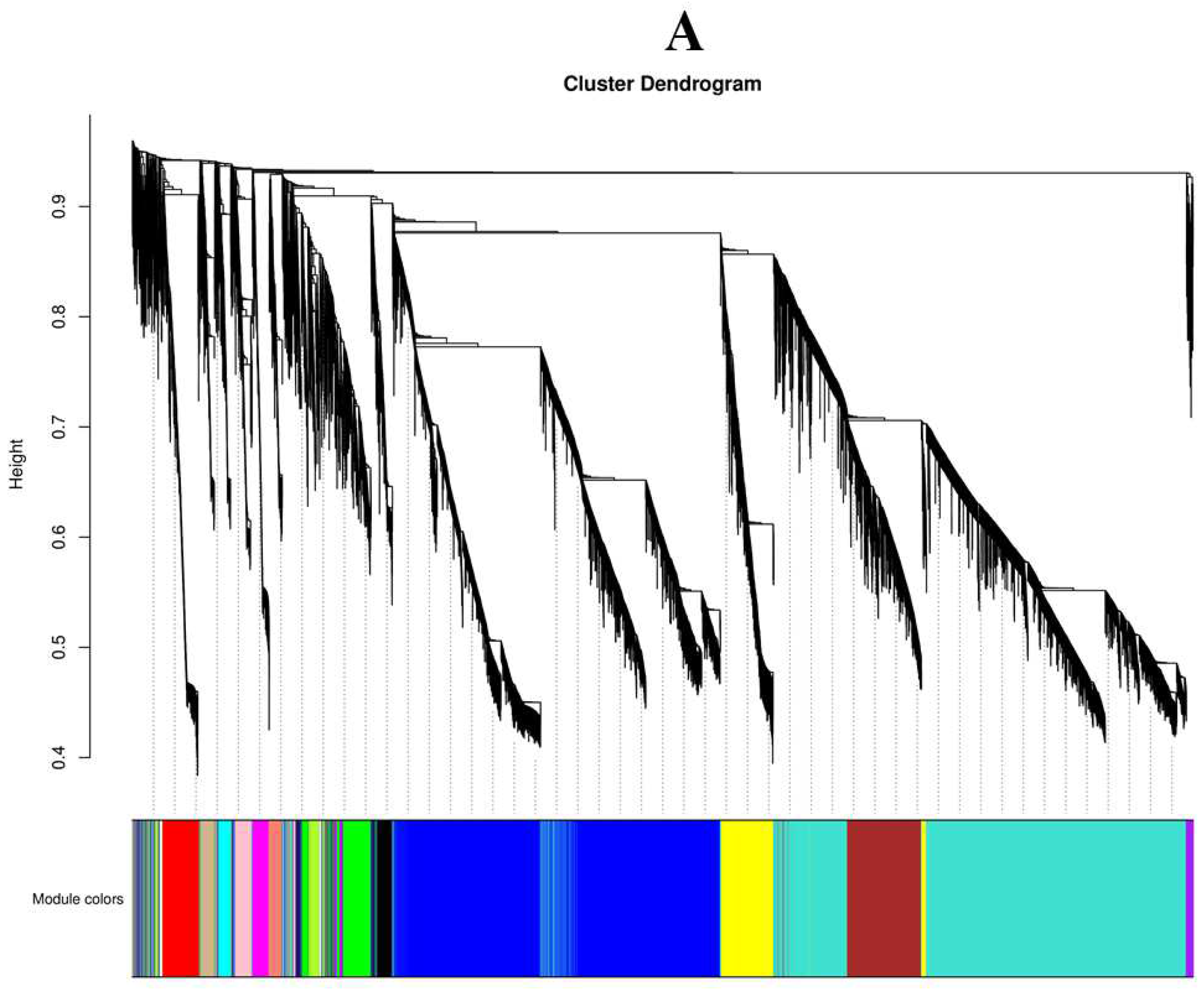
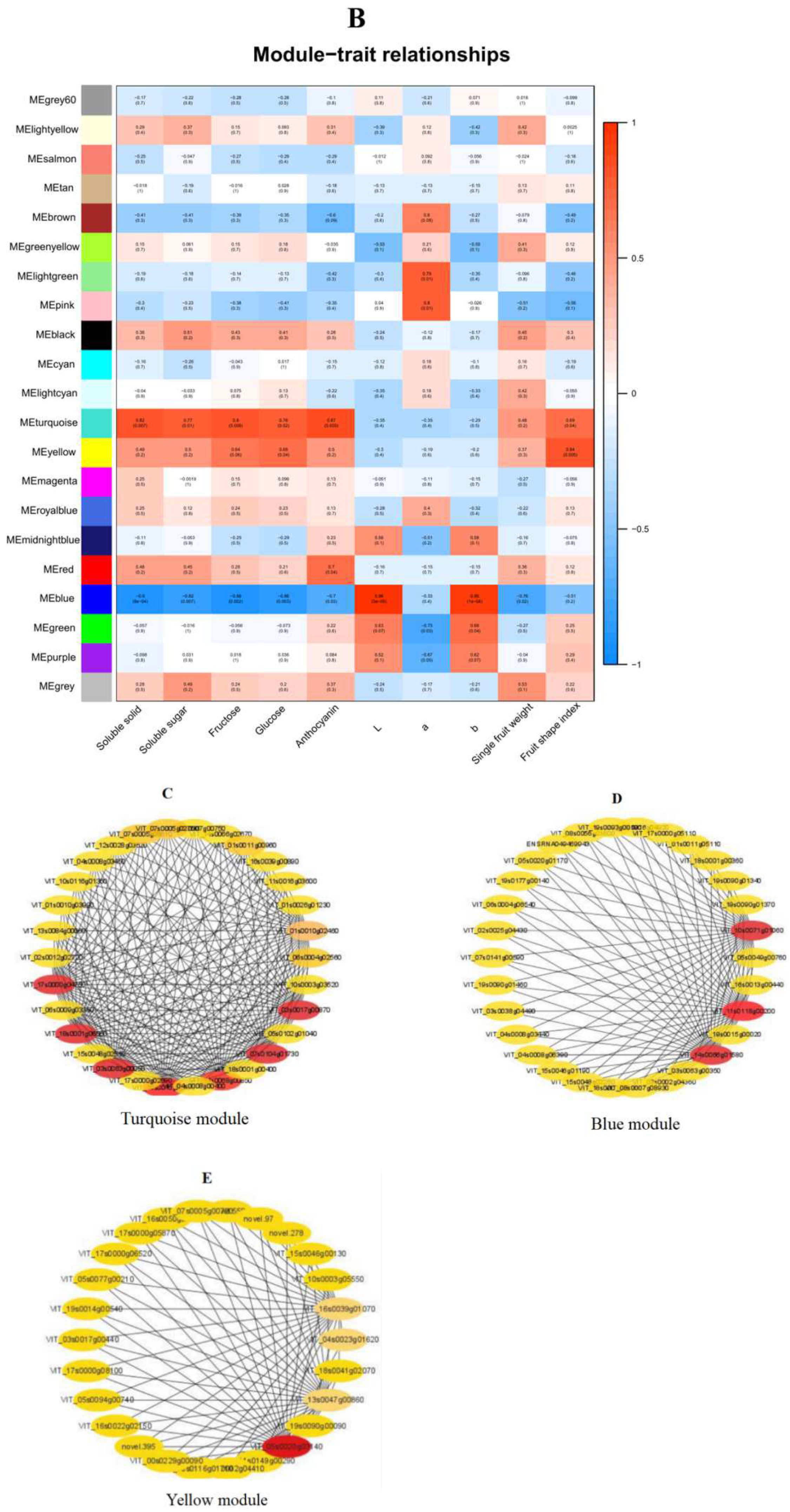
1.9. Construction of Gene Co-Expression Network Module
1.10. Gene Expression Analysis by Quantitative RT-PCR (RT-qPCR)
1.11. Data Statistics and Analysis
2. Results
2.1. Grape Fruit Potassium Content
2.2. Soil Potassium Content
2.3. Anthocyanin and Soluble Solids Content
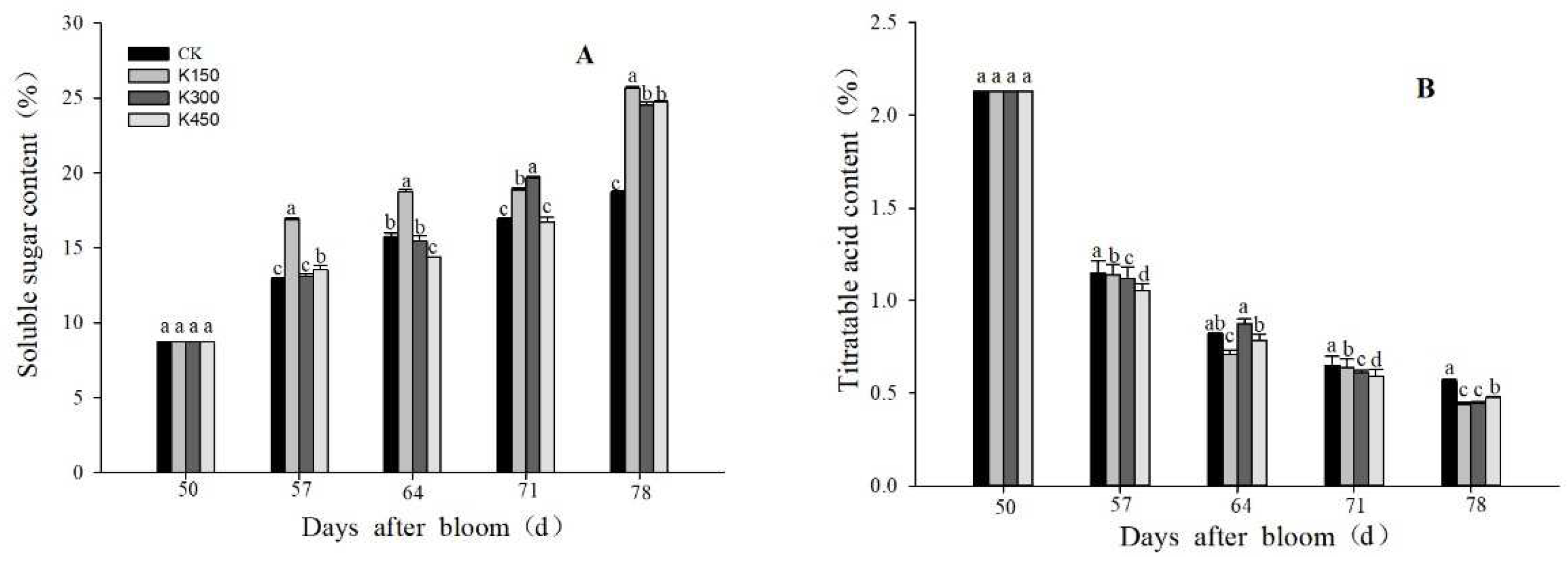
2.4. Soluble Sugars and TA Content
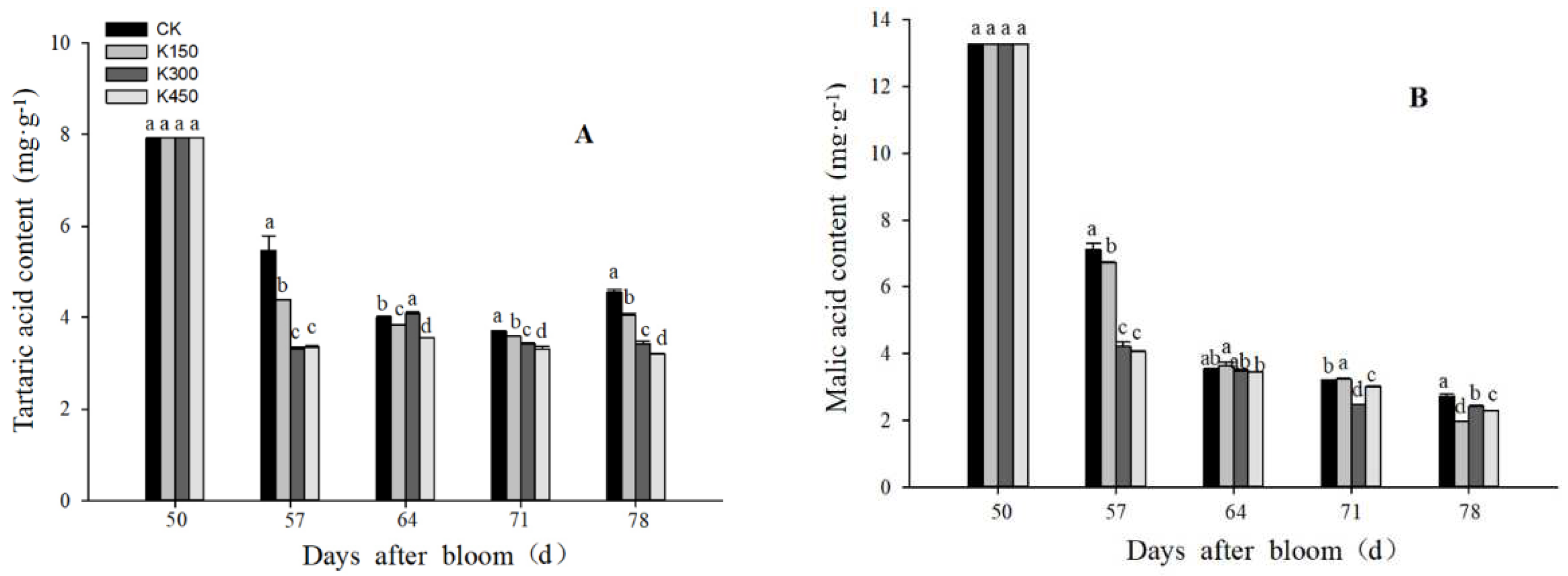
2.5. Glucose, Fructose, Tartaric Acid, and Malic Acid Content
2.6. Berry Skin Color
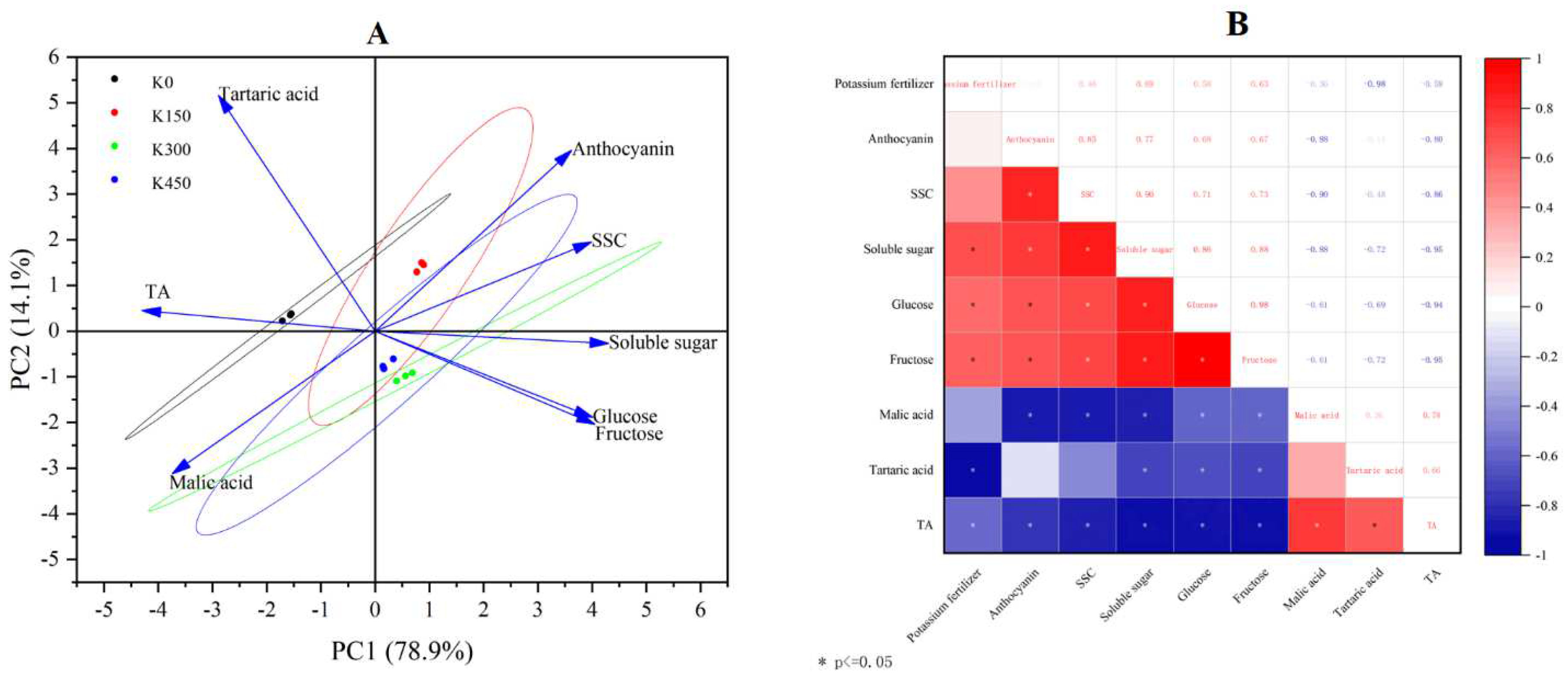
2.7. Principal Component Analysis (PCA) and Correlation Analysis
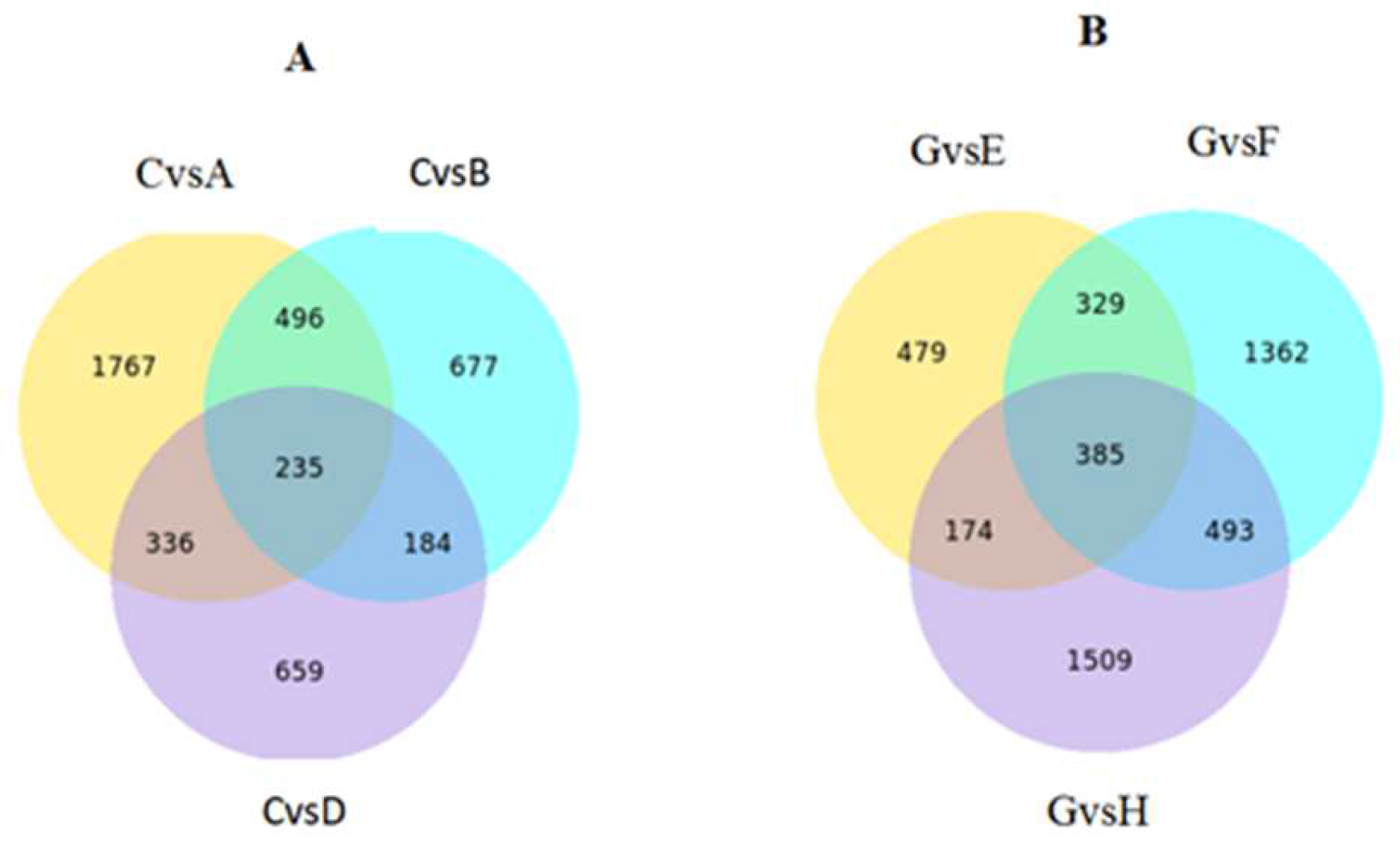
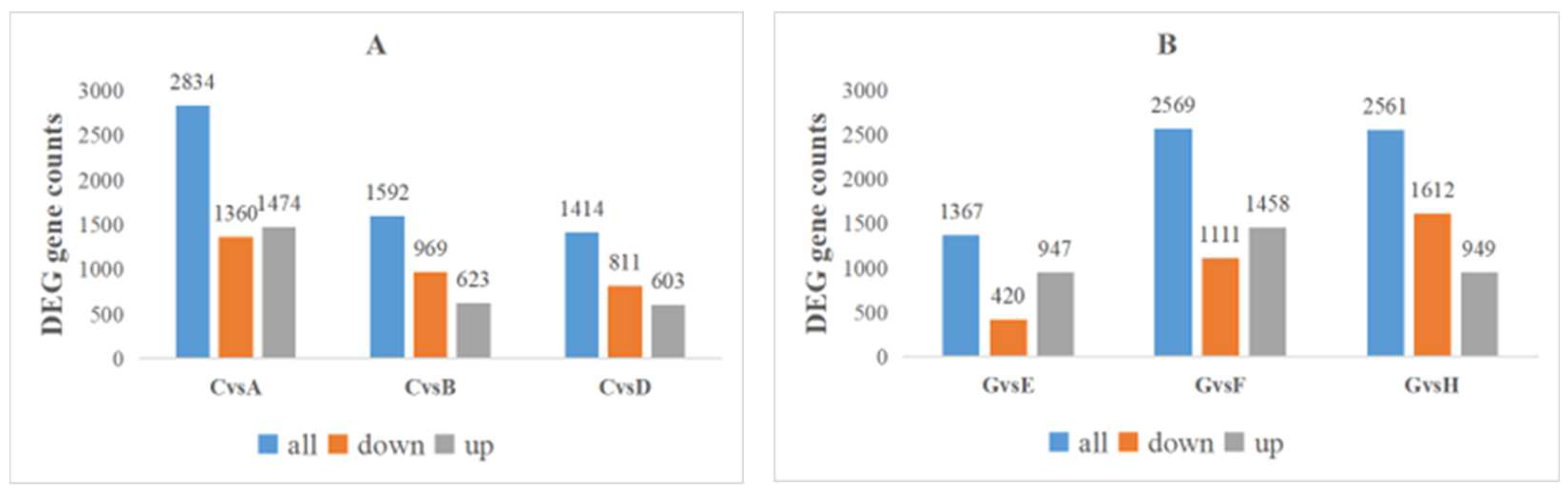
2.8. DEG Identification after K Treatment
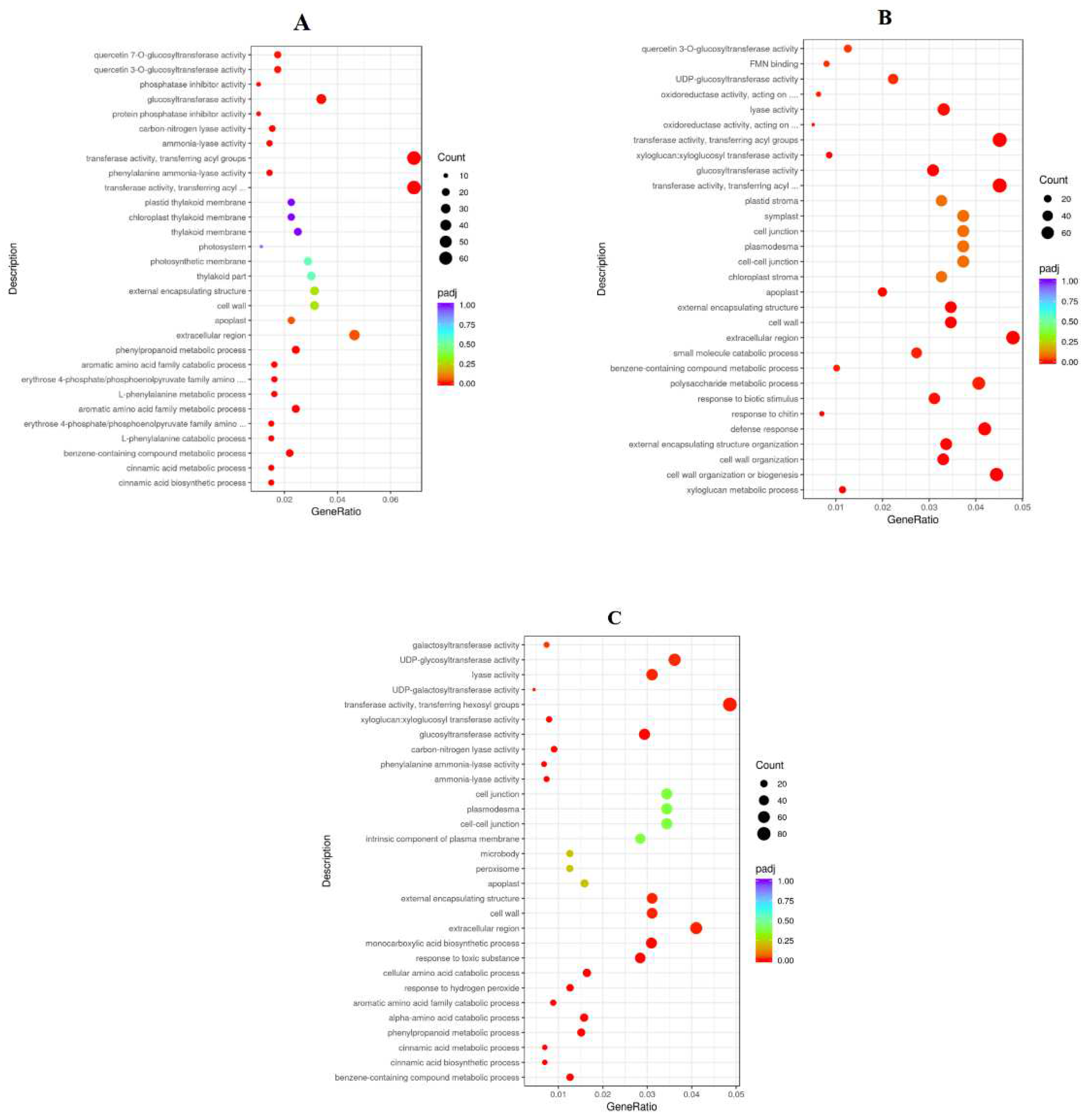
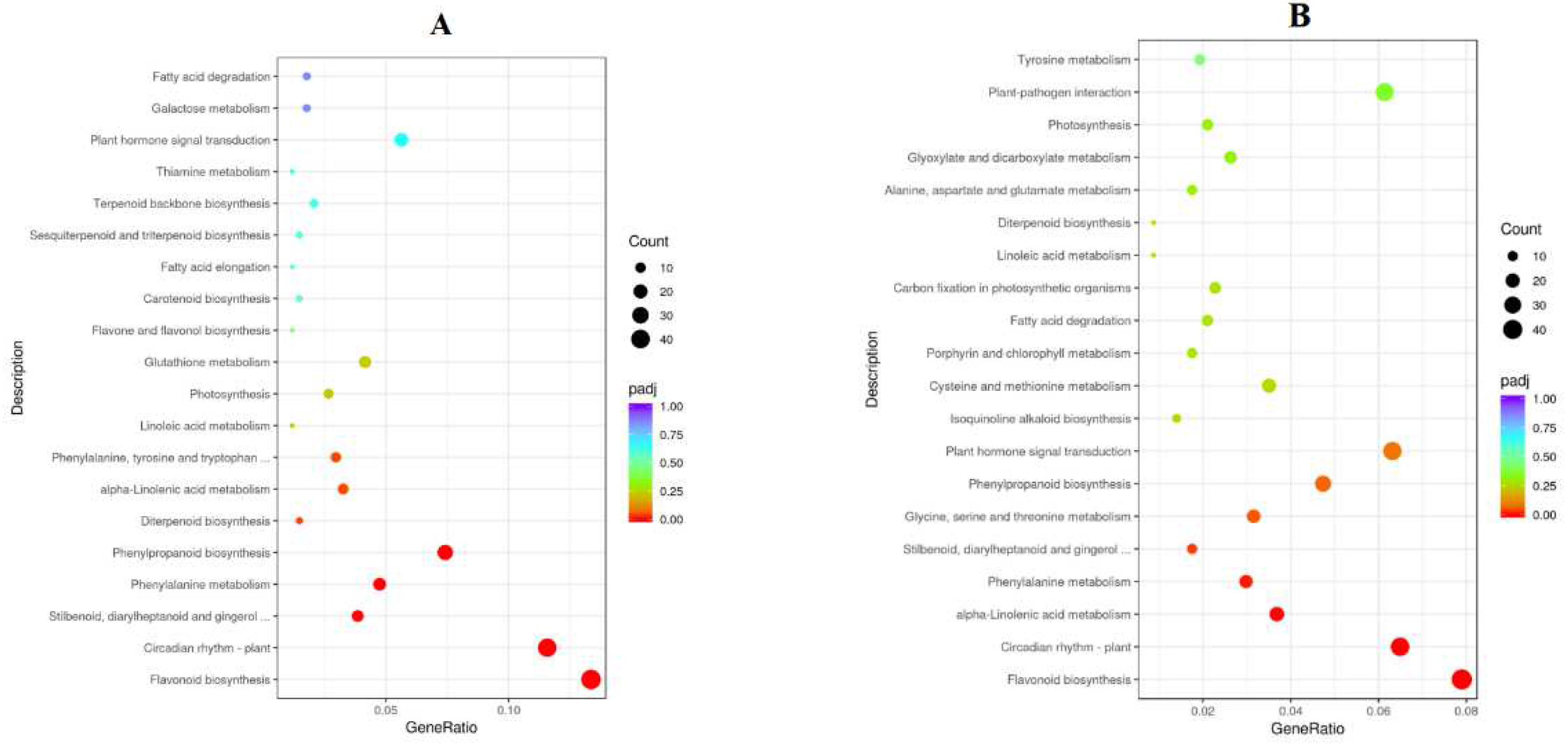
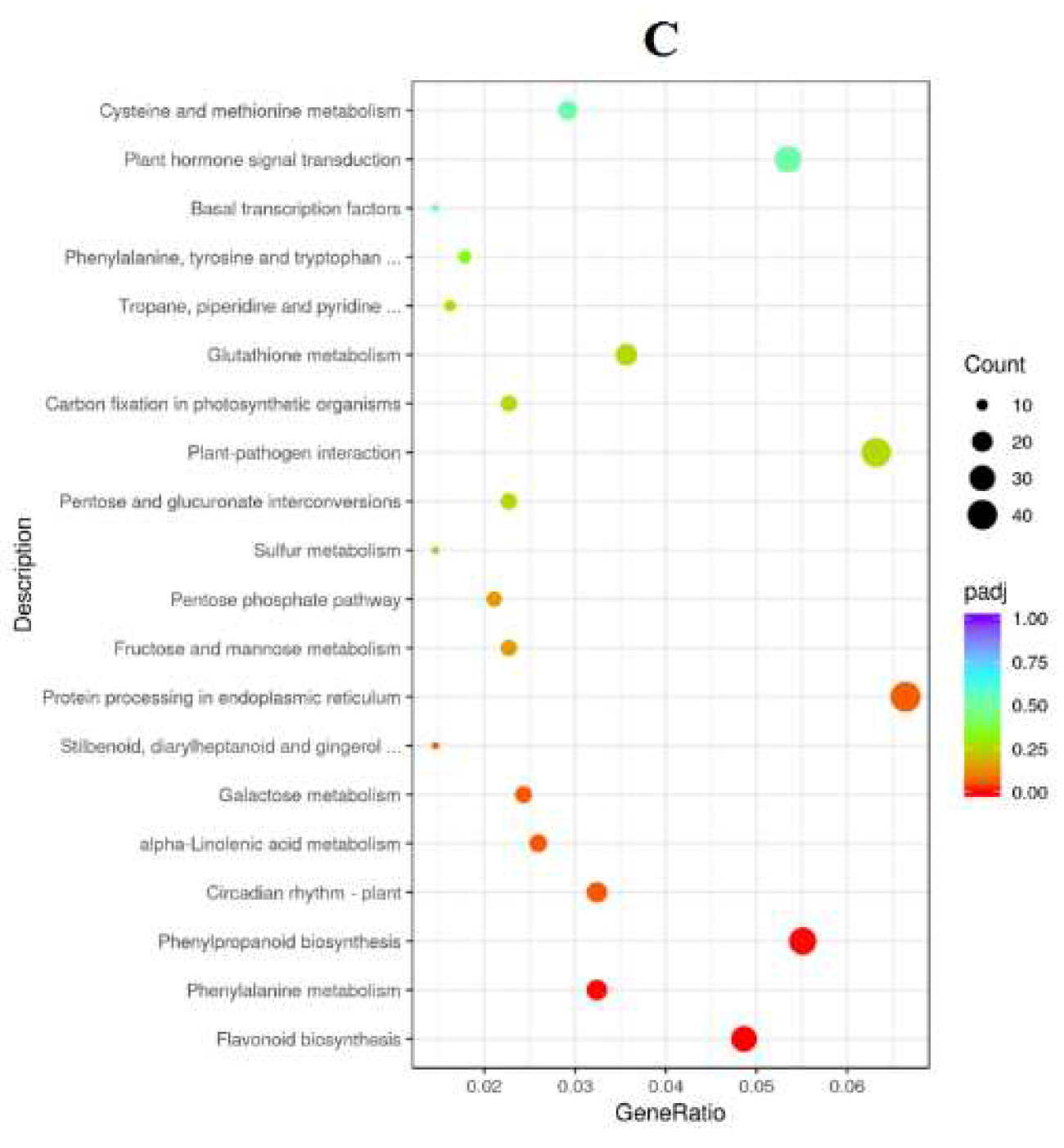
2.9. Functional Classification of DEGs
2.10. Constructing the Co-Expression Network and Identification of Key Candidates
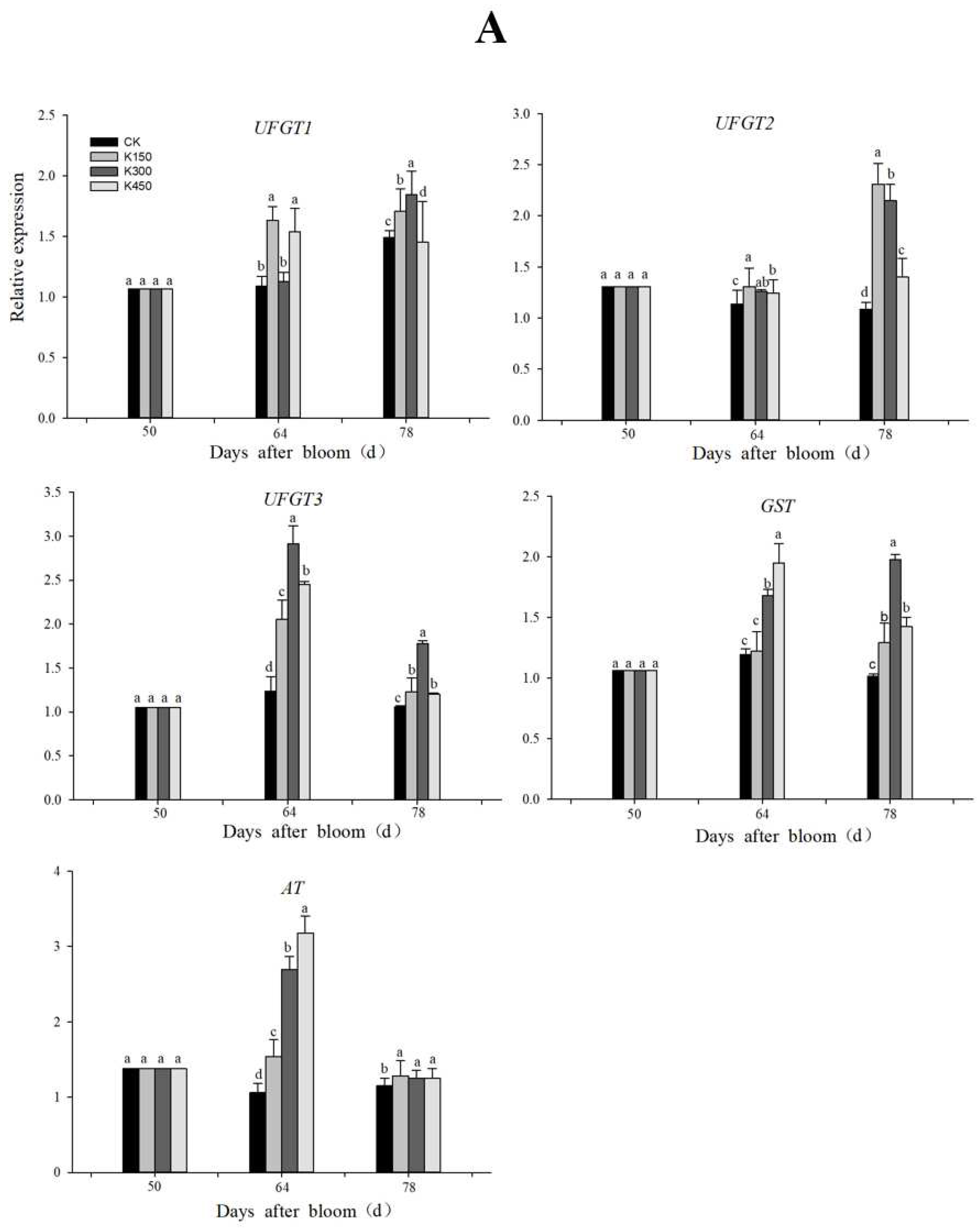
2.11. RT-qPCR Analysis of Differentially Expressed Genes
3. Discussion
3.1. Anthocyanin Synthesis-Related Genes Responses to K Treatment
3.2. Sugar Metabolism-Related Genes Responses to K Treatment
3.3. Potassium Transporter-Related Genes Responses to K Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic Acid Is a Major Regulator of Grape Berry Ripening Onset: New Insights into ABA Signaling Network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- FAO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 May 2022).
- Colombo, R.C.; Roberto, S.R.; da Cruz, M.A.; de Carvalho, D.U.; Yamamoto, L.Y.; Nixdorf, S.L.; Perez-Navarro, J.; Gomez-Alonso, S.; Shahab, M.; Ahmed, S.; et al. Characterization of the phenolic ripening development of ‘BRS Vitoria’ seedless table grapes using HPLC-DAD-ESI-MS/MS. J. Food Compos. Anal. 2021, 95, 103693. [Google Scholar] [CrossRef]
- Villette, J.; Cuellar, T.; Verdeil, J.-L.; Delrot, S.; Gaillard, I. Grapevine Potassium Nutrition and Fruit Quality in the Context of Climate Change. Front. Plant Sci. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.-F.; Wu, W.-H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Pushpavathi, Y.; Satisha, J.; Satisha, G.C.; Shivashankara, K.S.; Reddy, M.L.; Sriram, S. Influence of different sources and methods of potassium application on the quality of grapes cv. Sharad Seedless (Vitis vinifera L.). Curr. Sci. 2020, 118, 639–643. [Google Scholar] [CrossRef]
- Strydom, J. The Effect of Foliar Potassium and Seaweed Products in Combination with a Leonardite Fertigation Product on Flame Seedless Grape Quality. S. Afr. J. Enol. Vitic. 2014, 35, 283–291. [Google Scholar] [CrossRef]
- Obenland, D.; Feliziani, E.; Zhu, S.; Zhao, X.; Margosan, D.A.; Gabler, F.M.; Van Zyl, S.; Romanazzi, G.; Smilanick, J.L.; Beno-Moualem, D.; et al. Potassium application to table grape clusters after veraison increases soluble solids by enhancing berry water loss. Sci. Hortic. 2015, 187, 58–64. [Google Scholar] [CrossRef]
- Feliziani, E.; Smilanick, J.L.; Margosan, D.A.; Mansour, M.F.; Romanazzi, G.; Gu, S.; Gohil, H.L.; Ames, Z.R. Preharvest Fungicide, Potassium Sorbate, or Chitosan Use on Quality and Storage Decay of Table Grapes. Plant Dis. 2013, 97, 307–314. [Google Scholar] [CrossRef]
- Yao, X.; Horie, T.; Xue, S.; Leung, H.-Y.; Katsuhara, M.; Brodsky, D.E.; Wu, Y.; Schroeder, J.I. Differential sodium and potassium transport selectivities of the rice OsHKT2;1 and OsHKT2;2 transporters in plant cells. Plant Physiol. 2009, 152, 341–355. [Google Scholar] [CrossRef]
- Camarena, L.; Bruno, V.; Euskirchen, G.; Poggio, S.; Snyder, M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010, 6, e1000834. [Google Scholar] [CrossRef]
- Venturini, L.; Ferrarini, A.; Zenoni, S.; Tornielli, G.B.; Fasoli, M.; Dal Santo, S.; Minio, A.; Buson, G.; Tononi, P.; Zago, E.D.; et al. De novo transcriptome characterization of Vitis vinifera cv. Corvina unveils varietal diversity. BMC Genom. 2013, 14, 41. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Xia, H.; Wang, X.; Su, W.; Jiang, L.; Lin, L.; Deng, Q.; Wang, J.; Deng, H.; Hu, R.; Liao, M.; et al. Changes in the carotenoids profile of two yellow-fleshed kiwifruit cultivars during storage. Postharvest Biol. Technol. 2020, 164, 111162. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of Exogenous Application of Melatonin on Quality and Sugar Metabolism in ‘Zaosu’ Pear Fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragariaxananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of key gene networks controlling organic acid and sugar metabolism during watermelon fruit development by integrating metabolic phenotypes and gene expression profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Gurak, P.D.; Ferreira Marczak, L.D.; Tessaro, I.C. Tracking bioactive compounds with colour changes in foods—A review. Dye. Pigment. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Zoerb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-L.; Wu, W.-H.; Wang, Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Ling, Q.; Fan, L.; Li, Y.; Hu, F.; Chen, J.; Huang, Z.; Deng, H.; Li, Q.; Qi, Y. Transcriptome Profiling of Sugarcane Roots in Response to Low Potassium Stress. PLoS ONE 2015, 10, e0126306. [Google Scholar] [CrossRef]
- Cao, T.; Lahiri, I.; Singh, V.; Louis, J.; Shah, J.; Ayre, B.G. Metabolic engineering of raffinose-family oligosaccharides in the phloem reveals alterations in carbon partitioning and enhances resistance to green peach aphid. Front. Plant Sci. 2013, 4, 263. [Google Scholar] [CrossRef]
- Smith, C.R.; Knowles, V.L.; Plaxton, W.C. Purification and characterization of cytosolic pyruvate kinase from Brassica napus (rapeseed) suspension cell cultures: Implications for the integration of glycolysis with nitrogen assimilation. Eur. J. Biochem. 2000, 267, 4477–4485. [Google Scholar] [CrossRef]
- Armengaud, P.; Sulpice, R.; Miller, A.J.; Stitt, M.; Amtmann, A.; Gibon, Y. Multilevel Analysis of Primary Metabolism Provides New Insights into the Role of Potassium Nutrition for Glycolysis and Nitrogen Assimilation in Arabidopsis Roots. Plant Physiol. 2009, 150, 772–785. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef]
- Chen, G.; Feng, H.; Hu, Q.; Qu, H.; Chen, A.; Yu, L.; Xu, G. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol. J. 2015, 13, 833–848. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Hu, Y.; Wu, F.; Hu, Q.; Chen, G.; Cai, J.; Wu, T.; Moran, N.; Yu, L.; et al. The Role of a Potassium Transporter OsHAK5 in Potassium Acquisition and Transport from Roots to Shoots in Rice at Low Potassium Supply Levels. Plant Physiol. 2014, 166, 945–959. [Google Scholar] [CrossRef]
- Philippar, K.; Buchsenschutz, K.; Abshagen, M.; Fuchs, I.; Geiger, D.; Lacombe, B.; Hedrich, R. The K+ channel KZM1 mediates potassium uptake into the phloem and guard cells of the C4 grass Zea mays. J. Biol. Chem. 2003, 278, 16973–16981. [Google Scholar] [CrossRef]



| Gene Number | Reference Gene Number in NCBI | Forward Primer (5′-3′) | TM (°C) | Reverse Primer (5′-3′) | TM (°C) |
|---|---|---|---|---|---|
| VIT_11s0118g00200 | XM_002265437 | TACTTGGAGGCGATTCTGGA | 55.4 | GTGGGACTGAATCTCCCTCT | 56.5 |
| VIT_03s0063g00050 | XM_002285067 | CTCGATGTGGAGAAGACAGC | 57.5 | CCTGAGACCTCGTCAATTCG | 57.5 |
| VIT_14s0068g00850 | XM_010662357 | GAAACCCTTCCCAGCTTTCA | 57.5 | AAAGTGGTGAAGTGCTCAGG | 57.5 |
| VIT_03s0017g00870 | XM_010649854 | GATGGATTCACTGCTGCTCA | 55.4 | TGGCAAGAAAGAGGCCAAAT | 54.4 |
| VIT_10s0071g01060 | XM_010657659 | GCCAGTAGTTACTGCCACTC | 57.5 | CTCTCCACTGAGCATGACAC | 57.5 |
| VIT_14s0066g01580 | XM_010662640 | GCTTGCTGATGAGGAACTGT | 55.4 | AGCCAGAACAAGCAATACCC | 55.4 |
| VIT_07s0104g01730 | NM_001280973 | TGAGGTGATGGCAGAGAAAG | 55.4 | TTGTGTGGAATGTCAAATACCTT | 54.4 |
| VIT_19s0015g02680 | XM_003634701 | GCTTCTTGAGATGAACCCGA | 57.5 | GGTATGGGTCACTAGGCAAC | 57.5 |
| VIT_05s0020g03140 | NM_001281278 | CGTCTCTATATCTTGCGGGC | 57.5 | CGGTATTAAGCACCACTCCC | 57.5 |
| VIT_18s0001g06060 | XM_002285734 | CCAAACTCAACGACAGAGGT | 55.4 | ACTGCACATAGGCCATGAAG | 55.4 |
| VIT_17s0000g04750 | XM_002268053 | GGGCGTGTCACATATTGTCT | 56.5 | GGGGAATTGGGAATGCTAGG | 57.5 |
| VvGAPDH | GU585870 | TTCTCGTTGAGGGCTATTCCA | 57.5 | CCACAGACTTCATCGGTGACA | 57.5 |
| Days | Treatment | |||
|---|---|---|---|---|
| K0-CK | K150 | K300 | K450 | |
| 50 DAB | 1.135 ± 0.029 a | 1.135 ± 0.029 a | 1.135 ± 0.029 a | 1.135 ± 0.029 a |
| 64 DAB | 1.142 ± 0.002 b | 1.154 ± 0.006 b | 1.256 ± 0.017 a | 1.255 ± 0.002 a |
| 78 DAB | 1.188 ± 0.013 d | 1.332 ± 0.002 b | 1.370 ± 0.019 a | 1.241 ± 0.020 c |
| Days | Treatment | |||
|---|---|---|---|---|
| K0-CK | K150 | K300 | K450 | |
| 78 DAB | 27.239 ± 0.156 a | 24.184 ± 0.099 d | 25.080 ± 0.086 c | 25.565 ± 0.166 b |
| Treatment | L* | a* | b* |
|---|---|---|---|
| K0-CK | 23.18 ± 0.20 a | 1.27 ± 0.02 b | −1.02 ± 0.14 a |
| K150 | 22.43 ± 0.12 b | −1.69 ± 0.06 d | −1.35 ± 0.08 b |
| K300 | 21.48 ± 0.16 c | 1.00 ± 0.08 c | −1.88 ± 0.09 c |
| K450 | 22.41 ± 0.25 b | 1.47 ± 0.03 a | −1.48 ± 0.02 b |
| Days | Sample | Total Raw Reads | Total Clean Reads | Clean Bases | GC Percentage | Q20 Percentage | Q30 Percentage |
|---|---|---|---|---|---|---|---|
| 50 DAB | T-CK | 47158728 | 45794224 | 6.87 G | 46.93 | 97.66 | 93.59 |
| 64 DAB | A-CK | 46651772 | 45547160 | 6.83 G | 47.04 | 97.89 | 94.11 |
| B-K150 | 47439556 | 45496384 | 6.82 G | 47.12 | 97.37 | 92.73 | |
| C-K300 | 45645408 | 43878926 | 6.58 G | 47.08 | 97.71 | 93.76 | |
| D-K450 | 46533625 | 44951584 | 6.74 G | 47.84 | 97.28 | 92.85 | |
| 78 DAB | E-CK | 47322815 | 45934790 | 6.89 G | 47.69 | 97.42 | 93.11 |
| F-K150 | 49139536 | 47400020 | 7.11 G | 46.85 | 97.56 | 93.38 | |
| G-K300 | 43948610 | 42425047 | 6.37 G | 48.40 | 97.17 | 92.95 | |
| H-K450 | 45027932 | 43493211 | 6.52 G | 47.24 | 97.56 | 93.54 |
| KEGG ID | Metabolic Pathways | 64 DAB | 78 DAB | Total Number of Differentially Expressed Genes for Pathway Annotations | ||||
|---|---|---|---|---|---|---|---|---|
| AVSC | BVSC | CVSD | EVSG | FVSG | GVSH | |||
| vvi0480 | Glutathione metabolism | 15 | 16 | 5 | 14 | 18 | 22 | 137 |
| vvi0052 | Galactose metabolism | 17 | 13 | 9 | 6 | 6 | 15 | 90 |
| vvi04075 | Plant hormone signal transduction | 36 | 20 | 17 | 19 | 36 | 15 | 66 |
| vvi0051 | Fructose and mannose metabolism | 14 | 7 | 6 | 6 | 7 | 14 | 143 |
| vvi00010 | Glycolysis/Gluconeogenesis | 30 | 13 | 22 | 8 | 9 | 19 | 54 |
| vvi00500 | Starch and sucrose metabolism | 24 | 20 | 16 | 8 | 19 | 19 | 101 |
| vvi04626 | Plant-pathogen interaction | 30 | 15 | 15 | 16 | 35 | 39 | 106 |
| vvi00061 | Fatty acid biosynthesis | 4 | 4 | 4 | 3 | 7 | 5 | 150 |
| vvi00941 | Flavonoid biosynthesis | 9 | 4 | 4 | 45 | 45 | 30 | 27 |
| Gene Number | Module | Weight | Annotation |
|---|---|---|---|
| VIT_10s0071g01060 | blue | 0.3923 | Pyruvate kinase (PK) |
| VIT_11s0118g00200 | blue | 0.4789 | Sucrose-phosphate synthase (SPS) |
| VIT_05s0020g03140 | yellow | 0.5734 | Hexose transporter (HT) |
| VIT_03s0063g00050 | turquoise | 0.5236 | Glycosyltransferase (UFGT1) |
| VIT_17s0000g04750 | turquoise | 0.4572 | Glycosyltransferase (UFGT2) |
| VIT_19s0015g02680 | turquoise | 0.5267 | Glutathione-S-transferase (GST1) |
| VIT_14s0068g00850 | turquoise | 0.5182 | Potassium transporter (KUP1) |
| VIT_14s0066g01580 | blue | 0.4959 | Potassium transporter (KUP2) |
| VIT_03s0017g00870 | turquoise | 0.4123 | Anthocyanin acyltransferase (AT) |
| VIT_07s0104g01730 | turquoise | 0.5097 | Potassium transporter (KUP3) |
| VIT_18s0001g06060 | turquoise | 0.4996 | Glycosyltransferase (UFGT3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Zhao, X.; Xiao, Q.; Hu, W.; Wang, P.; Luo, Y.; Xia, H.; Lin, L.; Lv, X.; Liang, D.; et al. Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes. Int. J. Mol. Sci. 2023, 24, 1218. https://doi.org/10.3390/ijms24021218
Huang H, Zhao X, Xiao Q, Hu W, Wang P, Luo Y, Xia H, Lin L, Lv X, Liang D, et al. Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes. International Journal of Molecular Sciences. 2023; 24(2):1218. https://doi.org/10.3390/ijms24021218
Chicago/Turabian StyleHuang, Hong, Xiaoyan Zhao, Qiao Xiao, Wenjie Hu, Pei Wang, Yuanyou Luo, Hui Xia, Lijin Lin, Xiulan Lv, Dong Liang, and et al. 2023. "Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes" International Journal of Molecular Sciences 24, no. 2: 1218. https://doi.org/10.3390/ijms24021218
APA StyleHuang, H., Zhao, X., Xiao, Q., Hu, W., Wang, P., Luo, Y., Xia, H., Lin, L., Lv, X., Liang, D., & Wang, J. (2023). Identification of Key Genes Induced by Different Potassium Levels Provides Insight into the Formation of Fruit Quality in Grapes. International Journal of Molecular Sciences, 24(2), 1218. https://doi.org/10.3390/ijms24021218






