Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue
Abstract
1. Introduction
- The determination of the possibility of NaYF4:20%Yb3+,2%Er3+ UCNPs internalization
- Cytotoxicity evaluation and ultrastructural changes analysis after exposure to NaYF4:20%Yb3+,2%Er3+ UCNPs
2. Results
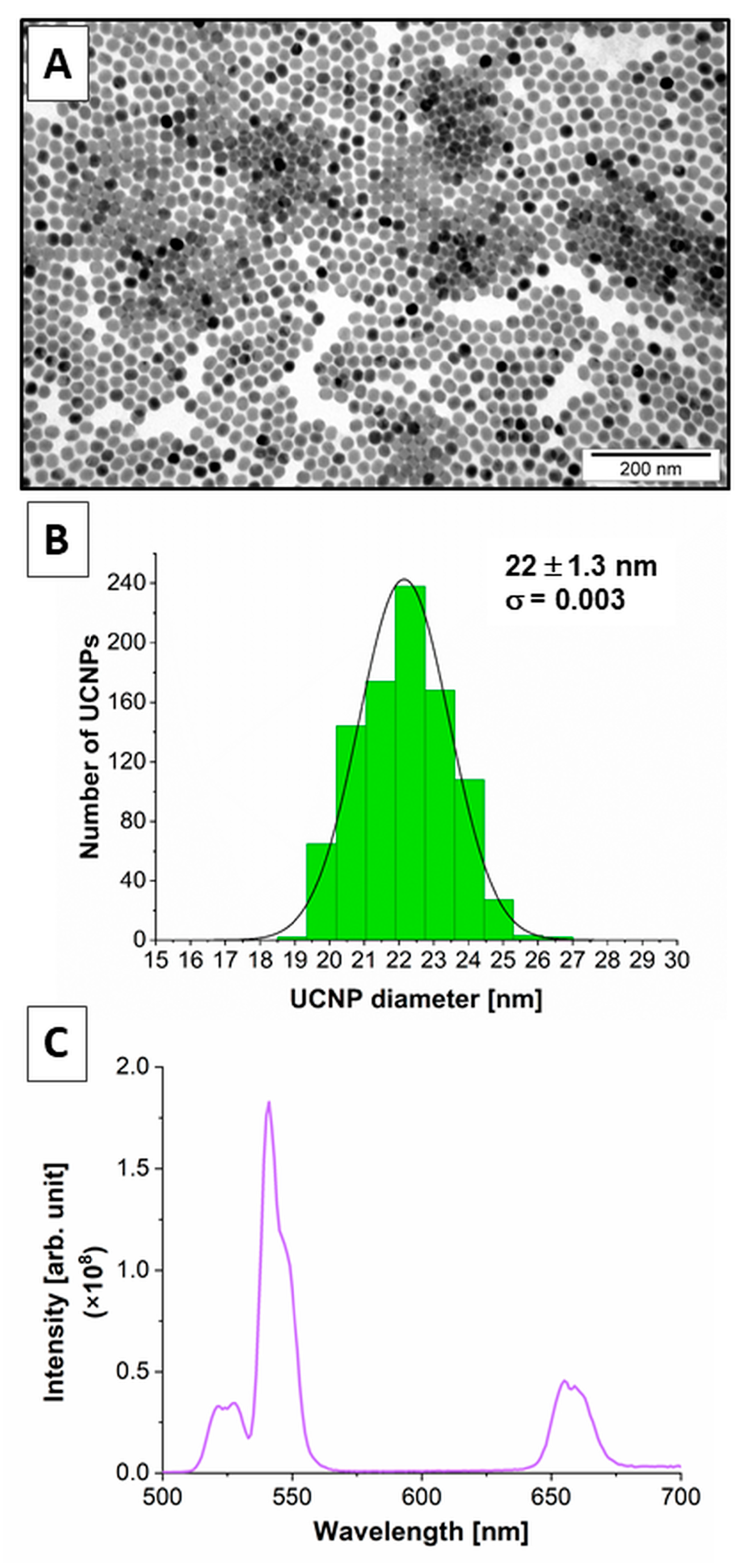
2.1. Physicochemical Properties of the β-NaYF4:20%Yb3+,2%Er3+ UCNPs
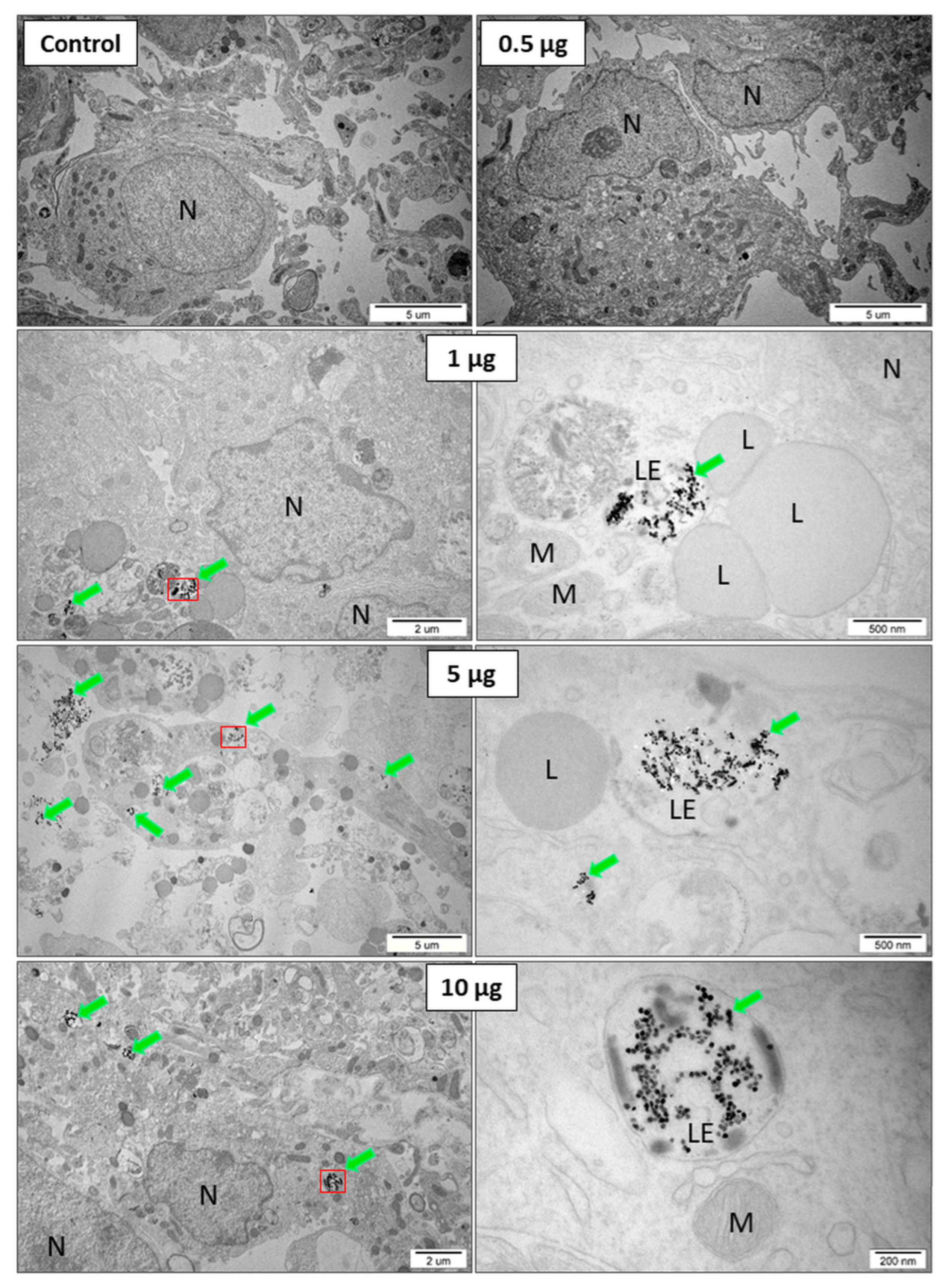
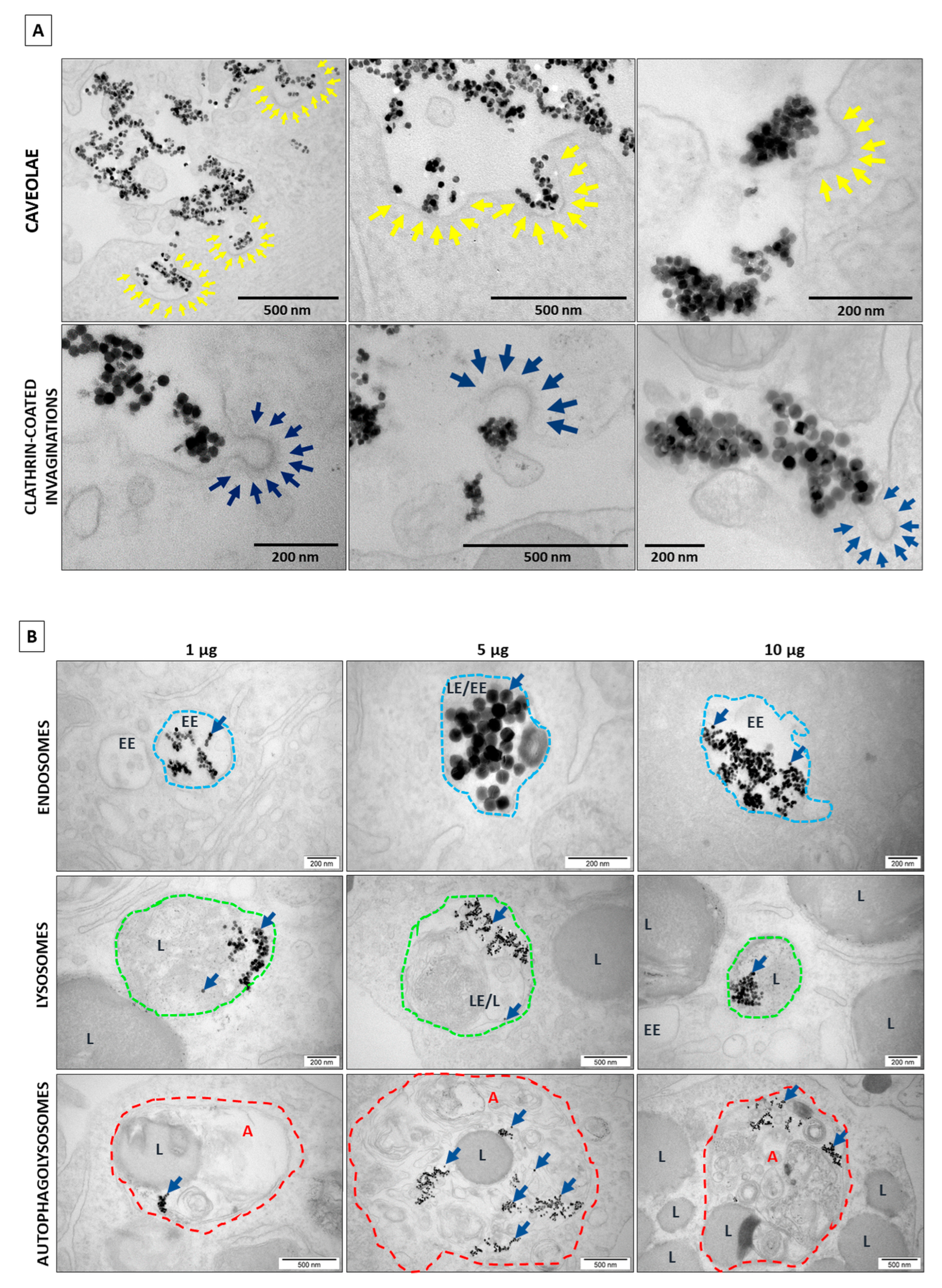
2.2. TEM Results and Analysis
- (1)
- The UCNPs were added to 1 mL of medium (0.5; 1; 5 and 10 μg mL−1, 24 h incubation);
- (2)
- The UCNPs were added to 2 mL of medium with immersed slices (0.5; 1; 5 and 10 μg mL−1, 1 h incubation);
- (3)
- The UCNPs were applied in droplets directly to the top of the slice surface (0.5; 1; 5 and 10 μg 4μL−1, 2 h incubation).
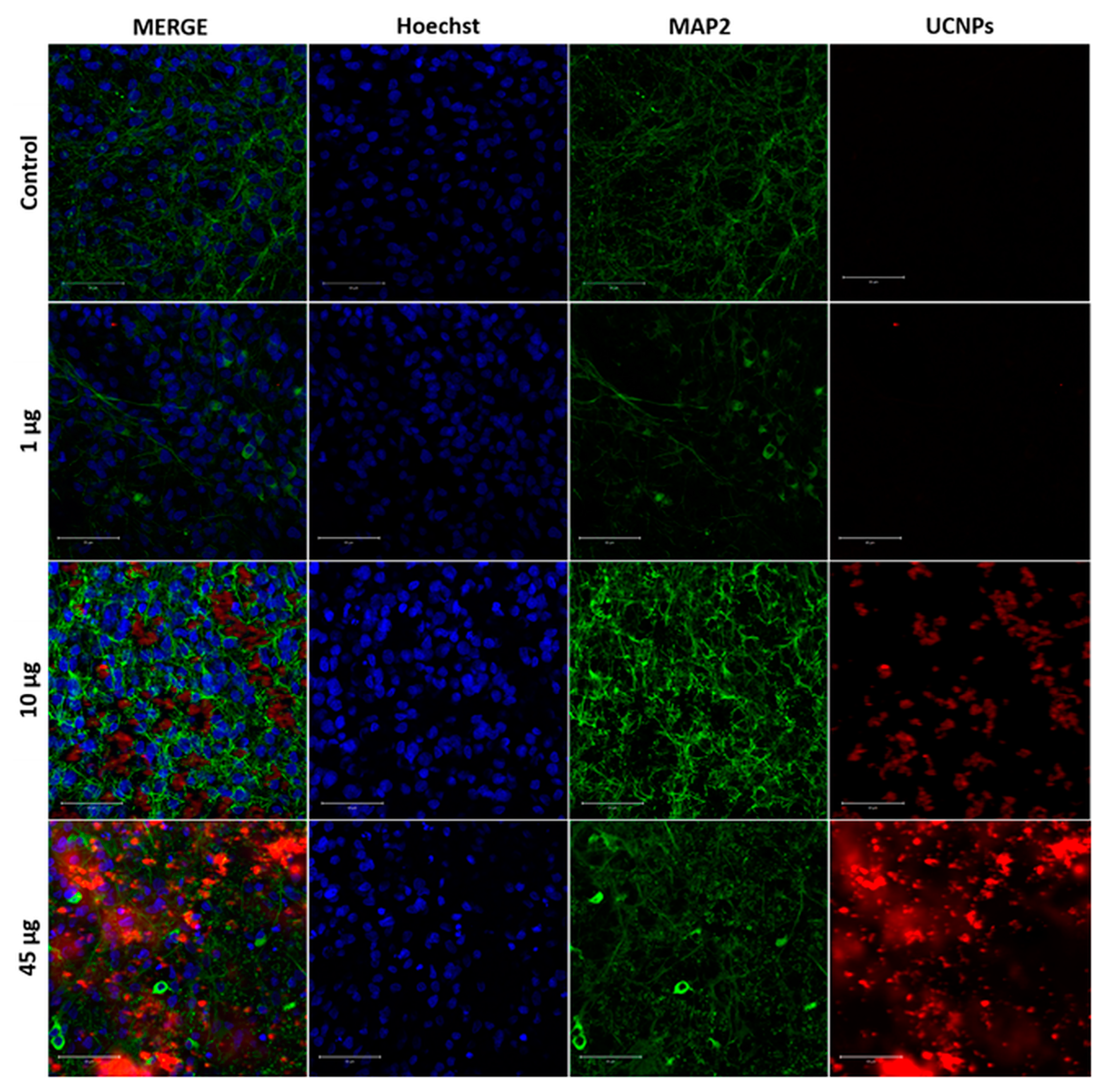
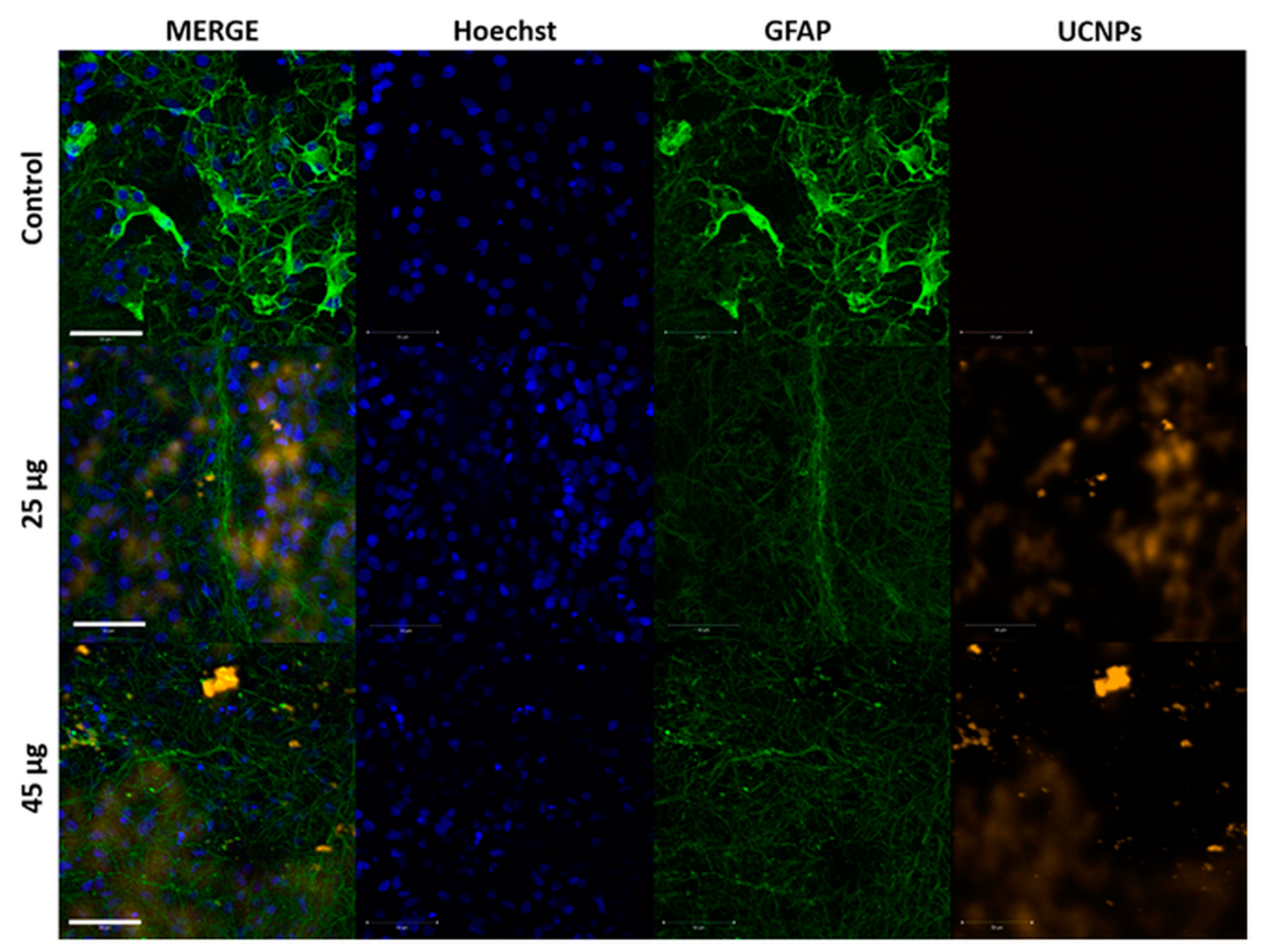
2.3. The Confocal Microscope Imaging Results
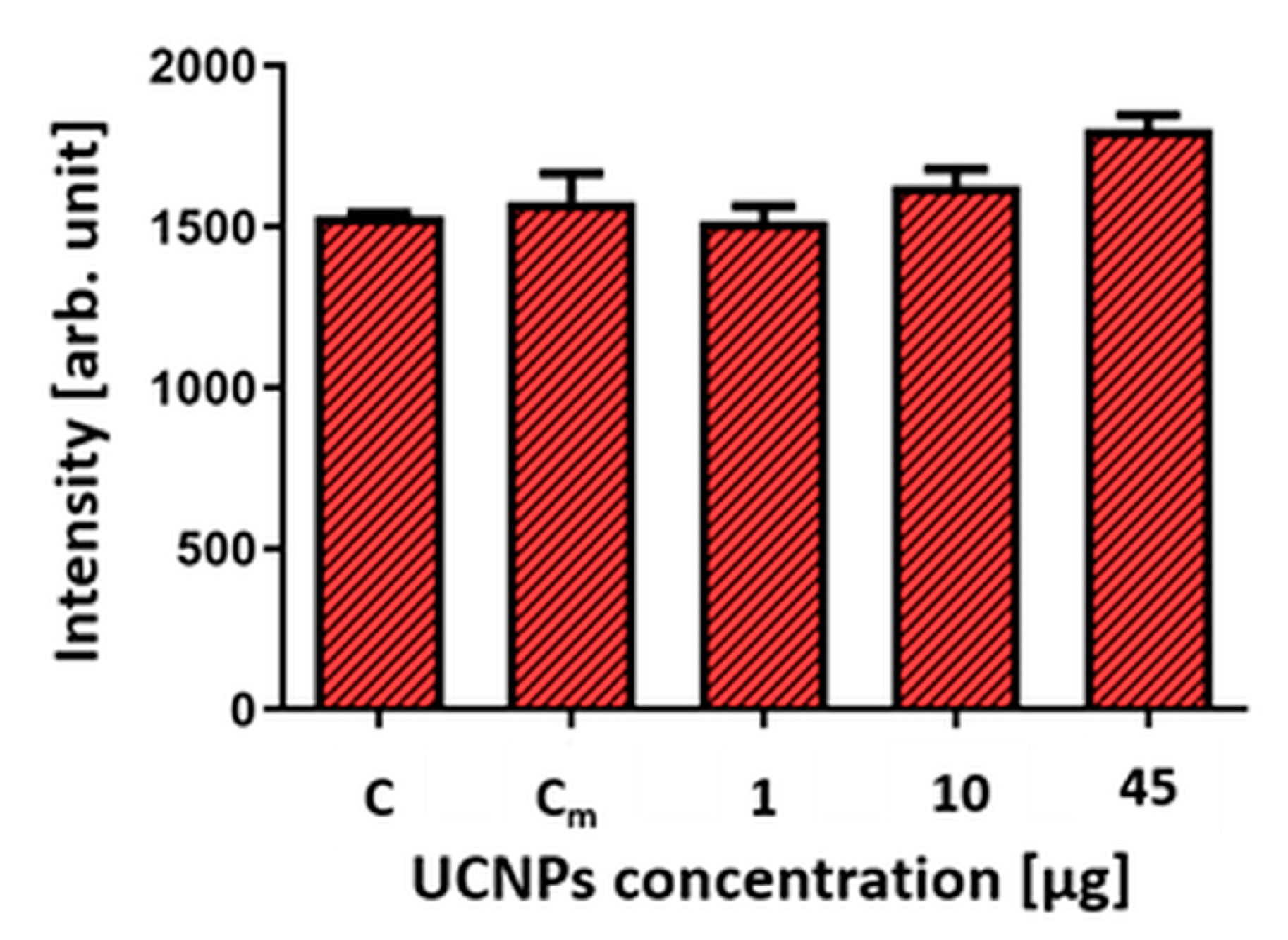
2.4. Cell Viability after Exposure to UCNPs
3. Discussion
4. Materials and Methods
4.1. Synthesis of β-NaYF4:20%Yb3+,2%Er3+ UCNPs
4.2. Physicochemical Characteristics of β-NaYF4:20%Yb3+,2%Er3+ UCNPs
4.3. Ex Vivo Culture of Organotypic Hippocampal Slices (OHSCs)
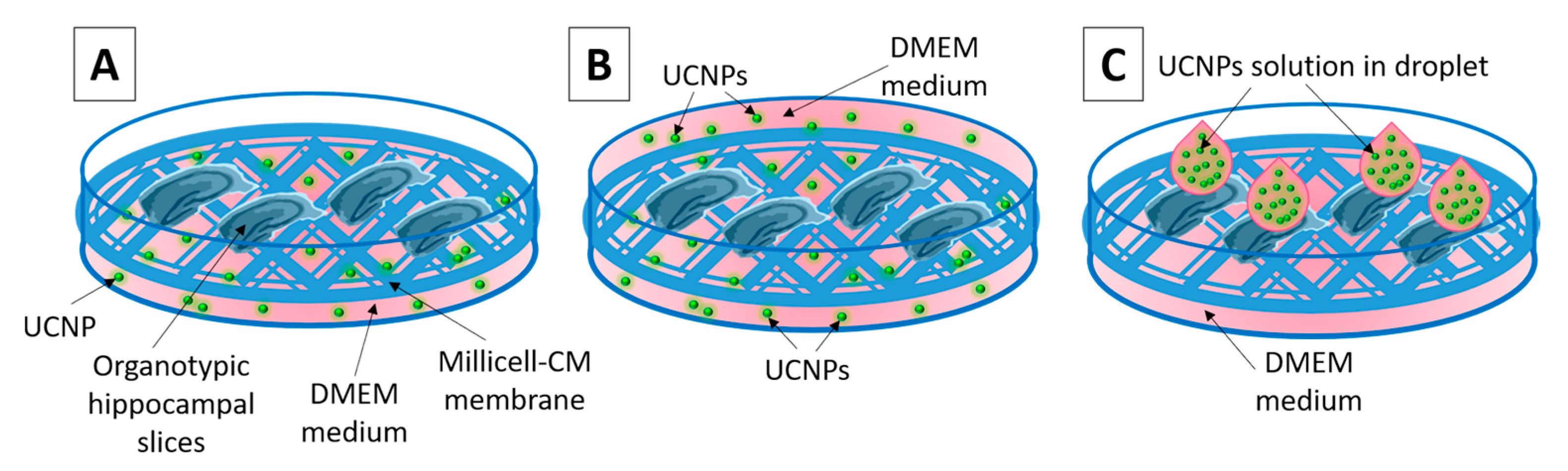
4.4. UCNPs Administration to OHSC
- (1)
- The method of adding the UCNPs solution directly to the medium (UCNPs should be internalized by cells from the medium) (Figure 2A).
- (2)
- The method of immersion of OHSCs in the medium with UCNPs. Under these conditions, they were incubated for 1 h (Figure 2B).
- (3)
- The method of the UCNPs solution applying by droplet (4 μL) directly to the surface of the slice and incubating for 2 or 24 h (Figure 2C).
4.5. TEM Analysis
4.6. Immunohistochemical Staining
4.7. Cell Viability Analysis Using Propidium Iodide
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.K. Exploitation of antimicrobial nanoparticles and their applications in biomedical engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Elsayed, H.H.; Al-Sherbini, A.S.A.; Abd-Elhady, E.E.; Ahmed, K.A.E.A. Treatment of anemia progression via magnetite and folate nanoparticles in vivo. Int. Sch. Res. Not. 2014, 2014, 287575. [Google Scholar]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular uptake of nanoparticles versus small molecules: A matter of size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Soler, M.A.G.; Lima, E.C.D.; da Silva, S.W.; Melo, T.F.O.; Pimenta, A.C.M.; Sinnecker, J.P.; Azevedo, R.B.; Garg, V.K.; Oliveira, A.C.; Novak, M.A.; et al. Aging investigation of cobalt ferrite nanoparticles in low pH magnetic fluid. Langmuir 2007, 23, 9611–9617. [Google Scholar] [CrossRef] [PubMed]
- Lacava, L.M.; Lacava, Z.G.M.; Da Silva, M.F.; Silva, O.; Chaves, S.B.; Azevedo, R.B.; Pelegrini, F.; Gansau, C.; Buske, N.; Sabolovic, D.; et al. Magnetic Resonance of a Dextran-Coated Magnetic Fluid Intravenously Administered in Mice. Biophys. J. 2001, 80, 2483–2486. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Garcia, M.P.; Tavares, M.C.H.; Valois, C.R.A.; Lima, E.C.D.; Teixeira, D.S.; Morais, P.C.; Tomaz, C.; Azevedo, R.B. Biodistribution and biocompatibility of DMSA-stabilized maghemite magnetic nanoparticles in nonhuman primates (Cebus spp.). Nanomedicine 2011, 6, 1529–1544. [Google Scholar] [CrossRef]
- Lacava, Z.G.M.; Garcia, V.A.P.; Lacava, L.M.; Azevedo, R.B.; Silva, O.; Pelegrini, F.; De Cuyper, M.; Morais, P.C. Biodistribution and biocompatibility investigation in magnetoliposome treated mice. Spectroscopy 2004, 18, 597–603. [Google Scholar] [CrossRef]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.; Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Simko, M.; Mattsson, M.O. Interactions between nanosized materials and the brain. Curr. Med. Chem. 2014, 21, 4200–4214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood-brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting nanoparticles to the brain by exploiting the blood–brain barrier impermeability to selectively label the brain endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Yu, D.X.; Xiao, Z.; He, Z. Application of nanodiagnostics and nanotherapy to CNS diseases. Nanomedicine 2018, 13, 2341–2371. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Pampaloni, N.P.; Giugliano, M.; Scaini, D.; Ballerini, L.; Rauti, R. Advances in nano neuroscience: From nanomaterials to nanotools. Front. Neurosci. 2019, 12, 953. [Google Scholar] [CrossRef]
- Woolf, N.J.; Priel, A.; Tuszynski, J.A. Nanotechnology, Nanostructure, and Nervous System Disorders. In Nanoneuroscience; Springer: Berlin/Heidelberg, Germany, 2009; pp. 177–226. [Google Scholar]
- Mullen, T.J.; Zhang, M.; Feng, W.; El-khouri, R.J.; Sun, L.-D.; Yan, C.–H.; Patten, T.E.; Liu, G. Fabrication and Characterization of Rare-Earth-Doped Nanostructures on Surfaces. ACS Nano 2011, 5, 6539–6545. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Ren, J.; Qu, X. Near-Infrared Upconversion Controls Photocaged Cell Adhesion. J. Am. Chem. Soc. 2014, 136, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, D.; Zhu, H.; Ma, E.; Chen, X. Lanthanide-doped luminescent nano-bioprobes: From fundamentals to biodetection. Nanoscale 2013, 5, 1369–1384. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Ju, Q.; Chen, X.; Ma, R.; Chen, B. Lanthanide-Doped Energy Cascade Nanoparticles: Full Spectrum Emission by Single Wavelength Excitation. Chem. Mater. 2015, 27, 3115–3120. [Google Scholar] [CrossRef]
- Liu, S.; De, G.; Xu, Y.; Wang, X.; Liu, Y.; Cheng, C.; Wang, J. Size, phase-controlled synthesis, the nucleation and growth mechanisms of NaYF4:Yb/Er nanocrystals. J. Rare Earths 2018, 3, 1060–1066. [Google Scholar] [CrossRef]
- Li, C.; Yang, D.; Ma, P.; Chen, Y.; Wu, Y.; Hou, Z.; Dai, Y.; Zhao, J.; Sui, C.; Lin, J. Multifunctional Upconversion Mesoporous Silica Nanostructures for Dual Modal Imaging and In Vivo Drug Delivery. Small 2013, 9, 4150–4159. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Auzel, F.E. Materials and devices using double-pumped-phosphors with energy transfer. Proc. IEEE 1973, 61, 758–786. [Google Scholar] [CrossRef]
- Boyer, J.C.; Cuccia, L.A.; Capobianco, J.A. Synthesis of Colloidal Upconverting NaYF4: Er3+/Yb3+ and Tm3+/Yb3+ Monodisperse Nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, G.-Y.; Sun, L.-D.; Xiao, J.-W.; Zhou, J.-C.; Yan, C.-H. Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect. ACS Nano 2013, 7, 7200–7206. [Google Scholar] [CrossRef]
- Kwon, O.S.; Song, H.S.; Conde, J.; Kim, H.I.; Artzi, N.; Kim, J.-H. Dual-color emissive upconversion nanocapsules for differential cancer bioimaging in vivo. ACS Nano 2016, 10, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; La, H.; Zhu, R.; El-Banna, G.; Wei, Y.; Han, G. Upconverting NIR photons for bioimaging. Nanomaterials 2015, 5, 2148–2168. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.Y.; Lim, M.E.; Ong, L.C.; Zhang, Y. Applications of upconversion nanoparticles in imaging, detection and therapy. Nanomedicine 2011, 6, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Li, J.; Liu, F.; Padmanabhan, P.; Yeow, E.K.; Xing, B. Recent advance of biological molecular imaging based on lanthanide-doped upconversion-luminescent nanomaterials. Nanomaterials 2014, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, C.; Shi, Z. Current advances in lanthanide-doped upconversion nanostructures for detection and bioapplication. Adv. Sci. 2016, 3, 1600029. [Google Scholar] [CrossRef]
- Bettinelli, M.; Carlos, L.; Liu, X. Lanthanide-doped. Phys. Today 2015, 68, 38. [Google Scholar] [CrossRef]
- Rostami, I.; Alanagh, H.R.; Hu, Z.; Shahmoradian, S.H. Breakthroughs in medicine and bioimaging with up-conversion nanoparticles. Int. J. Nanomed. 2019, 14, 7759–7780. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol 2020, 18, 154. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z.; Lv, L.; Yin, D.; Chen, D.; Han, Z.; Ma, Y.; Zhang, M.; Yang, M.; Gu, Y. Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics 2016, 6, 1131–1144. [Google Scholar] [CrossRef]
- Xing, Y.; Li, L.; Ai, X.; Fu, L. Polyaniline-coated upconversion nanoparticles with upconverting luminescent and photothermal conversion properties for photothermal cancer therapy. Int. J. Nanomed. 2016, 11, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, Q.; Lau, J.W.; Xing, B. Lanthanide-doped upconversion nanoparticles meet the needs for cutting-edge bioapplications: Recent progress and perspectives. ACS Mater. Lett. 2020, 2, 1516–1531. [Google Scholar] [CrossRef]
- Shah, S.; Liu, J.J.; Pasquale, N.; Lai, J.; McGowan, H.; Pang, Z.P.; Lee, K.-B. Hybrid upconversion nanomaterials for optogenetic neuronal control. Nanoscale 2015, 7, 16571–16577. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, W.; Teoh, C.L.; Han, S.; Kim, B.; Samanta, A.; Er, J.C.; Wang, L.; Yuan, L.; Liu, X.; et al. High-efficiency in vitro and in vivo detection of Zn2+ by dye-assembled upconversion nanoparticles. J. Am. Chem. Soc. 2015, 137, 2336–2342. [Google Scholar] [CrossRef]
- Chamanzar, M.; Garfield, D.J.; Iafrati, J.; Chan, E.M.; Sohal, V.; Cohen, B.E.; Schuck, P.J.; Maharbiz, M.M. Upconverting nanoparticle micro-lightbulbs designed for deep tissue optical stimulation and imaging. Biomed. Opt. Express 2018, 9, 4359–4371. [Google Scholar] [CrossRef]
- Zhao, J.; Ellis-Davies, G.C. Intracellular photoswitchable neuropharmacology driven by luminescence from upconverting nanoparticles. Chem. Commun. 2020, 56, 9445–9448. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Wang, Y.; Si, Y.; Zhang, H.; Li, X.; Zhang, Z.; Yan, B.; Jiang, S.; Wang, F.; et al. Near-infrared manipulation of multiple neuronal populations via trichromatic upconversion. Nat. Commun. 2021, 12, 5662. [Google Scholar] [CrossRef]
- Qi, X.R.; Verwer, R.W.; Bao, A.M.; Balesar, R.A.; Luchetti, S.; Zhou, J.N.; Swaab, D.F. Human brain slice culture: A useful tool to study brain disorders and potential therapeutic compounds. Neurosci. Bull. 2019, 35, 244–252. [Google Scholar] [CrossRef]
- Saba, N.; Pangha, A.; Flora, S.J.S. Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar]
- Aragon, F.H.; Coaquira, J.A.H.; Villegas-Lelovsky, L.; da Silva, S.W.; Cesar, D.F.; Nagamine, L.C.C.M.; Cohen, R.; Menendez-Proupin, E.; Morais, P.C.J. Evolution of the doping regimes in the Al-doped SnO2 nanoparticles prepared by a polymer precursor method. J. Phys. Condens. Matter 2015, 27, 095301. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Kamińska, I.; Fronc, K.; Borodziuk, A.; Duda, M.; Wojciechowski, T.; Sobczak, K.; Kalinowska, D.; Klepka, M.T.; Sikora, B. The ROS-generating photosensitizer-free NaYF4:Yb, Tm@SiO2 upconverting nanoparticles for photodynamic therapy application. Nanotechnology 2021, 32, 475101. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Elbaum, D.; Mikulski, J.; Fronc, K.; Kamińska, I.; Morais, P.C.; de Souza, P.E.; Nunes, R.B.; Veiga-Souza, F.H.; Gruzeł, G.; et al. Upconversion fluorescence imaging of HeLa cells using ROS generating SiO2-coated lanthanide-doped NaYF4 nanoconstructs. RSC Adv. 2017, 7, 30262–30273. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Sikora, B.; Kowalik, P.; Mikulski, J.; Fronc, K.; Kamińska, I.; Szewczyk, M.; Konopka, A.; Zajdel, K.; Minikayev, R.; Sobczak, K.; et al. Mammalian cell defence mechanisms against the cytotoxicity of NaYF4:(Er,Yb,Gd) nanoparticles. Nanoscale 2017, 9, 14259–14271. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.; Kraig, R.P.; Medintz, I.; Delehanty, J.B.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Dawson, P.E.; Dawson, G. Nanoparticle targeting to neurons in a rat hippocampal slice culture model. ASN Neuro 2012, 4, 383–392. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kursungoz, C.; Taş, S.T.; Sargon, M.F.; Sara, Y.; Ortaç, B. Toxicity of internalized laser generated pure silver nanoparticles to the isolated rat hippocampus cells. Toxicol. Ind. Health 2017, 33, 555–563. [Google Scholar] [CrossRef]
- Thellung, S.; Corsaro, A.; Nizzari, M.; Barbieri, F.; Florio, T. Autophagy Activator Drugs: A New Opportunity in Neuroprotection from Misfolded Protein Toxicity. Int. J. Mol. Sci. 2019, 20, 901. [Google Scholar] [CrossRef]
- Phiwchai, I.; Chariyarangsitham, W.; Phatruengdet, T.; Pilapong, C. Ferric-Tannic Nanoparticles Increase Neuronal Cellular Clearance. ACS Chem. Neurosci. 2019, 10, 4136–4144. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Nedzvetsky, V.S.; Ağca, C.A.; Kirici, M. Pristine C60 Fullerene Nanoparticles Ameliorate Hyperglycemia-Induced Disturbances via Modulation of Apoptosis and Autophagy Flux. Neurochem. Res. 2020, 45, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Sypecka, J.; Sarnowska, A. The neuroprotective effect exerted by oligodendroglial progenitors on chemically impaired hippocampal cells. Mol. Neurobiol. 2014, 49, 685–701. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajdel, K.; Janowska, J.; Frontczak-Baniewicz, M.; Sypecka, J.; Sikora, B. Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue. Int. J. Mol. Sci. 2023, 24, 1122. https://doi.org/10.3390/ijms24021122
Zajdel K, Janowska J, Frontczak-Baniewicz M, Sypecka J, Sikora B. Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue. International Journal of Molecular Sciences. 2023; 24(2):1122. https://doi.org/10.3390/ijms24021122
Chicago/Turabian StyleZajdel, Karolina, Justyna Janowska, Małgorzata Frontczak-Baniewicz, Joanna Sypecka, and Bożena Sikora. 2023. "Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue" International Journal of Molecular Sciences 24, no. 2: 1122. https://doi.org/10.3390/ijms24021122
APA StyleZajdel, K., Janowska, J., Frontczak-Baniewicz, M., Sypecka, J., & Sikora, B. (2023). Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue. International Journal of Molecular Sciences, 24(2), 1122. https://doi.org/10.3390/ijms24021122







