Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology
Abstract
:1. Introduction
2. Carbon Dots
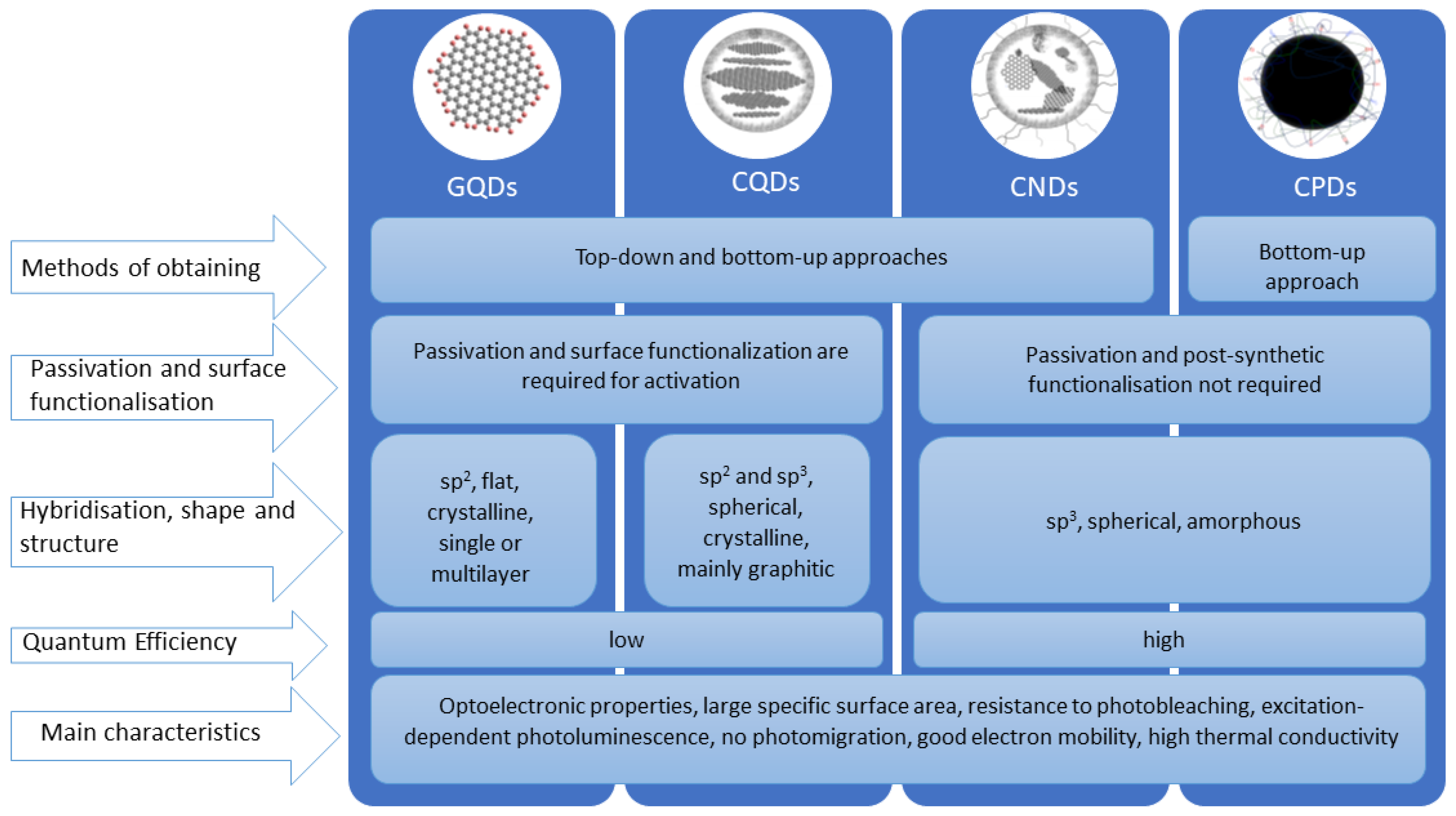
2.1. Types of Carbon Dots
2.2. Obtaining Carbon Quantum Dots (CQDs)
2.2.1. Microwave-Assisted Synthesis
2.2.2. Laser Ablation Technique
2.3. Applications of Carbon Dots
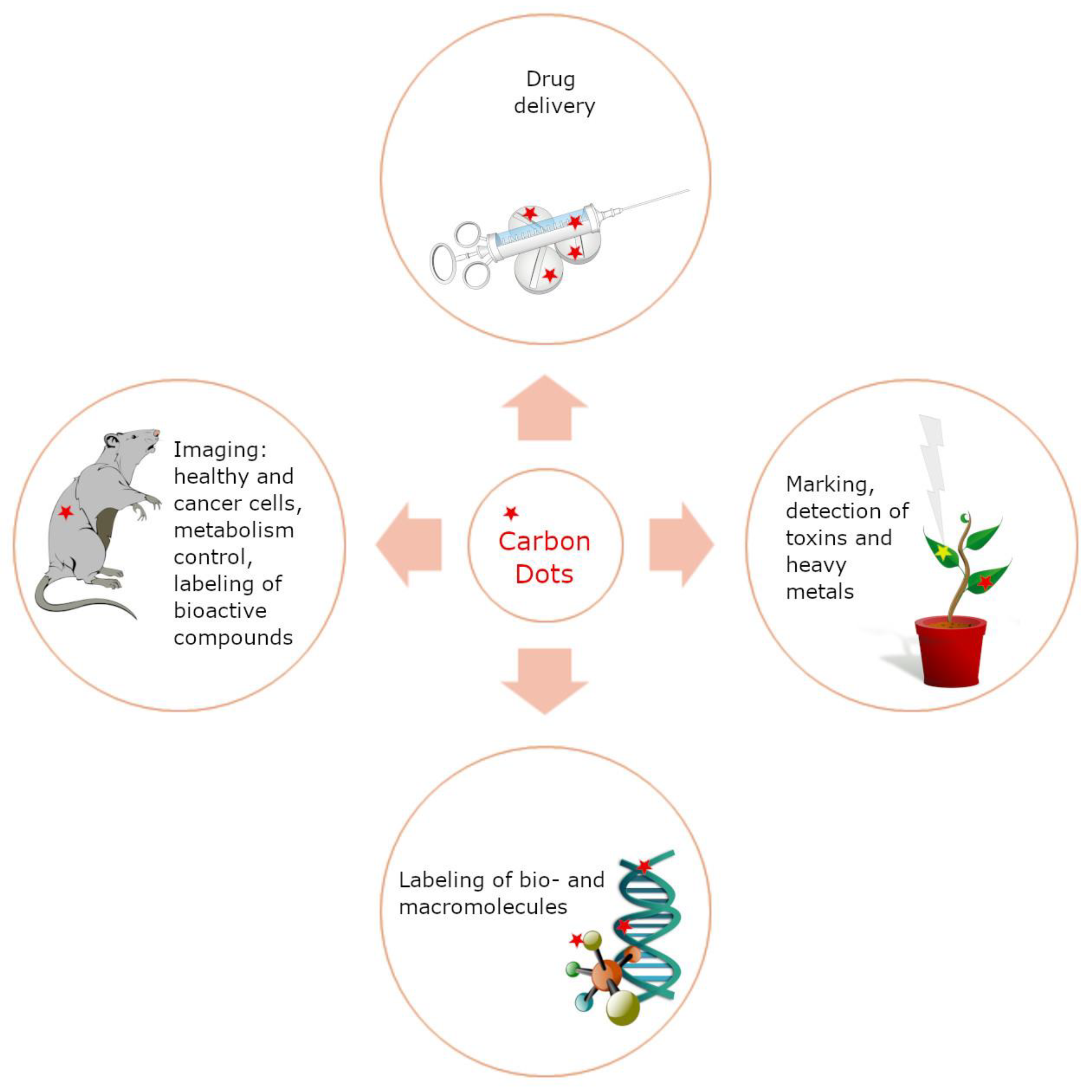
2.3.1. Applications of Carbon Dots in Biotechnology
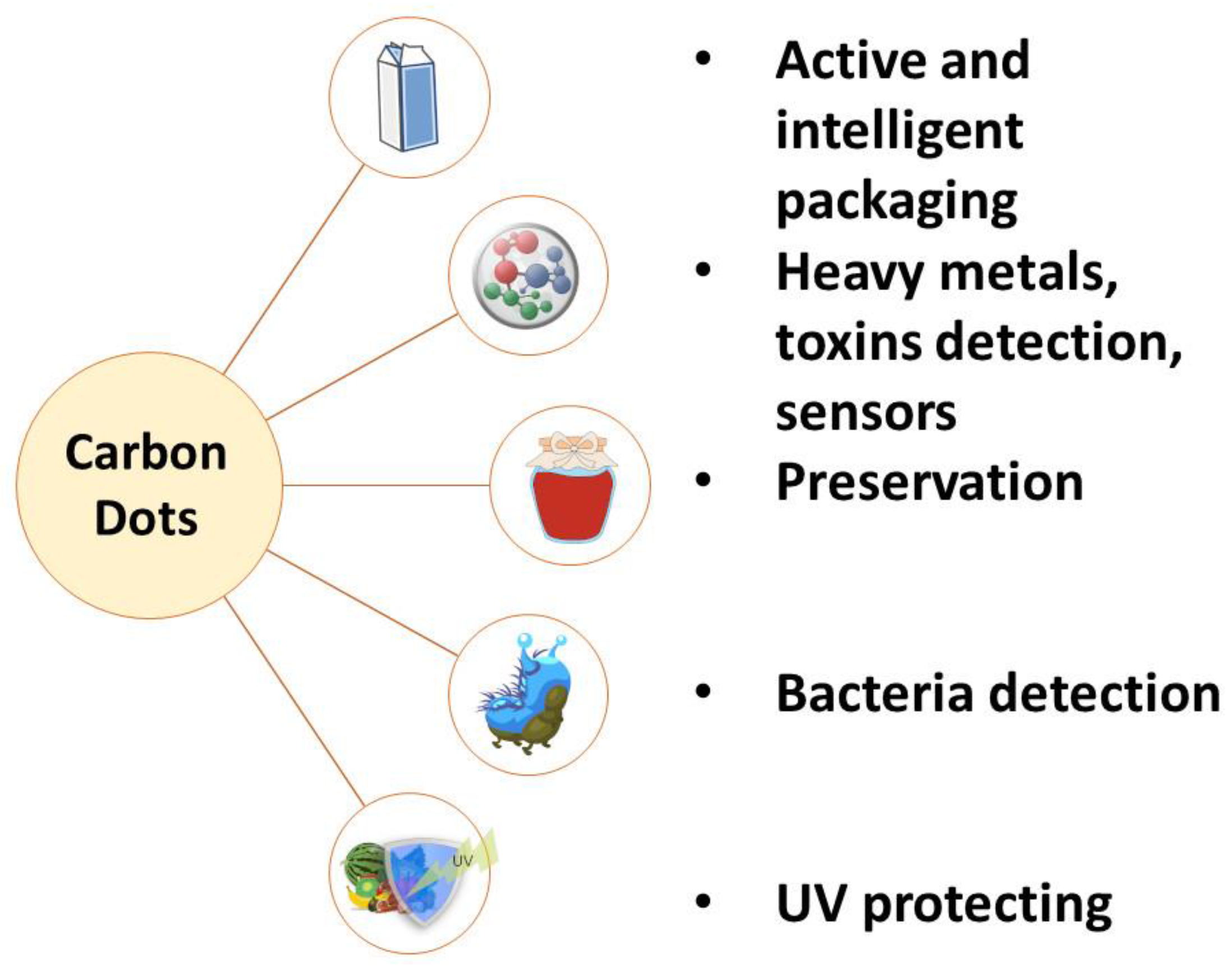
2.3.2. Application of Carbon Dots in Food Technology
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Luthra, G. Fundamental approaches and applications of nanotechnology: A mini review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Bhushan, B. Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Khachatryan, G.; Khachatryan, K.; Szczepankowska, J.; Krzan, M.; Krystyjan, M. Design of Carbon Nanocomposites Based on Sodium Alginate/Chitosan Reinforced with Graphene Oxide and Carbon Nanotubes. Polymers 2023, 15, 925. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Krzan, M.; Ciesielski, W.; Zarska, S.; Szczepankowska, J. Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers 2022, 14, 948. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Futaba, D.N.; Chen, G.; Chen, C.; Zhou, K.; Zhang, Q.; Li, M.; Ye, Z.; Xu, M. Structural design and fabrication of multifunctional nanocarbon materials for extreme environmental applications. Adv. Mater. 2022, 34, 2201046. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; Olojede, S.O.; Sahoo, S.; Kumar, R. Recent advances in modification of novel carbon-based composites: Synthesis, properties, and biotechnological/biomedical applications. Chem.-Biol. Interact. 2023, 379, 110517. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Fluorescent quantum dots-based hydrogels: Synthesis, Fabrication and multimodal biosensing. Talanta Open 2023, 8, 100243. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Zhang, T.; Zhang, W.; Chen, W.; Cao, Y.; Chen, M.; Zhou, X. Orange-emissive carbon quantum dots: Toward application in wound pH monitoring based on colorimetric and fluorescent changing. Small 2019, 15, 1902823. [Google Scholar] [CrossRef]
- Tan, T.L.; Nulit, R.; Jusoh, M.; Rashid, S.A. Recent developments, applications and challenges for carbon quantum dots as a photosynthesis enhancer in agriculture. RSC Adv. 2023, 13, 25093–25117. [Google Scholar]
- Pourmadadi, M.; Nouralishahi, A.; Shalbaf, M.; Shabani Shayeh, J.; Nouralishahi, A. An electrochemical aptasensor for detection of prostate-specific antigen-based on carbon quantum dots-gold nanoparticles. Biotechnol. Appl. Biochem. 2023, 70, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, A.; Farhadian, N.; Abnous, K.; Matin, M.M.; Ziaee, N.; Yaghoobi, E. N doped-carbon quantum dots with ultra-high quantum yield photoluminescent property conjugated with folic acid for targeted drug delivery and bioimaging applications. J. Photochem. Photobiol. A Chem. 2023, 444, 114972. [Google Scholar] [CrossRef]
- Ponduru, H.K.; Gurubilli, C.S.R.; Mudunuru, S.; Banisetti, D.K. Green synthesis of nano particles from biodegradable waste extracts and their applications. Int. J. Pharmacogn. Chem. 2023, 4, 46–54. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S. Revolutionizing Food Safety with Quantum Dot–Polymer Nanocomposites: From Monitoring to Sensing Applications. Foods 2023, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Chellasamy, G.; Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Green synthesized carbon quantum dots from maple tree leaves for biosensing of Cesium and electrocatalytic oxidation of glycerol. Chemosphere 2022, 287, 131915. [Google Scholar] [CrossRef]
- Ezati, P.; Priyadarshi, R.; Rhim, J.-W. Prospects of sustainable and renewable source-based carbon quantum dots for food packaging applications. Sustain. Mater. Technol. 2022, 33, e00494. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Kang, H.; Kim, D.Y.; Cho, J. Top-Down Fabrication of Luminescent Graphene Quantum Dots Using Self-Assembled Au Nanoparticles. ACS Omega 2023, 8, 5885–5892. [Google Scholar] [CrossRef]
- Wei, X.; Yang, J.; Hu, L.; Cao, Y.; Lai, J.; Cao, F.; Gu, J.; Cao, X. Recent advances in room temperature phosphorescent carbon dots: Preparation, mechanism, and applications. J. Mater. Chem. C 2021, 9, 4425–4443. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, properties, synthesis, characterization, and applications in health care—An updated review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, R.; Vignesh, N.S.; Vinothini, K.; Rajan, M.; Ashokkumar, B.; Brindhadevi, K.; Chi, N.T.L.; Pugazhendhi, A.; Varalakshmi, P. Carbon quantum dots (CQD) fabricated from Exiguobacterium sp. VK2 exopolysaccharide (EPS) using hydrothermal reaction and its biodiesel applications. Fuel 2023, 333, 126426. [Google Scholar] [CrossRef]
- Kumar, L.; Gaikwad, K.K. Carbon dots for food packaging applications. Sustain. Food Technol. 2023, 1, 185–199. [Google Scholar]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Jana, J.; Ngo, Y.-L.T.; Chung, J.S.; Hur, S.H. Contribution of carbon dot nanoparticles in electrocatalysis: Development in energy conversion process. J. Electrochem. Sci. Technol. 2020, 11, 220–237. [Google Scholar] [CrossRef]
- Siwal, S.S.; Kaur, H.; Saini, A.K.; Thakur, V.K. Recent Progress in Carbon Dots-Based Materials for Electrochemical Energy Storage Toward Environmental Sustainability. Adv. Energy Sustain. Res. 2022, 3, 2200062. [Google Scholar] [CrossRef]
- Torres, S.; Bogireddy, N.K.R.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Agarwal, S.; Bhatia, S. Carbon dots: Emerging green nanoprobes and their diverse applications. In Functionalized Nanomaterials for Catalytic Application; Whiley: Hoboken, NJ, USA, 2021; pp. 417–492. [Google Scholar]
- MOOSA, A.; ABED, M. Graphene preparation and graphite exfoliation. Turk. J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef]
- Draude, A.P.; Dierking, I. Thermotropic liquid crystals with low-dimensional carbon allotropes. Nano Express 2021, 2, 012002. [Google Scholar] [CrossRef]
- Pandey, D.; Xiao, S.; Wubs, M. Graphene multilayers for coherent perfect absorption: Effects of interlayer separation. Opt. Express 2022, 30, 44504–44517. [Google Scholar] [CrossRef] [PubMed]
- Kokorina, A.A.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. Luminescent carbon nanoparticles separation and purification. Adv. Colloid Interface Sci. 2019, 274, 102043. [Google Scholar] [CrossRef] [PubMed]
- Başoğlu, A.; Ocak, Ü.; Gümrükçüoğlu, A. Synthesis of microwave-assisted fluorescence carbon quantum dots using roasted–chickpeas and its applications for sensitive and selective detection of Fe3+ ions. J. Fluoresc. 2020, 30, 515–526. [Google Scholar] [CrossRef]
- Banger, A.; Gautam, S.; Jadoun, S.; Jangid, N.K.; Srivastava, A.; Pulidindi, I.N.; Dwivedi, J.; Srivastava, M. Synthetic Methods and Applications of Carbon Nanodots. Catalysts 2023, 13, 858. [Google Scholar] [CrossRef]
- Koutamehr, M.E.; Moradi, M.; Tajik, H.; Molaei, R.; Heshmati, M.K.; Alizadeh, A. Sour whey-derived carbon dots; synthesis, characterization, antioxidant activity and antimicrobial performance on foodborne pathogens. LWT 2023, 184, 114978. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Behi, M.; Gholami, L.; Naficy, S.; Palomba, S.; Dehghani, F. Carbon dots: A novel platform for biomedical applications. Nanoscale Adv. 2022, 4, 353–376. [Google Scholar] [CrossRef]
- Ma, H.; Guan, L.; Chen, M.; Zhang, Y.; Wu, Y.; Liu, Z.; Wang, D.; Wang, F.; Li, X. Synthesis and enhancement of carbon quantum dots from Mopan persimmons for Fe3+ sensing and anti-counterfeiting applications. Chem. Eng. J. 2023, 453, 139906. [Google Scholar] [CrossRef]
- Kechagias, A.; Lykos, C.; Karabagias, V.K.; Georgopoulos, S.; Sakavitsi, V.; Leontiou, A.; Salmas, C.E.; Giannakas, A.E.; Konstantinou, I. Development and Characterization of N/S-Carbon Quantum Dots by Valorizing Greek Crayfish Food Waste. Appl. Sci. 2023, 13, 8730. [Google Scholar] [CrossRef]
- Chan, M.-H.; Chen, B.-G.; Ngo, L.T.; Huang, W.-T.; Li, C.-H.; Liu, R.-S.; Hsiao, M. Natural carbon nanodots: Toxicity assessment and theranostic biological application. Pharmaceutics 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon quantum dots for energy applications: A review. ACS Appl. Nano Mater. 2021, 4, 6515–6541. [Google Scholar] [CrossRef]
- Bruno, F.; Sciortino, A.; Buscarino, G.; Soriano, M.L.; Ríos, Á.; Cannas, M.; Gelardi, F.; Messina, F.; Agnello, S. A comparative study of top-down and bottom-up carbon nanodots and their interaction with mercury ions. Nanomaterials 2021, 11, 1265. [Google Scholar] [CrossRef] [PubMed]
- Crista, D.M.; Esteves da Silva, J.C.; Pinto da Silva, L. Evaluation of different bottom-up routes for the fabrication of carbon dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Farouk Elsadek, M.; Mohamed Ahmed, B.; Fawzy Farahat, M.; Mosleh, K.N.; Taher, M.M. Recent developments in carbon quantum dots: Properties, fabrication techniques, and bio-applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Kolawole, S.Y.; Mubarak, L.A.; Soleimani, H.; Afolabi, L.O.; Toyin, A.-O.O. Advance and prospect of carbon quantum dots synthesis for energy conversion and storage application: A comprehensive review. J. Energy Storage 2023, 60, 106556. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Lakshmi, B.A.; Kim, S.; Kim, J. Preparation of shape-specific (trilateral and quadrilateral) carbon quantum dots towards multiple color emission. Nanoscale 2020, 12, 11947–11959. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent carbon quantum dots—Synthesis, functionalization and sensing application in food analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711. [Google Scholar] [CrossRef]
- Yadav, A.R.; Mohite, S.K. A brief review: Microwave chemistry and its applications. Res. J. Pharm. Dos. Forms Technol. 2020, 12, 191–197. [Google Scholar] [CrossRef]
- Gartshore, A.; Kidd, M.; Joshi, L.T. Applications of microwave energy in medicine. Biosensors 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, R.; Singh, D.; Savu, R.; Moshkalev, S. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, H.; An, Y.; Hou, T.; Chang, Q.; Huang, L.; Li, J.; Su, R.; Zhou, P. Fiber laser development enabled by machine learning: Review and prospect. PhotoniX 2022, 3, 16. [Google Scholar] [CrossRef]
- Brand, J.; Rode, A.V.; Madden, S.; Wain, A.; King, P.L.; Rapp, L. Ultrashort pulsed laser ablation of granite for stone conservation. Opt. Laser Technol. 2022, 151, 108057. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Janus, Ł. Synteza i Badanie Właściwości Węglowych Kropek Kwantowych do Zastosowań w Medycynie. Ph.D. Thesis, Politechnika Krakowska, Kraków, Poland, 2022. [Google Scholar]
- Li, Z.; Lv, J.; Zhu, X.; Cui, Y.; Jian, X. Development of high frequency piezocomposite with hexagonal pillars via cold ablation process. Ultrasonics 2021, 114, 106404. [Google Scholar] [CrossRef]
- Moreddu, R.; Nasrollahi, V.; Kassanos, P.; Dimov, S.; Vigolo, D.; Yetisen, A.K. Lab-on-a-Contact Lens Platforms Fabricated by Multi-Axis Femtosecond Laser Ablation. Small 2021, 17, 2102008. [Google Scholar] [CrossRef]
- Garcia-Herrera, J.; Henao, J.; Espinosa-Arbelaez, D.; Gonzalez-Carmona, J.; Felix-Martinez, C.; Santos-Fernandez, R.; Corona-Castuera, J.; Poblano-Salas, C.; Alvarado-Orozco, J. Laser cladding deposition of a Fe-based metallic glass on 304 stainless steel substrates. J. Therm. Spray Technol. 2022, 31, 968–979. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.-S.; Pasha, S.K.; Ashokkumar, M.; Choi, M.Y. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo-and electrocatalytic applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- Espina-Casado, J.; Fontanil, T.; Fernández-González, A.; Cal, S.; Obaya, Á.J.; Díaz-García, M.E.; Badia-Laino, R. Carbon dots as multifunctional platform for intracellular pH sensing and bioimaging. In vitro and in vivo studies. Sens. Actuators B Chem. 2021, 346, 130555. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Rajabzadeh-Khosroshahi, M.; Samadi, A.; Behzadmehr, R.; Rahdar, A.; Ferreira, L.F.R. Properties and application of carbon quantum dots (CQDs) in biosensors for disease detection: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 80, 104156. [Google Scholar] [CrossRef]
- Prakash, A.; Yadav, S.; Yadav, U.; Saxena, P.S.; Srivastava, A. Recent advances on nitrogen-doped carbon quantum dots and their applications in bioimaging: A review. Bull. Mater. Sci. 2023, 46, 7. [Google Scholar] [CrossRef]
- Sheikh Mohd Ghazali, S.A.I.; Fatimah, I.; Zamil, Z.N.; Zulkifli, N.N.; Adam, N. Graphene quantum dots: A comprehensive overview. Open Chem. 2023, 21, 20220285. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Can, V.; Onat, B.; Cirit, E.S.m.; Sahin, F.; Canbek Ozdil, Z.C. Metal-Enhanced Fluorescent Carbon Quantum Dots via One-Pot Solid State Synthesis for Cell Imaging. ACS Appl. Bio Mater. 2023, 6, 1798–1805. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, F.; Hu, J.; Gao, W.; Zhang, M. A mini review on pH-sensitive photoluminescence in carbon nanodots. Front. Chem. 2021, 8, 605028. [Google Scholar] [CrossRef]
- Tao, X.; Liao, M.; Wu, F.; Jiang, Y.; Sun, J.; Shi, S. Designing of biomass-derived carbon quantum dots@ polyvinyl alcohol film with excellent fluorescent performance and pH-responsiveness for intelligent detection. Chem. Eng. J. 2022, 443, 136442. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Kong, T.; Hao, L.; Wei, Y.; Cai, X.; Zhu, B. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-Y.; Gedda, G.; Girma, W.M.; Yen, C.-L.; Ling, Y.-C.; Chang, J.-Y. Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liao, T.; Wan, L.; Kuang, Y.; Liu, C.; Duan, J.; Xu, X.; Xu, Z.; Jiang, B.; Li, C. Dual-stimuli responsive near-infrared emissive carbon dots/hollow mesoporous silica-based integrated theranostics platform for real-time visualized drug delivery. Nano Res. 2021, 14, 4264–4273. [Google Scholar] [CrossRef]
- Wang, L.; Pan, H.; Gu, D.; Sun, H.; Chen, K.; Tan, G.; Pan, W. A novel carbon dots/thermo-sensitive in situ gel for a composite ocular drug delivery system: Characterization, ex-vivo imaging, and in vivo evaluation. Int. J. Mol. Sci. 2021, 22, 9934. [Google Scholar] [CrossRef]
- Chung, H.K.; Wongso, V.; Sambudi, N.S.; Isnaeni. Biowaste-derived carbon dots/hydroxyapatite nanocomposite as drug delivery vehicle for acetaminophen. J. Sol-Gel Sci. Technol. 2020, 93, 214–223. [Google Scholar] [CrossRef]
- John, T.S.; Yadav, P.K.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence 2020, 35, 913–923. [Google Scholar] [CrossRef]
- Aguilar Cosme, J.R.; Bryant, H.E.; Claeyssens, F. Carbon dot-protoporphyrin IX conjugates for improved drug delivery and bioimaging. PLoS ONE 2019, 14, e0220210. [Google Scholar] [CrossRef]
- Yang, T.; Huang, J.L.; Wang, Y.T.; Zheng, A.Q.; Shu, Y.; Wang, J.H. β-Cyclodextrin-Decorated carbon dots serve as nanocarriers for targeted drug delivery and controlled release. ChemNanoMat 2019, 5, 479–487. [Google Scholar] [CrossRef]
- Tejwan, N.; Kundu, M.; Ghosh, N.; Chatterjee, S.; Sharma, A.; Singh, T.A.; Das, J.; Sil, P.C. Synthesis of green carbon dots as bioimaging agent and drug delivery system for enhanced antioxidant and antibacterial efficacy. Inorg. Chem. Commun. 2022, 139, 109317. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Wang, L.; Yu, J.; Wang, B.; Hu, Y.; Zang, S.Q.; Yang, B.; Lu, S. Solid-State Red Laser with a Single Longitudinal Mode from Carbon Dots. Angew. Chem. Int. Ed. 2021, 60, 25514–25521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef] [PubMed]
- Zhi, B.; Cui, Y.; Wang, S.; Frank, B.P.; Williams, D.N.; Brown, R.P.; Melby, E.S.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H. Malic acid carbon dots: From super-resolution live-cell imaging to highly efficient separation. ACS Nano 2018, 12, 5741–5752. [Google Scholar] [CrossRef]
- He, H.; Chen, X.; Feng, Z.; Liu, L.; Wang, Q.; Bi, S. Nanoscopic imaging of nucleolar stress enabled by protein-mimicking carbon dots. Nano Lett. 2021, 21, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Serafín, V.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Asadpour-Zeynali, K. Ultrasensitive determination of receptor tyrosine kinase with a label-free electrochemical immunosensor using graphene quantum dots-modified screen-printed electrodes. Anal. Chim. Acta 2018, 1011, 28–34. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, G.; Lu, N.; Yuan, X.; Li, B. A miniaturized electrochemical toxicity biosensor based on graphene oxide quantum dots/carboxylated carbon nanotubes for assessment of priority pollutants. J. Hazard. Mater. 2017, 324, 272–280. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Cranberry beans derived carbon dots as a potential fluorescence sensor for selective detection of Fe3+ ions in aqueous solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef]
- Kong, B.; Zhu, A.; Ding, C.; Zhao, X.; Li, B.; Tian, Y. Carbon dot-based inorganic–organic nanosystem for two-photon imaging and biosensing of pH variation in living cells and tissues. Adv. Mater. 2012, 24, 5844–5848. [Google Scholar] [CrossRef]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Dong, C.; Shuang, S. A High Catalytic Activity Nanozyme Based on Cobalt-Doped Carbon Dots for Biosensor and Anticancer Cell Effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef]
- Disha; Kumari, P.; Patel, M.K.; Kumar, P.; Nayak, M.K. Carbon Dots Conjugated Antibody as an Effective FRET-Based Biosensor for Progesterone Hormone Screening. Biosensors 2022, 12, 993. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Wu, H.; He, H.; Jin, Y. Ionic liquid-functionalized fluorescent carbon nanodots and their applications in electrocatalysis, biosensing, and cell imaging. Langmuir 2014, 30, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Hou, Y.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Nanosensor composed of nitrogen-doped carbon dots and gold nanoparticles for highly selective detection of cysteine with multiple signals. Anal. Chem. 2015, 87, 2195–2203. [Google Scholar] [CrossRef]
- Xu-Cheng, F.; Xuan-Hua, L.; Jin, J.-Z.; Zhang, J.; Wei, G. Facile synthesis of bagasse waste derived carbon dots for trace mercury detection. Mater. Res. Express 2018, 5, 065044. [Google Scholar] [CrossRef]
- Thongsai, N.; Tanawannapong, N.; Praneerad, J.; Kladsomboon, S.; Jaiyong, P.; Paoprasert, P. Real-time detection of alcohol vapors and volatile organic compounds via optical electronic nose using carbon dots prepared from rice husk and density functional theory calculation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 278–287. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gore, A.H.; Naik, V.M.; Pawar, S.P.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Carbon dots as a dual sensor for the selective determination of d-penicillamine and biological applications. Opt. Mater. 2019, 88, 134–142. [Google Scholar] [CrossRef]
- Gunjal, D.B.; Gurav, Y.M.; Gore, A.H.; Naik, V.M.; Waghmare, R.D.; Patil, C.S.; Sohn, D.; Anbhule, P.V.; Shejwal, R.V.; Kolekar, G.B. Nitrogen doped waste tea residue derived carbon dots for selective quantification of tetracycline in urine and pharmaceutical samples and yeast cell imaging application. Opt. Mater. 2019, 98, 109484. [Google Scholar] [CrossRef]
- Babar, D.G.; Garje, S.S. Nitrogen and Phosphorus Co-Doped Carbon Dots for Selective Detection of Nitro Explosives. ACS Omega 2020, 5, 2710–2717. [Google Scholar] [CrossRef]
- Du, F.; Zeng, Q.; Lai, Z.; Cheng, Z.; Ruan, G. Silicon doped graphene quantum dots combined with ruthenium(iii) ions as a fluorescent probe for turn-on detection of triclosan. New J. Chem. 2019, 43, 12907–12915. [Google Scholar] [CrossRef]
- Yahaya Pudza, M.; Zainal Abidin, Z.; Abdul Rashid, S.; Md Yasin, F.; Noor, A.S.M.; Issa, M.A. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Deng, J.; Wang, S. A novel fluorescent “turn-on” aptasensor based on nitrogen-doped graphene quantum dots and hexagonal cobalt oxyhydroxide nanoflakes to detect tetracycline. Anal. Bioanal. Chem. 2020, 412, 1343–1351. [Google Scholar] [CrossRef]
- Jalili, R.; Khataee, A.; Rashidi, M.-R.; Razmjou, A. Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem. 2020, 314, 126172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, M.; Wu, F.; Li, Q.; Wei, L.; Ma, L. Amino-functionalized CdSe/ZnS quantum dot-based lateral flow immunoassay for sensitive detection of aflatoxin B1. Anal. Methods 2018, 10, 3582–3588. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, W.; Zhao, Y.; Liu, H.; Sun, B. Single, dual and multi-emission carbon dots based optosensing for food safety. Trends Food Sci. Technol. 2021, 111, 388–404. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Li, T.; Li, Z.; Huang, T.; Tian, L. Carbon quantum dot-based sensors for food safety. Sens. Actuators A Phys. 2021, 331, 113003. [Google Scholar] [CrossRef]
- Sobiech, M.; Luliński, P.; Wieczorek, P.P.; Marć, M. Quantum and carbon dots conjugated molecularly imprinted polymers as advanced nanomaterials for selective recognition of analytes in environmental, food and biomedical applications. TrAC Trends Anal. Chem. 2021, 142, 116306. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Fu, Z.; Gao, W.; Zhang, C.; Zhao, Q.; Deng, F.; Lu, X. Review on carbon dots in food safety applications. Talanta 2019, 194, 809–821. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, X.; Tang, W.; Yue, T.; Li, Z. Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends Food Sci. Technol. 2020, 95, 149–161. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.-H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sens. Actuators B Chem. 2019, 286, 206–213. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.-Y.; Zhou, Z.-J.; Wu, Y.-M.; Li, Y.; Li, J.-C.; Bai, Y.-H.; Wang, J.-L. Application Progress of Fluorescent Carbon Quantum Dots in Food Analysis. Chin. J. Anal. Chem. 2020, 48, 1288–1296. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Hao, S.; Yang, Y.; Li, X.; Luo, X.; Fang, G.; Liu, J.; Wang, S. Perspective on recent developments of nanomaterial based fluorescent sensors: Applications in safety and quality control of food and beverages. J. Food Drug Anal. 2020, 28, 486. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Song, Y.; Zhu, B.-W.; Tan, M. Universal existence of fluorescent carbon dots in beer and assessment of their potential toxicity. Nanotoxicology 2019, 13, 160–173. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Bhandari, B.; Bai, B. Microbial and quality improvement of boiled gansi dish using carbon dots combined with radio frequency treatment. Int. J. Food Microbiol. 2020, 334, 108835. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Wang, H.; Devahastin, S. Effect of addition of carbon dots to the frying oils on oxidative stabilities and quality changes of fried meatballs during refrigerated storage. Meat Sci. 2022, 185, 108715. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Kousheh, S.A.; Guimarães, J.T.; McClements, D.J. Carbon dots synthesized from microorganisms and food by-products: Active and smart food packaging applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1943–1959. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Zhang, Z.; Zhang, J. “Off-on” fluorescence probe based on green emissive carbon dots for the determination of Cu2+ ions and glyphosate and development of a smart sensing film for vegetable packaging. Microchim. Acta 2022, 189, 131. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef]
- Min, S.; Ezati, P.; Rhim, J.-W. Gelatin-based packaging material incorporated with potato skins carbon dots as functional filler. Ind. Crops Prod. 2022, 181, 114820. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.; Valcárcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Zheng, E.; Chen, L.; Wang, X.; Kong, L.; You, C.; Ruan, Y.; Weng, X. Hybrid carbon source for producing nitrogen-doped polymer nanodots: One-pot hydrothermal synthesis, fluorescence enhancement and highly selective detection of Fe (III). Nanoscale 2013, 5, 8015–8021. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Chen, M.; An, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Solvent-free preparation of tannic acid carbon dots for selective detection of Ni2+ in the Environment. Int. J. Mol. Sci. 2022, 23, 6681. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Khan, A.; Rhim, J.-W.; Kim, J.T.; Molaei, R. pH-Responsive strips integrated with resazurin and carbon dots for monitoring shrimp freshness. Colloids Surf. B Biointerfaces 2023, 221, 113013. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sivanesan, S.; Panneerselvam, P. Turn-On fluorescence sensor based detection of heavy metal ion using carbon dots@ graphitic-carbon nitride nanocomposite probe. J. Photochem. Photobiol. A Chem. 2020, 389, 112204. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Park, T.J.; Kailasa, S.K. Glutathione-capped Syzygium cumini carbon dot-amalgamated agarose hydrogel film for naked-eye detection of heavy metal ions. J. Anal. Sci. Technol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, M.; Zhang, L.; Zou, M.; Chen, D.; Huang, Y.; Zhao, S. Unique approach to develop carbon dot-based nanohybrid near-infrared ratiometric fluorescent sensor for the detection of mercury ions. Anal. Chem. 2017, 89, 8044–8049. [Google Scholar] [CrossRef]
- Lu, Z.; Su, T.; Feng, Y.; Jiang, S.; Zhou, C.; Hong, P.; Sun, S.; Li, C. Potential application of nitrogen-doped carbon quantum dots synthesized by a solvothermal method for detecting silver ions in food packaging. Int. J. Environ. Res. Public Health 2019, 16, 2518. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Shi, Y.; Wang, Y.; Sun, Y.; Hu, J.; Ni, P.; Li, Z. A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sens. Actuators B Chem. 2014, 202, 201–208. [Google Scholar] [CrossRef]
- Naksen, P.; Jarujamrus, P.; Anutrasakda, W.; Promarak, V.; Zhang, L.; Shen, W. Old silver mirror in qualitative analysis with new shoots in quantification: Nitrogen-doped carbon dots (N-CDs) as fluorescent probes for “off-on” sensing of formalin in food samples. Talanta 2022, 236, 122862. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chowdhuri, A.R.; Mahto, T.K.; Samui, A.; kumar Sahu, S. One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv. 2016, 6, 72471–72478. [Google Scholar] [CrossRef]
- Jampasa, S.; Ngamrojanavanich, N.; Rengpipat, S.; Chailapakul, O.; Kalcher, K.; Chaiyo, S. Ultrasensitive electrochemiluminescence sensor based on nitrogen-decorated carbon dots for Listeria monocytogenes determination using a screen-printed carbon electrode. Biosens. Bioelectron. 2021, 188, 113323. [Google Scholar] [CrossRef]
- Choi, C.A.; Mazrad, Z.A.I.; Lee, G.; In, I.; Lee, K.D.; Park, S.Y. Boronate-based fluorescent carbon dot for rapid and selectively bacterial sensing by luminescence off/on system. J. Pharm. Biomed. Anal. 2018, 159, 1–10. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, J.; Zhang, Z. Exonuclease I-assisted fluorescent method for ochratoxin A detection using iron-doped porous carbon, nitrogen-doped graphene quantum dots, and double magnetic separation. Anal. Bioanal. Chem. 2019, 411, 2405–2414. [Google Scholar] [CrossRef]
- Huang, S.; Yao, J.; Chu, X.; Liu, Y.; Xiao, Q.; Zhang, Y. One-step facile synthesis of nitrogen-doped carbon dots: A ratiometric fluorescent probe for evaluation of acetylcholinesterase activity and detection of organophosphorus pesticides in tap water and food. J. Agric. Food Chem. 2019, 67, 11244–11255. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Jiao, Y.; Gao, Y.; Qiao, J.; Mozneb, M.; Shuang, S.; Dong, C.; Li, C.-Z. Bright yellow fluorescent carbon dots as a multifunctional sensing platform for the label-free detection of fluoroquinolones and histidine. ACS Appl. Mater. Interfaces 2018, 10, 42915–42924. [Google Scholar] [CrossRef] [PubMed]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green synthesized carbon dots embedded in silica molecularly imprinted polymers, characterization and application as a rapid and selective fluorimetric sensor for determination of thiabendazole in juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef]
- Yang, M.; Liu, M.; Wu, Z.; He, Y.; Ge, Y.; Song, G.; Zhou, J. Carbon dots co-doped with nitrogen and chlorine for “off-on” fluorometric determination of the activity of acetylcholinesterase and for quantification of organophosphate pesticides. Microchim. Acta 2019, 186, 585. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zeng, Y.; Li, L.; Luo, F.; Qiu, B.; Lin, Z.; Guo, L. Ratiometric fluorescent hydrogel test kit for on-spot visual detection of nitrite. ACS Sens. 2019, 4, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ezati, P.; Rhim, J.-W.; Kim, J.T.; Molaei, R. pH-sensitive green tea-derived carbon quantum dots for real-time monitoring of shrimp freshness. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131242. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N, S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, L.; Rao, S.-q.; Yang, Z.-q.; Li, T.; Gong, X. Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem. 2019, 280, 195–202. [Google Scholar] [CrossRef]
- Chen, K.; Qing, W.; Hu, W.; Lu, M.; Wang, Y.; Liu, X. On-off-on fluorescent carbon dots from waste tea: Their properties, antioxidant and selective detection of CrO42−, Fe3+, ascorbic acid and L-cysteine in real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 228–234. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Miao, H.; Wang, L.; Wu, S.; Yang, X. Fluorescent carbon dots derived from lactose for assaying folic acid. Sci. China Chem. 2016, 59, 487–492. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J. Novel fluorescence resonance energy transfer optical sensors for vitamin B 12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501–507. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosens. Bioelectron. 2016, 79, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhai, X.; Shi, J.; Zou, X.; Xue, Y.; Sun, Y.; Sun, W.; Zhang, J.; Huang, X.; Li, Z.; et al. A ratiometric fluorescence amine sensor based on carbon quantum dot-loaded electrospun polyvinylidene fluoride film for visual monitoring of food freshness. Food Chem. 2024, 434, 137423. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.-W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Fan, D.; Jiang, F. Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified-atmosphere-packaged fresh-cut cucumber. J. Sci. Food Agric. 2019, 99, 6032–6041. [Google Scholar] [CrossRef]
- Kousheh, S.A.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, Y.; Jiang, Y.; Miao, M.; Cao, S.; Fang, J. Use of carbon dots to enhance UV-blocking of transparent nanocellulose films. Carbohydr. Polym. 2017, 161, 253–260. [Google Scholar] [CrossRef]
| Area | Application | References |
|---|---|---|
| Drug delivery system | Fluorescent CDs with a carboxyl-rich surface used as a drug delivery system for localized therapy in vivo. | [73] |
| Highly fluorescent carbon dots for delivery of doxorubicin and drug delivery system for cancer therapy. | [74] | |
| Magnetofluorescent carbon dots obtained from waist crab shell for bioimaging and drug delivery. | [75] | |
| Carbon dots as a model for nanodrug delivery for glioblastoma brain tumors. | [76] | |
| Carbon dots/hollowmesoporous silica-based platform for near-infrared fluorescent imaging and therapeutic functions. | [77] | |
| Carbon dots/thermo-sensitive in situ gel as drug delivery agent. | [78] | |
| Biowaste-derived carbon dots/hydroxyapatite nanocomposite used as drugcarrier for acetaminophen. | [79] | |
| CDs conjugated with amoxicillin used as a drug delivery agent for bacterial inhibition. | [80] | |
| Carbon dot-protoporphyrin IX conjugates as carriers in drug delivery and bioimaging applications | [81] | |
| Nanoplatform of doxorubicin and carbon dots for targeted delivery and controlled discharge of anti-cancer medicines. | [82] | |
| Bioimaging | Carbon dots, synthesized from the root extract of red Korean ginseng used as bioimaging and drug delivery agent to improve antioxidant and antimicrobial activity. | [83] |
| Red-emissive carbon dots from aliphatic precursors applied in solid-state micro/nano lasers. | [84] | |
| CDs used in vivo bio-imaging and in fluorescence bio-labeling of Escherichia coli bacteria | [85] | |
| Malic acid carbon dots used for super-resolution fluorescence localization microscopy. | [86] | |
| Protein-mimicking CDs for nucleolar stress studies in cell diagnostics and therapeutics. | [87] | |
| Biosensing | CDs as an electrochemical immunosensor for the selective and sensitive determination of the biomarker receptor tyrosine kinase (AXL) in human serum. | [88] |
| Biosensor for the assessment of contaminant toxicity in the aquatic environment. | [89] | |
| Fluorescence detector for the specific detection of Fe3+ ions in aqueous solution. | [90] | |
| CDs-based two-photon fluorescent sensor for monitoring pH variation in living cells and tissues. | [91] | |
| A nanozyme with high catalytic activity derived from cobalt-doped carbon dots for biosensor and anti-tumour activity. | [92] | |
| Carbon dot conjugated antibody as a biosensor for progesterone hormone screening. | [93] | |
| Fluorescent carbon nanodroplets functionalised with ionic liquid for use in electrocatalysis, biosensors and cell imaging. | [94] | |
| Nitrogen-doped CDs and gold nanoparticles for the detection of cysteine in human serum. | [95] | |
| Nitrogen doped fluorescent carbon dots to the detection of Hg2+ in real water samples. | [96] | |
| Carbon dots prepared from rice husk to detecting alcohol vapour and of distinguishing between methanol, ethanol, and certain volatile organic compounds | [97] | |
| CDs from Mahogany fruit shell by chemical oxidation method for the selective detection of d-Penicillamine (D-PA). | [98] | |
| Carbon dots derived from tea residues, doped with nitrogen, for the detection of tetracycline in urine and pharmaceutical samples and for yeast cell imaging. | [99] | |
| Carbon dots doped with nitrogen and phosphorus for the identification of nitro explosives. | [100] | |
| Graphene quantum dots/silicon/ruthenium ion as a fluorescent agent for the detection of triclosan. | [101] | |
| Carbon dots from tapioca use in wastewater detection and treatment, bioimaging and chemical detection. | [102] | |
| Aptamer-functionalized nitrogen-doped graphene quantum dots (N-GQDs-aptamer) coupled with cobalt oxyhydroxide (CoOOH) nanoflakes for the detection of tetracycline (antibiotic) residues in foodstuffs. | [103] | |
| Dual-emission carbon dots for detection of antibiotic residues—penicillin in dairy products. | [104] | |
| Amino-functionalized QDs combine with for detection of aflatoxin B1. | [105] |
| Area | Application | Form of CDs | References |
|---|---|---|---|
| Food safety | colorimetric detection of H2O2 and glucose | nitrogen-doped graphene quantum dots (N-GQDs) | [130] |
| detection of Ag+ ions in water | luminescent Carbon Quantum Dot hydrogels (CQDGs) | [125] | |
| Cr (VI), Cu (II) and Pb (II) detection | carbon dots@graphitic-carbon nitride (CDs@g-C3N4) nanocomposite | [131] | |
| Mn2+ detection | glutathione carbon dots-agarose hydrogel film | [132] | |
| Hg2+ detection | CD-based nanohybrid sensor | [133] | |
| Ag+ detection | Nitrogen-Doped Carbon Quantum Dots | [134] | |
| biosensor for melamine detection | amino-functionalized carbon dots | [135] | |
| Formalin detection in Fish, shrimp cauliflower, apple | nitrogen-doped carbon dots | [136] | |
| histamine detection in fish | fluorescence biosensor based on CDs and synthetic peptides | [106] | |
| Pesticide detection | fluorescence emitting CDs synthesized from cauliflower juice | [137] | |
| E. coli detection | magnetite-carbon dots by lemon and grape fruit extracts | [138] | |
| amikacin modified fluorescent carbon dots | [139] | ||
| Listeria monocytogenes detection | nitrogen—carbon dots | [140] | |
| E. coli and Staphylococcus aureus | boronate-based fluorescent carbon dot | [141] | |
| bioimaging of bacterial and fungal cells (E. coli, Aspergillus aculeatus and Fomitopsis sp.) | carbon dots from Manilkara zapota fruits | [142] | |
| ochratoxin detection | iron-doped porous carbon (MPC) and aptamer-functionalized nitrogen-doped graphene quantum dots (NGQDs-Apt) | [143] | |
| organophosphorus pesticides in tap water and food detection | nitrogen-doped carbon dots (N-CDs) | [144] | |
| fluoroquinolones (FQs) and histidine (His) detection in milk | bright yellow fluorescent CDs | [145] | |
| thiabendazole (TBZ) detection in food | carbon dots (CDs) from Rosemary leaves modified with molecularly imprinted polymers | [146] | |
| acetylcholinesterase (AChE) activity and organophosphate pesticides (OPs) detection | Nitrogen and chlorine dually-doped carbon dots | [147] | |
| detection of nitrite (NO2−) | CDs synthesize from m-phenylenediamine | [148] | |
| shrimp freshness monitoring | pH-sensitive green tea-derived carbon quantum dots | [149] | |
| Food control | ascorbic acid in common fruits determination | N, S co-doped carbon dots | [150] |
| curcumin determination in food | nitrogen and chlorine dual-doped carbon nanodots (N,Cl-CDs) | [151] | |
| CrO42−, Fe3+, ascorbic acid and L-Cysteine detection in food samples | carbon dots synthesis of waste tea extract | [152] | |
| folic acid detection | carbon dots derived from lactos | [153] | |
| Vitamin B12 detection | hermally-reduced carbon dot | [154] | |
| Thiamine detection | Monodispersed CDs synthesize from coconut water | [155] | |
| monitoring of food freshness | dual-emission carbon quantum dots | [156] | |
| Food storage | active food packaging | chitosan-based carbon quantum dots | [157] |
| active and smart food packaging | carbon dots synthesized from microorganisms and food by-products | [121] | |
| active food packaging | carbon dots with chitosan coating | [158] | |
| antimicrobial/ultraviolet protective film | nanocellulose film with carbon dots synthesized from lactic acid bacteria | [159] | |
| UV-blocking of transparent nanocellulose films | CDs-ONC (oxidized nanocellulose) composites | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepankowska, J.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M. Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology. Int. J. Mol. Sci. 2023, 24, 14984. https://doi.org/10.3390/ijms241914984
Szczepankowska J, Khachatryan G, Khachatryan K, Krystyjan M. Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology. International Journal of Molecular Sciences. 2023; 24(19):14984. https://doi.org/10.3390/ijms241914984
Chicago/Turabian StyleSzczepankowska, Joanna, Gohar Khachatryan, Karen Khachatryan, and Magdalena Krystyjan. 2023. "Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology" International Journal of Molecular Sciences 24, no. 19: 14984. https://doi.org/10.3390/ijms241914984
APA StyleSzczepankowska, J., Khachatryan, G., Khachatryan, K., & Krystyjan, M. (2023). Carbon Dots—Types, Obtaining and Application in Biotechnology and Food Technology. International Journal of Molecular Sciences, 24(19), 14984. https://doi.org/10.3390/ijms241914984









