A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression
Abstract
1. Introduction
2. Results
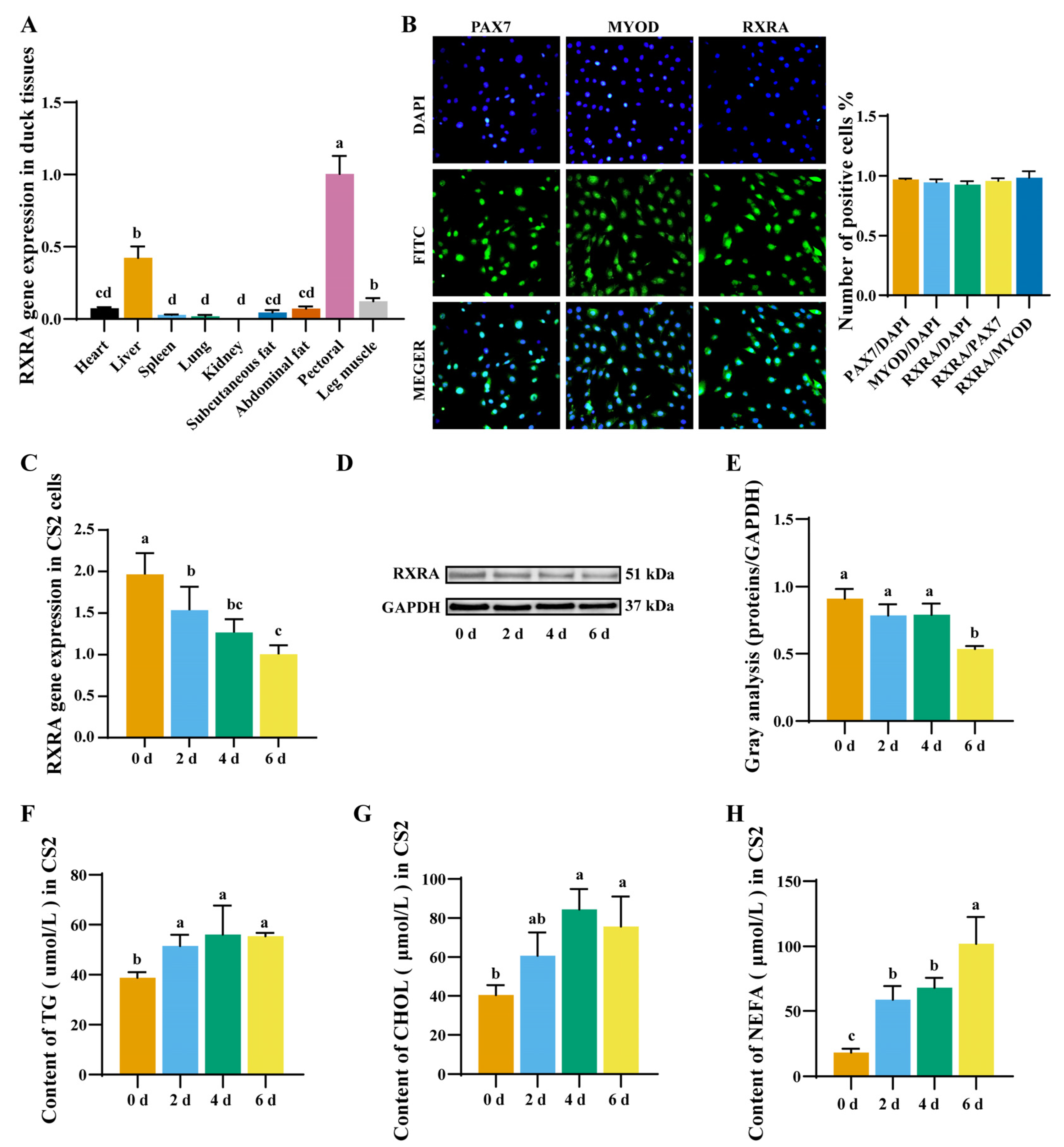
2.1. Expression Pattern of RXRA Gene in Duck Myoblasts
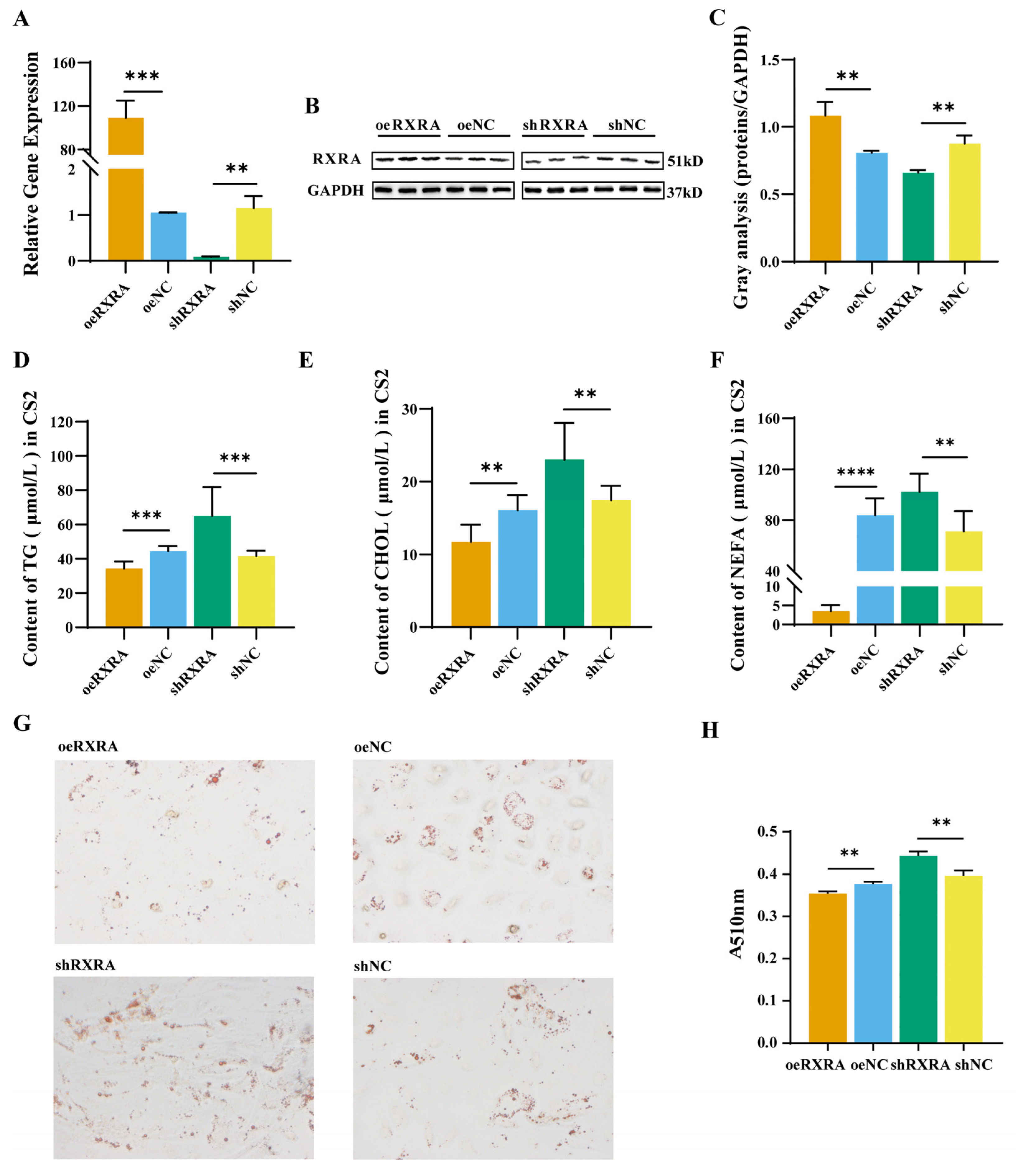
2.2. The RXRA Gene Inhibits the Accumulation of TGs, CHOL, and NEFAs in Myoblasts
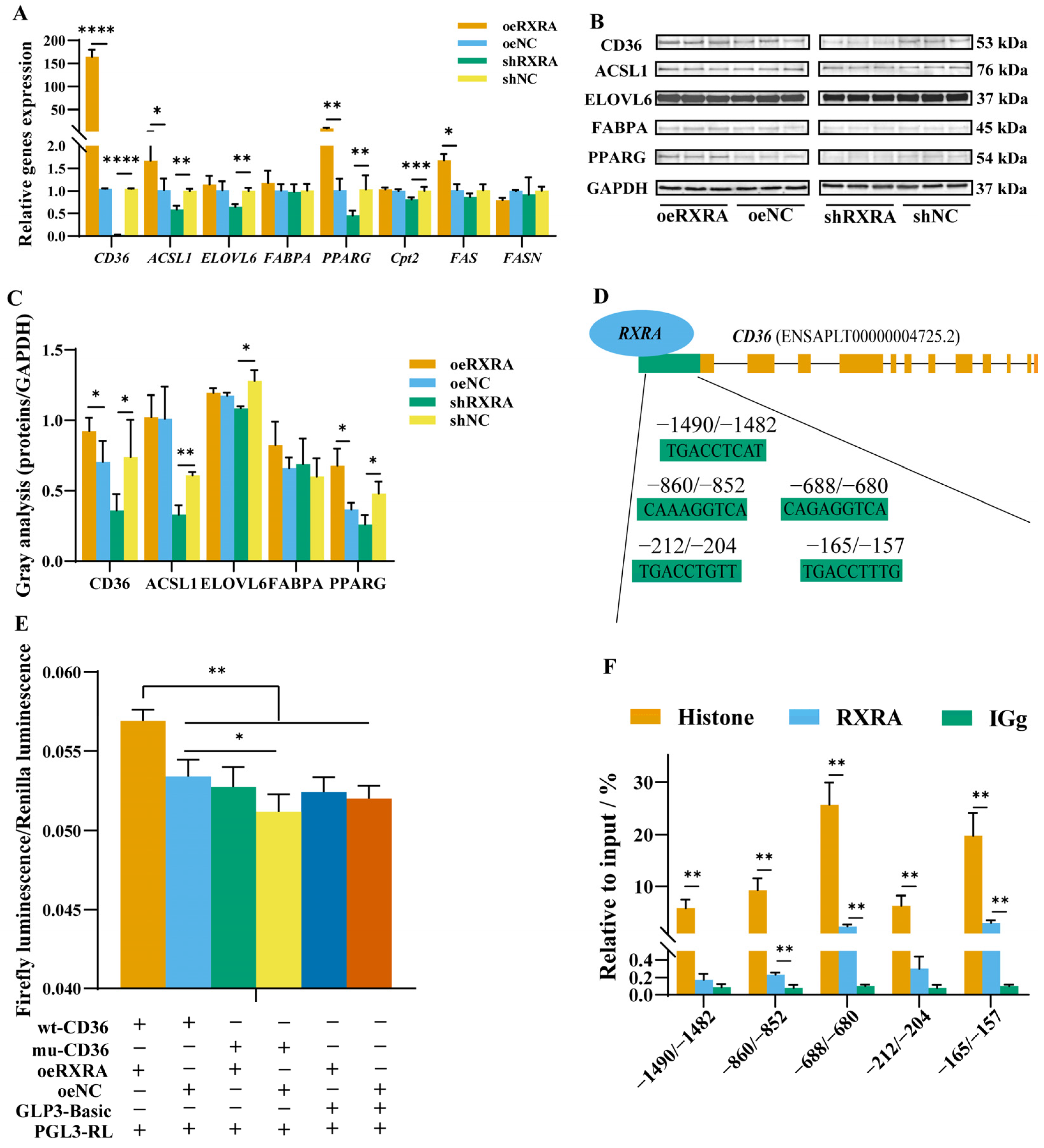
2.3. RXRA Promotes Gene Expression in PPAR-Signaling Pathway
2.4. RXRA Promotes CD36 Expression by Binding to the CD36 Promoter
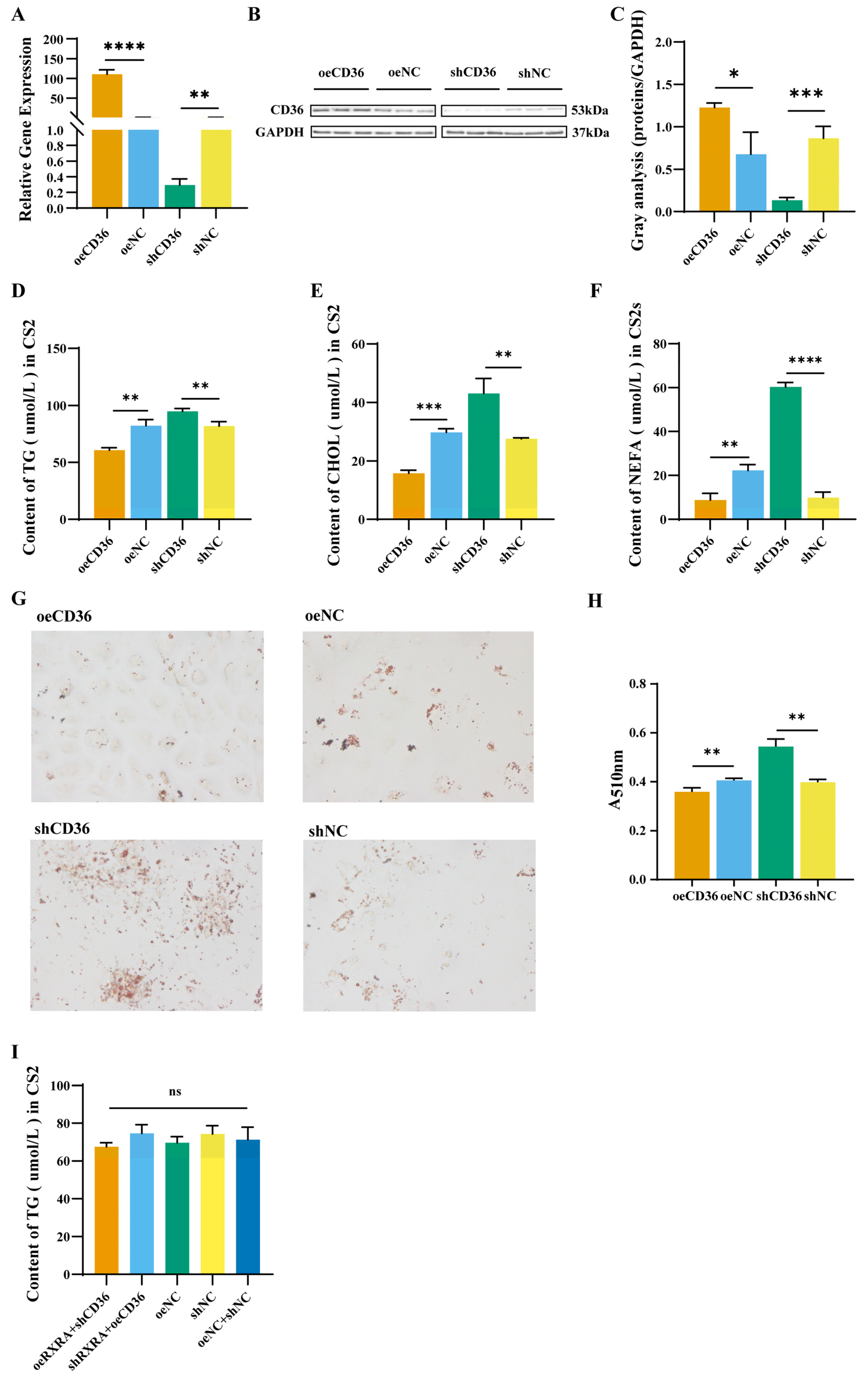
2.5. The CD36 Inhibits the Accumulation of TGs, CHOL, and NEFAs in Myoblasts
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA Isolation and Quantitative Real-Time PCR (RT-PCR)
4.3. Primary Myoblasts Separation, Culture, and Differentiation
4.4. Immunofluorescence
4.5. Western Blotting
4.6. Analyses of the Intracellular Triglyceride (TG), Cholesterol (CHOL), and Nonesterified Fatty Acid (NEFA) Contents
4.7. Vector Construction
4.8. Oil Red O Staining
4.9. Luciferase Reporter Assay
4.10. Chromatin Immunoprecipitation (CHIP)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gariglio, M.; Dabbou, S.; Gai, F.; Trocino, A.; Xiccato, G.; Holodova, M.; Gresakova, L.; Nery, J.; Bellezza Oddon, S.; Biasato, I.; et al. Black Soldier Fly Larva in Muscovy Duck Diets: Effects on Duck Growth, Carcass Property, and Meat Quality. Poult. Sci. 2021, 100, 101303. [Google Scholar] [CrossRef]
- Fan, W.; Liu, W.; Liu, H.; Meng, Q.; Xu, Y.; Guo, Y.; Wang, B.; Zhou, Z.; Hou, S. Dynamic Accumulation of Fatty Acids in Duck (Anas Platyrhynchos) Breast Muscle and Its Correlations with Gene Expression. BMC Genom. 2020, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Enser, M. Manipulating the Fatty Acid Composition of Meat to Improve Nutritional Value and Meat Quality. In New Aspects of Meat Quality; Woodhead Publishing: Sawston, UK, 2017; pp. 501–535. ISBN 978-0-08-100593-4. [Google Scholar]
- Daniel, A.; Sergey, T.; Qiu, B.; Evdokia, M.; Michelle, S.; Andrew, A.; Xie, H.; Celeste, S.M.; Kamphorst, J.J. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 2018, 24, 2596–2605.e5. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Relative Role of Phospholipids, Triacylglycerols, and Cholesterol Esters on Malonaldehyde Formation in Fat Extracted from Chicken Meat. J. Food Sci. 1984, 49, 704–708. [Google Scholar] [CrossRef]
- Laaksonen, D.E. Prediction of Cardiovascular Mortality in Middle-Aged Men by Dietary and Serum Linoleic and Polyunsaturated Fatty Acids. Arch. Intern. Med. 2005, 165, 193–199. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P. Dietary Monounsaturated Fatty Acids Are Protective Against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The Glucose Fatty-Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet 1963, 281, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Seo, Y.-K. Excess Accumulation of Lipid Impairs Insulin Sensitivity in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1949. [Google Scholar] [CrossRef]
- Stossi, F.; Dandekar, R.D.; Johnson, H.; Lavere, P.; Foulds, C.E.; Mancini, M.G.; Mancini, M.A. Tributyltin Chloride (TBT) Induces RXRA down-Regulation and Lipid Accumulation in Human Liver Cells. PLoS ONE 2019, 14, e0224405. [Google Scholar] [CrossRef]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors and Lipid Physiology: Opening the X-Files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Plutzky, J. The PPAR-RXR Transcriptional Complex in the Vasculature: Energy in the Balance. Circ. Res. 2011, 108, 1002. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Davies, P.J.A.; Crombie, D.L.; Bischoff, E.D.; Heyman, R.A. Sensitization of Diabetic and Obese Mice to Insulin by Retinoid X Receptor Agonists. J. Med. Chem. 1997, 386, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Benomar, Y.; Guédin, A.; Langlois, A.; Hennuyer, N.; Dumont, J.; Bouchaert, E.; Dacquet, C.; Pénicaud, L.; Casteilla, L.; et al. Proteasomal Degradation of Retinoid X Receptor Alpha Reprograms Transcriptional Activity of PPARgamma in Obese Mice and Humans. J. Clin. Investig. 2010, 120, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Lin, L. Small Molecules for Fat Combustion: Targeting Obesity. Acta Pharm. Sin. B 2019, 9, 220–236. [Google Scholar] [CrossRef]
- Haugen, B.R.; Jensen, D.R.; Vibha, S.; Pulawa, L.K.; Hays, W.R.; Wojciech, K.; Pierre, C.; Eckel, R.H. Retinoid X Receptor Gamma-Deficient Mice Have Increased Skeletal Muscle Lipoprotein Lipase Activity and Less Weight Gain When Fed a High-Fat Diet. Endocrinology 2004, 3679–3685. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.-M.; Kiens, B. Tuning Fatty Acid Oxidation in Skeletal Muscle with Dietary Fat and Ex Ercise. Nat. Rev. Endocrinol. 2020, 16, 683–696. [Google Scholar] [CrossRef]
- Kosters, A.; Sun, D.; Wu, H.; Tian, F.; Felix, J.C.; Li, W.; Karpen, S.J. Sexually Dimorphic Genome-Wide Binding of Retinoid X Receptor Alpha (RXRα) Determines Male-Female Differences in the Expression of Hepatic Lipid Processing Genes in Mice. PLoS ONE 2013, 8, e71538. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Umesono, K.; Noonan, D.J.; Heyman, R.A.; Evans, R.M. Convergence of 9-Cis Retinoic Acid and Peroxisome Proliferator Signalling Pathways through Heterodimer Formation of Their Receptors. Nature 1992, 358, 771–774. [Google Scholar] [CrossRef]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a Nuclear Receptor That Defines a Distinct Retinoid Response Pathway. Genes Dev. 1995, 9, 1033–1045. [Google Scholar] [CrossRef]
- Wan, Y.J.Y.; An, D.; Cai, Y.; Repa, J.J.; Hung-Po Chen, T.; Flores, M.; Postic, C.; Magnuson, M.A.; Chen, J.; Chien, K.R. Hepatocyte-Specific Mutation Establishes Retinoid X Receptor α as a Heterodimeric Integrator of Multiple Physiological Processes in the Liver. Mol. Cell. Biol. 2000, 20, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Hennuyer, N.; Staels, B.; Fruchart, J.C.; Auwerx, J. Alterations in Lipoprotein Metabolism in Peroxisome Proliferator-Activated Receptor α-Deficient Mice. J. Biol. Chem. 1997, 272, 27307–27312. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Haring, H.; Schick, F.; Stumvoll, M. Intramyocellular Lipids and Insulin Resistance. Diabetes Obes. Metab. 2004, 6, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Chai, H.-H.; Lee, S.-H.; Cho, Y.-M.; Choi, J.-W.; Kim, N.-K. Gene Expression Patterns Associated with Peroxisome Proliferator-Activated Receptor (PPAR) Signaling in the Longissimus Dorsi of Hanwoo (Korean Cattle). Asian Australas. J. Anim. Sci. 2015, 28, 1075–1083. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Meng, H.; Chen, L.; Meng, F. ACSL1 Affects Triglyceride Levels through the PPARγ Pathway. Int. J. Med. Sci. 2020, 17, 720–727. [Google Scholar] [CrossRef]
- Sunaga, H.; Matsui, H.; Anjo, S.; Syamsunarno, M.; Koitabashi, N.; Iso, T.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; Kurabayashi, M. Elongation of Long-Chain Fatty Acid Family Member 6 (Elovl6)-Driven Fatty Acid Metabolism Regulates Vascular Smooth Muscle Cell Phenotype Through AMP-Activated Protein Kinase/Krüppel-Like Factor 4 (AMPK/KLF4) Signaling. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e004014. [Google Scholar] [CrossRef]
- He, Q.; Luo, J.; Wu, J.; Li, Z.; Yao, W.; Zang, S.; Niu, H. ELOVL6 Promoter Binding Sites Directly Targeted by Sterol Regulatory Element Binding Protein 1 in Fatty Acid Synthesis of Goat Mammary Epithelial Cells. J. Dairy Sci. 2021, 104, 6253–6266. [Google Scholar] [CrossRef]
- Repa, J. Regulation of Absorption and ABC1-Mediated Efflux of Cholesterol by RXR Heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef]
- Brewer, H.B.; Santamarina, F.; Ojo, S. Clinical Significance of High-Density Lipoproteins and the Development of Atherosclerosis: Focus on the Role of the Adenosine Triphosphate-Binding Cassette Protein A1 Transporter. Am. J. Cardiol. 2003, 92, 10–16. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Brunham, L.R.; Visscher, H.; Kastelein, J.; Hayden, M.R. Efflux and Atherosclerosis The Clinical and Biochemical Impact of Variations in the ABCA1 Gene. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1322–1332. [Google Scholar] [CrossRef]
- Selva, D.M.; Hirsch-Reinshagen, V.; Burgess, B.; Zhou, S.; Wellington, C.L. The ATP-Binding Cassette Transporter 1 Mediates Lipid Efflux from Sertoli Cells and Influences Male Fertility. J. Lipid Res. 2004, 45, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Nebel, A.; Flachsbart, F.; Till, A.; Caliebe, A.; Blanché, H.; Arlt, A.; H?Sler, R.; Jacobs, G.; Kleindorp, R.; Franke, A. A Functional EXO1 Promoter Variant Is Associated with Prolonged Life Expectancy in Centenarians. Mech. Ageing Dev. 2009, 130, 691–699. [Google Scholar] [CrossRef]
- Rubinow, K.B.; Wall, V.Z.; Nelson, J.; Mar, D.; Bomsztyk, K.; Askari, B.; Lai, M.A.; Smith, K.D.; Han, M.S.; Vivekanandan-Giri, A.; et al. Acyl-CoA Synthetase 1 Is Induced by Gram-Negative Bacteria and Lipopolysaccharide and Is Required for Phospholipid Turnover in Stimulated Macrophages. J. Biol. Chem. 2013, 288, 9957–9970. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Duan, M.; Zhang, R.; Zeng, T.; Xu, W.; Feng, W.; Jiang, C.; Tian, Y.; Chen, L.; Lu, L. Probiotic Fermented Feed Alleviates Liver Fat Deposition in Shaoxing Ducks via Modulating Gut Microbiota. Front. Microbiol. 2022, 13, 928670. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.Y.; Schedlich, L.J.; Twigg, S.M.; Baxter, R.C. Inhibition of Adipocyte Differentiation by Insulin-like Growth Factor-Binding Protein-3. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E654–E663. [Google Scholar] [CrossRef]
- Maréchal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARγ Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.A.; Thomazy, V.A.; Evans, R.M. PPARγ Promotes Monocyte/Macrophage Differentiation and Uptake of Oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef]
- Serghides, L.; Kain, K.C. Peroxisome Proliferator-Activated Receptor γ-Retinoid X Receptor Agonists Increase CD36-Dependent Phagocytosis of Plasmodium Falciparum-Parasitized Erythrocytes and Decrease Malaria-Induced TNF-α Secretion by Monocytes/Macrophages1. J. Immunol. 2001, 166, 6742–6748. [Google Scholar] [CrossRef] [PubMed]
- McGilvray, I.D.; Serghides, L.; Kapus, A.; Rotstein, O.D.; Kain, K.C. Nonopsonic Monocyte/Macrophage Phagocytosis of Plasmodium Falciparum–Parasitized Erythrocytes: A Role for CD36 in Malarial Clearance. Blood 2000, 96, 3231–3240. [Google Scholar] [CrossRef]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in Chronic Kidney Disease: Novel Insights and Therapeutic Opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef]
- Garbacz, W.G.; Lu, P.; Miller, T.M.; Poloyac, S.M.; Eyre, N.S.; Mayrhofer, G.; Xu, M.; Ren, S.; Xie, W. Hepatic Overexpression of CD36 Improves Glycogen Homeostasis and Attenuates High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance. Mol. Cell. Biol. 2016, 36, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.-O.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting Metastasis-Initiating Cells through the Fatty Acid Receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Susztak, K.; Ciccone, E.; McCue, P.; Sharma, K.; Böttinger, E.P. Multiple Metabolic Hits Converge on CD36 as Novel Mediator of Tubular Epithelial Apoptosis in Diabetic Nephropathy. PLoS Med. 2005, 2, e45. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chou, C.-A.; Chen, W.-Y.; Yang, J.-L.; Lee, W.-C.; Chen, J.-B.; Lee, C.-T.; Li, L.-C. Empagliflozin Ameliorates Free Fatty Acid Induced-Lipotoxicity in Renal Proximal Tubular Cells via the PPARγ/CD36 Pathway in Obese Mice. Int. J. Mol. Sci. 2021, 22, 12408. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, A.; Bonen, A.; Blinn, W.D.; Hajri, T.; Li, X.; Zhong, K.; Cameron, R.; Abumrad, N.A. Muscle-Specific Overexpression of FAT/CD36 Enhances Fatty Acid Oxidation by Contracting Muscle, Reduces Plasma Triglycerides and Fatty Acids, and Increases Plasma Glucose and Insulin. J. Biol. Chem. 1999, 274, 26761–26766. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Shu, J.; Song, C.; Yan, H.U.; Chen, J.; Huifang, L.I. Culture and Identification of Myoblasts Isolated from Duck Embryos. Agric. Sci. Technol. 2014, 15, 1281–1284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Chen, X.; Wu, D.; Li, X.; Gao, W.; Li, G.; Du, G.; Zhang, C.; Jin, S.; Geng, Z. A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression. Int. J. Mol. Sci. 2023, 24, 1180. https://doi.org/10.3390/ijms24021180
Pan Z, Chen X, Wu D, Li X, Gao W, Li G, Du G, Zhang C, Jin S, Geng Z. A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression. International Journal of Molecular Sciences. 2023; 24(2):1180. https://doi.org/10.3390/ijms24021180
Chicago/Turabian StylePan, Ziyi, Xingyong Chen, Dongsheng Wu, Xuewen Li, Weifeng Gao, Guoyu Li, Guoqing Du, Cheng Zhang, Sihua Jin, and Zhaoyu Geng. 2023. "A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression" International Journal of Molecular Sciences 24, no. 2: 1180. https://doi.org/10.3390/ijms24021180
APA StylePan, Z., Chen, X., Wu, D., Li, X., Gao, W., Li, G., Du, G., Zhang, C., Jin, S., & Geng, Z. (2023). A Novel in Duck Myoblasts: The Transcription Factor Retinoid X Receptor Alpha (RXRA) Inhibits Lipid Accumulation by Promoting CD36 Expression. International Journal of Molecular Sciences, 24(2), 1180. https://doi.org/10.3390/ijms24021180





