Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs
Abstract
:1. Introduction
2. Results
2.1. Influence of Manufacturing Methods on the Properties of P(3HB) Microparticles
2.2. Influence of the Chemical Composition of PHAs on the Properties of Microparticles
2.3. Drug-Loaded PHA-Microparticle and Drug Release In Vitro
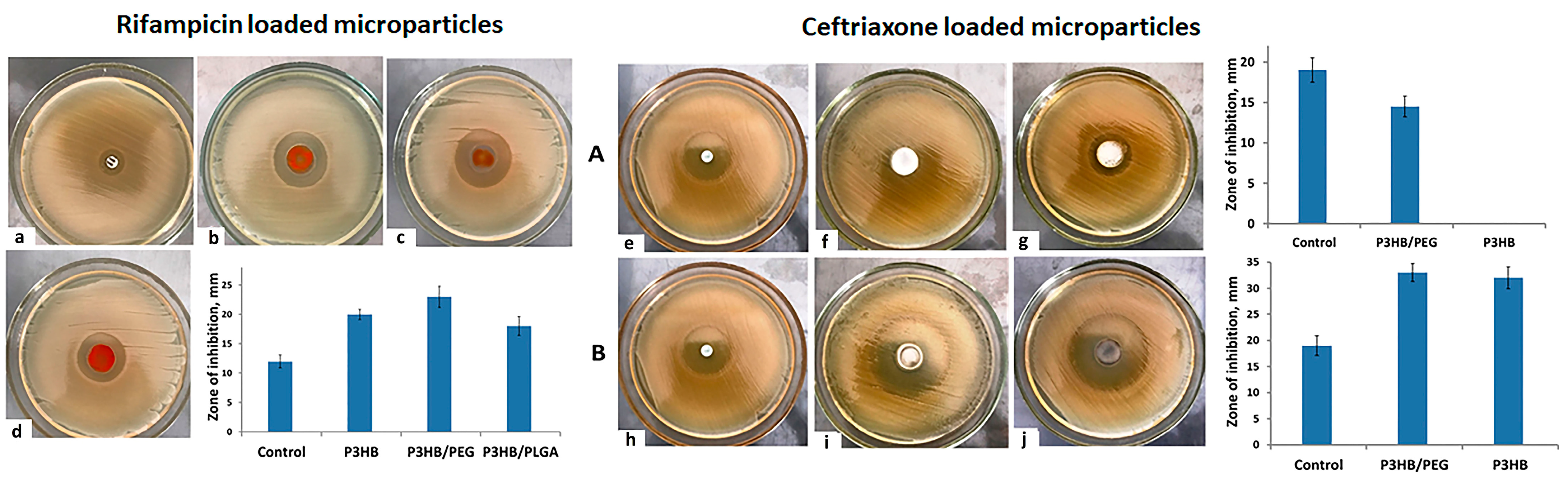
2.4. Antibacterial and Antitumor Efficacy of Drug-Loaded PHA Microparticles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods of Preparation of PHAs Microparticles
4.3. Characterization of Microparticles
4.3.1. Particle Size, Size Distribution and ζ Potential
4.3.2. Microscopic Analysis
4.3.3. Loading of Drugs and Encapsulation Efficiency
4.3.4. In Vitro Release Studies
4.4. Antimicrobial and Antitumor Efficacy of Drug-Loaded PHA Microparticles
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amass, W.; Amass, A.; Tighe, B. A Review of Biodegradale Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Poncelet, D. Microencapsulation: Fundamentals, methods and applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J., Ed.; Springer: Cham, The Netherlands, 2005; pp. 23–34. [Google Scholar]
- Torchilin, V. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.C. Drug carriers in pharmaceutical design: Promises and progress. Curr. Pharm. Des. 2007, 13, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Panarin, E.F.; Lavrov, N.A.; Solovskii, M.V.; Shalnova, L.I. Polymers—Carriers of biologically active substances; Publishing House of the COP “Profession”: St. Petersburg, Russia, 2014; p. 304. [Google Scholar]
- Jain, K.K. Drug Delivery Systems, Methods in Molecular Biology; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2020; Volume 2059. [Google Scholar] [CrossRef]
- Ambruosi, A.; Gelperina, S.; Khalansky, A. Influence of surfactants, polymer and doxorubicin loading on the anti-tumor effect of poly(butyl cyanoacrylate) nanoparticles in a rat glioma model. J. Microencapsul. 2006, 23, 582–592. [Google Scholar] [CrossRef]
- Jain, K. Drug Delivery Systems—An Overview. In Drug Delivery Systems; Jain, K.K., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 1–50. [Google Scholar]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer: New York, NY, USA, 2009; pp. 1–50. [Google Scholar]
- Maiti, S.; Sen, K.K. Introductory Chapter: Drug Delivery Concepts. In Advanced Technology for Delivering Therapeutics; Maiti, S., Sen, K.K., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Neubert, R.H. Potentials of New Nanocarriers for Dermal and Transdermal Drug Delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and Drug Delivery: A Mini Review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.; Park, T. Regylated recombinant human epidermal growth factor (rhEGF) for sustained release from biodegradable PLGA microspheres. Biomaterials 2002, 23, 2311–2317. [Google Scholar] [CrossRef]
- Yen, H.; Huang, Y. Injectable biodegradable polymeric implants for the prevention of postoperative infection. Am. J. Drug Deliv. 2003, 1, 149–156. [Google Scholar]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Scicluna, M.C.; Vella-Zarb, L. Evolution of Nanocarrier Drug-Delivery Systems and Recent Advancements in Covalent Organic Framework–Drug Systems. ACS Appl. Nano Mater. 2020, 3, 3097–3115. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.; Laurencin, C. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Modi, S.; Domb, A. Role of polyanhydrides as localized drug carriers. J. Control. Release 2005, 103, 541–563. [Google Scholar] [CrossRef]
- Goepferich, A.; Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 2002, 54, 911–931. [Google Scholar] [CrossRef]
- Ghosh, R.; Arun, Y.; Siman, P.; Domb, A.J. Synthesis of Aliphatic Polyanhydrides with Controllable and Reproducible Molecular Weight. Pharmaceutics 2022, 14, 1403. [Google Scholar] [CrossRef]
- Heller, J.; Barr, J.; Ng, S.Y. Poly(ortho esters) synthesis, characterization, properties and uses. Adv. Drug Deliv. Rev. 2002, 54, 1015–1039. [Google Scholar] [CrossRef]
- Li, H.; Palamoor, M.; Jablonski, M.M. Poly(ortho ester) nanoparticles targeted for chronic intraocular diseases: Ocular safety and localization after intravitreal injection. Nanotoxicology 2016, 10, 1152–1159. [Google Scholar] [CrossRef]
- Yun, Y.H.; Yun, Y.H.; Goetz, D.J.; Yellen, P. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials 2004, 25, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, S.; Michaelis, K.; Dinauer, N.; Van Briesen, H.; Kreuter, J.; Langer, K. Preparation and characterization of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials 2005, 26, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; F da Silva, C.; Andrade, L.N.; Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Luciano, B. Polyhydroxyalkanoates based systems: The future of drug delivery and tissue engineering devices. In Bio-Based Nanomaterials: Synthesis Protocols, Mechanisms and Applications; Mishra, A.K., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; 306p. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Chen, G.-Q. Plastics Completely Synthesized by Bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar]
- Sudesh, K.; Abe, H. Practical Guide to Microbial Polyhydroxyalkanoates; Ismithers: Shrewsbury, UK, 2010; ISBN 9781847351180. [Google Scholar]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications. In Degradable Polymers: Production, Properties, Applications; Nova Science Pub Inc.: Hauppauge, NY, USA, 2013; pp. 1–380. [Google Scholar]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward Cost Competitiveness and Functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Chen, G.; Xiang, H.; Han, J. An Updated Overview on the Regulatory Circuits of Polyhydroxyalkanoates Synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Ladhari, S.; Vu, N.-N.; Boisvert, C.; Saidi, A.; Nguyen-Tri, P. Recent Development of Polyhydroxyalkanoates (PHA)-Based Materials for Antibacterial Applications: A Review. ACS Appl. Bio Mater. 2023, 6, 1398–1430. [Google Scholar]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From Production to Nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar]
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate Blends: A Solution for Biodegradable Packaging? Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking Properties, Applications and End-of-Life Options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; de Micco, J.; Padamati, R.B.; O’Connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N.L. Polyhydroxyalkanoate Bio-Production and Its Rise as Biomaterial of the Future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef]
- Polyhydroxyalkanoate (PHA) Market by Type (Short chain length, Medium Chain Length), Production Method (Sugar Fermentation, Vegetable Oil Fermentation), Application (Packaging & Food Services, Biomedical) and Region—Global Forecast to 2028. Available online: https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html (accessed on 1 February 2023).
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Toluwalope Odediran, E.; Louis, H. Sustainable Synthesis and Applications of Polyhydroxyalkanoates (PHAs) from Biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.; Kenward, M.A.; Williams, C.D.; Radecka, I. Bacterial Synthesis of Biodegradable Polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The Application of Polyhydroxyalkanoates as Tissue Engineering Materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural-Based Polymers for Biomedical Applications; Apple Academic Press: Palm Bay, FL, USA, 2017. [Google Scholar]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Guo, W.; Yang, K.; Qin, X.; Luo, R.; Wang, H.; Huang, R. Polyhydroxyalkanoates in Tissue Repair and Regeneration. Eng. Regen. 2022, 3, 24–40. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical Applications of Microbially Engineered Polyhydroxyalkanoates: An Insight into Recent Advances, Bottlenecks, and Solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.; Gregory, D.A.; Fricker, A.; Marcello, E.; Paxinou, A.; Taylor, C.S.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates, Their Processing and Biomedical Applications. In The Handbook of Polyhydroxyalkanoates; CRC Press: Boca Raton, FL, USA, 2020; pp. 255–284. [Google Scholar]
- Pramanik, N. A tool for biomedical application: Synthesis and modification of polyhydroxyalkanoates. Sustain. Chem. Pharm. 2023, 32, 101041. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef]
- Aguilar-Rabiela, A.E.; Homaeigohar, S.; González-Castillo, E.I.; Sánchez, M.L.; Boccaccini, A.R. Comparison between the Astaxanthin Release Profile of Mesoporous Bioactive Glass Nanoparticles (MBGNs) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/MBGN Composite Microspheres. Polymers 2023, 15, 2432. [Google Scholar] [CrossRef]
- Alregib, A.H.; Tan, H.Y.; Wong, Y.H.; Kasbollah, A.; Wong, E.H.; Abdullah, B.J.J.; Perkins, A.C.; Yeong, C.H. Development and physicochemical characterization of a biodegradable microspheres formulation loaded with samarium-153 and doxorubicin for chemo radioembolization of liver tumours. J. Label Compd Radiopharm. 2023, 1, 13. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering Via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Mano, J.F. Polymer-based microparticles in tissue engineering and regenerative medicine. Biotechnol. Prog. 2011, 27, 897. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2012, 6, 628–647. [Google Scholar] [CrossRef]
- Amin, M.C.; Abadi, A.G.; Katas, H. Purification, characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydr. Polym. 2014, 99, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Strob, R.; Dräger-Gillessen, J.F.; Pieloth, D.; Schaldach, G.; Wiggers, H.; Thommes, M. Preparation of submicron drug particles via spray drying from organic solvents. Int. J. Pharm. 2019, 567, 118501. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K. Advantages and Challenges of the Spray-drying Technology for the Production of Pure Drug Particles and Drug-loaded Polymeric Carriers. Adv. Colloid Interf. Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Zimmermann, R.; Leal, B.B.J.; Braghirolli, D.I.; Pranke, P. Production of nanostructured systems: Main and innovative techniques. Drug Discov. Today. 2023, 28, 103454. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles. Polim Med. 2022, 52, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chawla, P.A.; Faruk, A.; Chawla, V. Spray Drying as an Effective Method in the Development of Solid Self- Emulsifying Drug Delivery Systems. Curr. Drug Deliv. 2022, 20, 508–525. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef]
- Fatnassi, M.; Jacquart, S.; Brouillet, F.; Rey, C.; Combes, C.; Fullana, S.G. Optimization of spray-dried hyaluronic acid microspheres to formulate drug-loaded bone substitute materials. Powder Technol. 2014, 255, 44–51. [Google Scholar] [CrossRef]
- Wan, F.; Yang, M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int. J. Pharm. 2016, 498, 82–95. [Google Scholar] [CrossRef]
- Re, M.-I. Formulating Drug Delivery Systems by Spray Drying. Drying Technol. 2006, 24, 433–446. [Google Scholar] [CrossRef]
- Zernov, A.L.; Bonartsev, A.P.; Yakovlev, S.G.; Myshkina, V.L.; Makhina, T.K.; Parshina, E.S.; Kharitonova, E.P.; Bonartseva, G.A.; Shaitan, K.V. Low molecular weight Poly(3-hydroxybutyrate) microparticles synthesized by piezoelectric spray drying for the sustained release of paclitaxel. Nanotechnologies Russ. 2017, 12, 218–225. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Goreva, A.V.; Voinova, O.N.; Inzhevatkin, E.V.; Khlebopros, R.G.; Volova, T.G. Evaluation of Antitumor Activity of Rubomycin Deposited in Absorbable Polymeric Microparticles. Bull. Exp. Biol. Med. 2008, 145, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Kuzmina, A.M.; Goreva, A.V.; Shishatskaya, E.I.; Hasirci, N.; Hasirci, V. In Vitro and Transdermal Penetration of PHBV Micro/Nanoparticles. J. Mater. Sci. Mater. Med. 2014, 25, 1471–1481. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Voinova, O.N.; Goreva, A.V.; Mogilnaya, O.A.; Volova, T.G. Biocompatability of polyhydroxybutyrate Microspheres: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2008, 19, 2493–2502. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Goreva, A.V.; Kalacheva, G.S.; Volova, T.G. Biocompatability and resorption of intravenously administered polymer microparticles in tissue of internalorgans of laboratory animals. J. Biomater. Sci. Polym. Ed. 2011, 22, 2185–2203. [Google Scholar] [CrossRef]
- Delgado, A.V.; Gonz’alez-Caballero, F.; Hunter, R.J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Mäkiläa, E.; Riikonen, J. Effect of isotonic solutions and peptide adsorption on zeta potential of porous silicon nanoparticle drug delivery formulations. Int. J. Pharmaceut. 2012, 431, 230–236. [Google Scholar] [CrossRef]
- Kovačević, A.B.; Müller, R.H.; Savić, S.D.; Vuleta, G.M.; Keck, C.M. Solid lipid nanoparticles (SLN) stabilized with polyhydroxy surfactants: Preparation, characterization and physical stability investigation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 15–25. [Google Scholar] [CrossRef]
- Morello, A.; Forbes, N.; Mathiowitz, E. Investigating the effects of surfactants on the size and hydrolytic stability of poly(adipic anhydride) particles. J. Microencapsul. 2007, 24, 40–56. [Google Scholar] [CrossRef]
- Berchane, N.; Jebrail, F.; Carson, K. About mean diameter and size distributions of poly(lactide-co-glycolide) (PLG) microspheres. J. Microencapsul. 2006, 23, 539–5552. [Google Scholar] [CrossRef]
- Vardhan, H.; Mittal, P.; Adena, S.K.R.; Upadhyay, M.; Mishra, B. Development of long-circulating docetaxel loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles: Optimization, pharmacokinetic, cytotoxicity and in vivo assessments. Int. J. Biol. Macromol. 2017, 103, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S. Microparticles and Nanoparticles for Drug Delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles and microparticles for drug and vaccine delivery. J. Anat. 1996, 189, 503–505. [Google Scholar] [PubMed]
- Zhang, X.; Liu, Y.; Yang, H.; Chen, J.; Lin, Y.; Han, S.; Cao, Q.; Zeng, H.; Ye, J. Polyhydroxyalkanoates-Based Carrier Platform of Bioactive Substances for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 798724. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Goreva, A.V.; Kuzmina, A.M. Study of the Efficiency of Doxorubicin Deposited in Microparticles from Resorbable Bioplastotane TM on Laboratory Animals with Ehrlich’s Solid Carcinoma. Bull. Exp. Biol. Med. 2013, 154, 773–777. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Robinson, J.R.; Lee, V.H.L. Controlled drug delivery. Fundamentals and Applications, 2nd ed.; Marcel Dekker: New York, NY, USA, 1987. [Google Scholar]
- Ranade, V.V.; Hollinger, M. Drug Delivery Systems—Pharmacology and Toxicology, 2nd ed.; Taylor&Francis Routledge: Boca Raton, FL, USA, 2004; 464p. [Google Scholar]
- Paude, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of Solid Dispersions of Poorly Water Soluble Drugs by Spray Drying: Formulation and Process Considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Feng, S.-S.; Huang, G. Effects of Emulsifiers on the Controlled Release of Paclitaxel (Taxol®) from Nanospheres of Biodegradable Polymers. J. Control. Rel. 2001, 71, 53–69. [Google Scholar] [CrossRef]
- Mlalila, N.; Swai, H.; Kalombo, L.; Hilonga, A. Effects of Spray-drying on w/o/w Multiple Emulsions Prepared from a Stearic Acid Matrix. Nanotechnol. Sci. Appl. 2014, 7, 105–112. [Google Scholar] [CrossRef]
- Tran, T.H.; Poudel, B.K.; Marasini, N.; Chi, S.C.; Choi, H.G.; Yong, C.S.; Kim, J.O. Preparation and Evaluation of Raloxifene-loaded Solid Dispersion Nanoparticle by Spray-drying Technique without an Organic Solvent. Int. J. Pharm. 2013, 443, 50–57. [Google Scholar] [CrossRef]
- Kumar, S.; Shen, J.; Burgess, D.J. Nano-amorphous Spray Dried Powder to Improve Oral Bioavailability of Itraconazole. J. Controlled Release 2014, 192, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Bigucci, F.; Corace, G.; Zecchi, V.; Luppi, B. Eudragit-coated Albumin Nanospheres Carrying Inclusion Complexes for Oral Administration of Indomethacin. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 129–136. [Google Scholar] [CrossRef]
- Oh, D.H.; Yan, Y.D.; Kim, D.W.; Kim, J.O.; Yong, C.S.; Choi, H.G. Development of Flurbiprofen Loaded Nanoparticles with a Narrow Size Distribution Using Sucrose. Drug Dev. Ind. Pharm. 2014, 40, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Citalingam, K.; Abas, F.; Lajis, N.; Othman, I.; Naidu, R. Anti-proliferative Effect and Induction of Apoptosis in Androgen-independent Human Prostate Cancer Cells by 1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one. Molecules 2015, 20, 3406–3430. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cho, C.H.; Park, E.K.; Jung, M.H.; Yoon, K.S.; Park, H.K. AFM-detected Apoptotic Changes in Morphology and Biophysical Property Caused by Paclitaxel in Ishikawa and Hela Cells. PLoS ONE 2012, 7, e30066. [Google Scholar]
- Kapoor, S.; Gupta, D.; Kumar, M.; Sharma, S.; Gupta, A.; Misroc, M.; Singh, H. Intracellular delivery of peptide cargos using polyhydroxybutyrate based biodegradable nanoparticles: Studies on antitumor efficacy of BCL-2 converting peptide, NuBCP-Inter. J. Pharm. 2016, 2, 876–889. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in Poly-(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Based Nanoparticles and Its in Vitro Application. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1054–1060. [Google Scholar] [CrossRef]
- Lipaikin, S.; Yaremenko, I.; Terent’ev, A.; Volova, T.; Shishatskaya, E. Development of Biodegradable Delivery Systems Containing Novel 1,2,4-Trioxolane Based on Bacterial Polyhydroxyalkanoates. Adv. Polym. Tech. 2022, 2022, 6353909. [Google Scholar] [CrossRef]
- Embelton, J.K.; Tighe, B.J. Polymers for biodegradable medical devices. IX: Microincapsulation studies: Effects of polymers composition and process parameters on polyhydroxybutyrate-hydroxyvalerate microcapsule morphology. Biomaterials 1992, 9, 73–87. [Google Scholar] [CrossRef]
- Rawat, M.; Saraf, S. Formulation optimization of double emulsification method for preparation of enzyme loaded Eudragit S100 microspheres. J. Microencapsul. 2009, 26, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Chotchindakun, K.; Pekkoh, J.; Ruangsuriya, J.; Zheng, K.; Unalan, I.; Boccaccini Aldo, R. Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration. Polymers 2021, 13, 1794. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Champion, J.; Katare, Y.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Karatas, A.; Sonakin, O.; Kilicarslan, M. Poly(ε-caprolactone) microspheres containing Levobunolol HCl prepared by a multiple emulsion (W/O/W) solvent evaporation technique: Effect of some formulation parameters on microparticle characteristics. J. Microencapsultion 2009, 26, 63–74. [Google Scholar] [CrossRef]
- Deepak, V.; Pandian, S.; Kalishwaralal, K. Purification, immobilization, and characterization of nattokinase on PHB nanoparticles. Bioresourse Technol. 2009, 100, 6644–6646. [Google Scholar] [CrossRef]
- Muller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.; Keshavarz, T.; Boccaccini, A.; Roy, I. Controlled Delivery of Gentamicin Using Poly(3-Hydroxybutyrate) Microspheres. Int. J. Mol. Sci. 2011, 12, 4294–4314. [Google Scholar] [CrossRef]
- Rentsch, C.; Rentsch, B.; Breier, A.; Hofmann, A.; Manthey, S.; Scharnweber, D.; Biewener, A.; Zwipp, H. Evaluation of the osteogenic potential and vascularization of 3D poly(3)hydroxybutyrate scaffolds subcutaneously implanted in nude rats. J. Biomed. Mater. Res. 2009, 92, 185–195. [Google Scholar] [CrossRef]
- Ulery, B.D.; Lakshmi, S.N.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Vinogradova, O.N.; Syrvacheva, D.A.; Shishatskaya, E.I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Microb. Ecol. 2017, 73, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Svasti, J.; Niamsiri, N. Development and Characterization of Bio-Derived Polyhydroxyalkanoate Nanoparticles as a Delivery System for Hydrophobic Photodynamic Therapy Agents. J. Mater. Sci. Mater. Med. 2016, 27, 40. [Google Scholar] [PubMed]
- Rezaie Shirmard, L.; Bahari Javan, N.; Khoshayand, M.R.; Kebriaee-Zadeh, A.; Dinarvand, R.; Dorkoosh, F.A. Nanoparticulate Fingolimod Delivery System Based on Biodegradable Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV): Design, Optimization, Characterization and in-Vitro Evaluation. Pharm. Dev. Technol. 2017, 22, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot Vidal, S.; Rojas, C.; Bouza Padin, R.; Perez Rivera, M.; Haensgen, A.; González, M.; Rodríguez-Llamazares, S. Synthesis and characterization of polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for encapsulation of quercetin. J. Bioact. Compat. Polym. 2016, 31, 439–452. [Google Scholar] [CrossRef]
- Cañadas, O.; García-García, A.; Prieto, M.A.; Pérez-Gil, J. Polyhydroxyalkanoate Nanoparticles for Pulmonary Drug Delivery:Interaction with Lung Surfactant. Nanomaterials 2021, 11, 1482. [Google Scholar] [CrossRef]
- Hu, J.; Wang, M.; Xiao, X.; Zhang, B.; Xie, Q.; Xu, X.; Li, S.; Zheng, Z.; Wei, D.; Zhang, X. A Novel Long-Acting Azathioprine Polyhydroxyalkanoate Nanoparticle Enhances Treatment Efficacy for Systemic Lupus Erythematosus with Reduced Side Effects. Nanoscale 2020, 12, 10799–10808. [Google Scholar] [CrossRef]
- Kilicay, E.; Karahaliloglu, Z.; Hazer, B.; Tekin, I.Ö.; Denkbas, E.B. Concanavaline A Conjugated Bacterial Polyester-Based PHBHHx Nanoparticles Loaded with Curcumin for Breast Cancer Therapy. J. Microencapsul. 2016, 33, 274–285. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Li, M.-C.; Zhu, X.-L.; Fan, F.; Wang, L.-L.; Ma, J.-G. Microbial Synthesized Biodegradable PHBHHxPEG Hybrid Copolymer as an Efficient Intracellular Delivery Nanocarrier for Kinase Inhibitor. BMC Biotechnol. 2014, 14, 4. [Google Scholar] [CrossRef]
- Kılıçay, E.; Demirbilek, M.; Türk, M.; Güven, E.; Hazer, B.; Denkbas, E.B. Preparation and Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHX) Based Nanoparticles for Targeted Cancer Therapy. Eur. J. Pharm. Sci. 2011, 44, 310–320. [Google Scholar] [CrossRef]
- Ullah, N.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amorphous Amphiphilic P(3HV-Co-4HB)-b-MPEG Block Copolymer Synthesized from Bacterial Copolyester via Melt Transesterification: Nanoparticle Preparation, Cisplatin-Loading for Cancer Therapy and in Vitro Evaluation. Eur. J. Pharm. Shah Biopharm. 2012, 80, 518–527. [Google Scholar]
- Shah, M.; Naseer, M.I.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amphiphilic PHA-MPEG Copolymeric Nanocontainers for Drug Delivery: Preparation, Characterization and in Vitro Evaluation. Int. J. Pharm. 2010, 400, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.A.B.; Mathews, P.D.; Madrid, R.R.M.; Garcia, I.T.S.; Rigoni, V.L.S.; Mertins, O. Antibacterial polypeptide-bioparticle for oral administration: Powder formulation, palatability and in vivo toxicity approach. Biomater. Adv. 2023, 153, 213525. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.S.; Panchal, F.; Munshi, R.; Madkaikar, M.; Malshe, V.C.; Devarajan, P.V. pH-responsive microparticles of rifampicin for augmented intramacrophage uptake and enhanced antitubercular efficacy. Int. J. Pharm. 2023, 635, 122729. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, N.; Price, J.C. Poly(hyrdoxybutyrate-co-hydroxyvalerate) microspheres containing progesterone: Preparation, morphology and release properties. J. Microencapsul. 1991, 8, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-C.; Yao, Y.-C.; Zhan, X.-Y.; Chen, G.-Q. Application of Polyhydroxyalkanoates Nanoparticles as Intracellular Sustained Drug-Release Vectors. J. Biomat. Sci. 2010, 21, 127–140. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-Mediated Poly(3-Hydroxybutyrate-Co-3-Hydroxyoctanoate) Nanoparticles for Targeting Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [Google Scholar] [CrossRef]
- Vilos, C.; Constandil, L.; Herrera, N.; Solar, P.; Escobar-Fica, J.; Velasquez, L.A. Ceftiofur-Loaded PHBV Microparticles: A Potential Formulation for a Long-Acting Antibiotic to Treat Animal Infections. Electron. J. Biotechnol. 2012, 15. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Synthesis and properties of polyhydroxyalkanoates on waste fish oil from the production of canned sprats. Processes 2023, 11, 2113. [Google Scholar] [CrossRef]
- Volova, T.G.; Syrvacheva, D.A.; Zhila, N.O.; Sukovatiy, A.G. Synthesis of P(3HB-co-3HHx) copolymers containing high molar fraction of 3-hydroxyhexanoate monomer by Cupriavidus eutrophus B10646. J. Chemical Technol. Biotechnol. 2016, 91, 416–425. [Google Scholar] [CrossRef]
- Zhila, N.O.; Shishatskaya, E.I. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable PHAs with different monomer compositions. Int Biol Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Cavalieri, S.J. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Washington, DC, USA, 2009; p. 241. [Google Scholar]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique, 5th ed.; John Wiley & Sons. Inc.: New York, NY, USA, 2005. [Google Scholar]
- Liu, K.; Liu, P.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
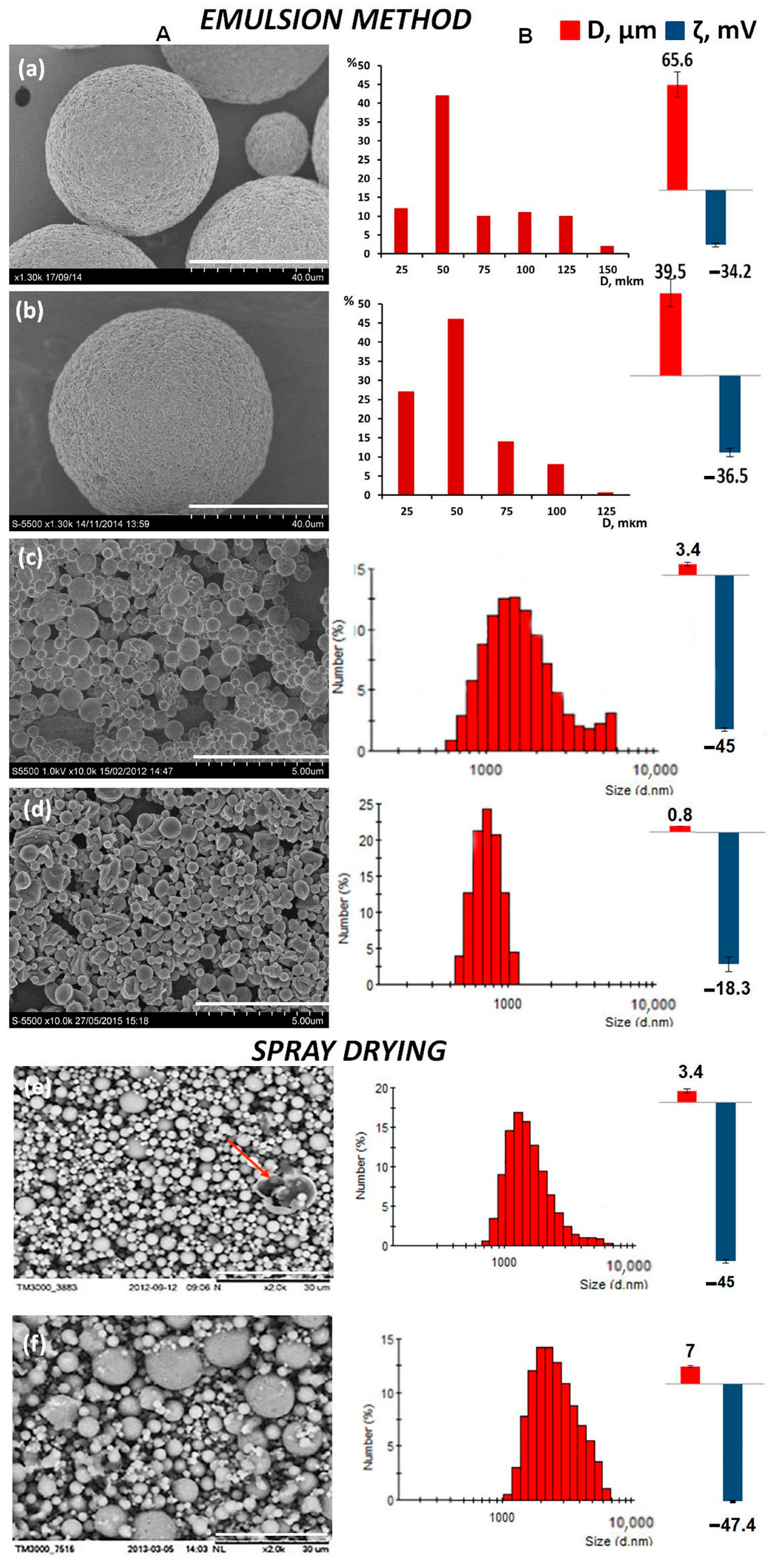
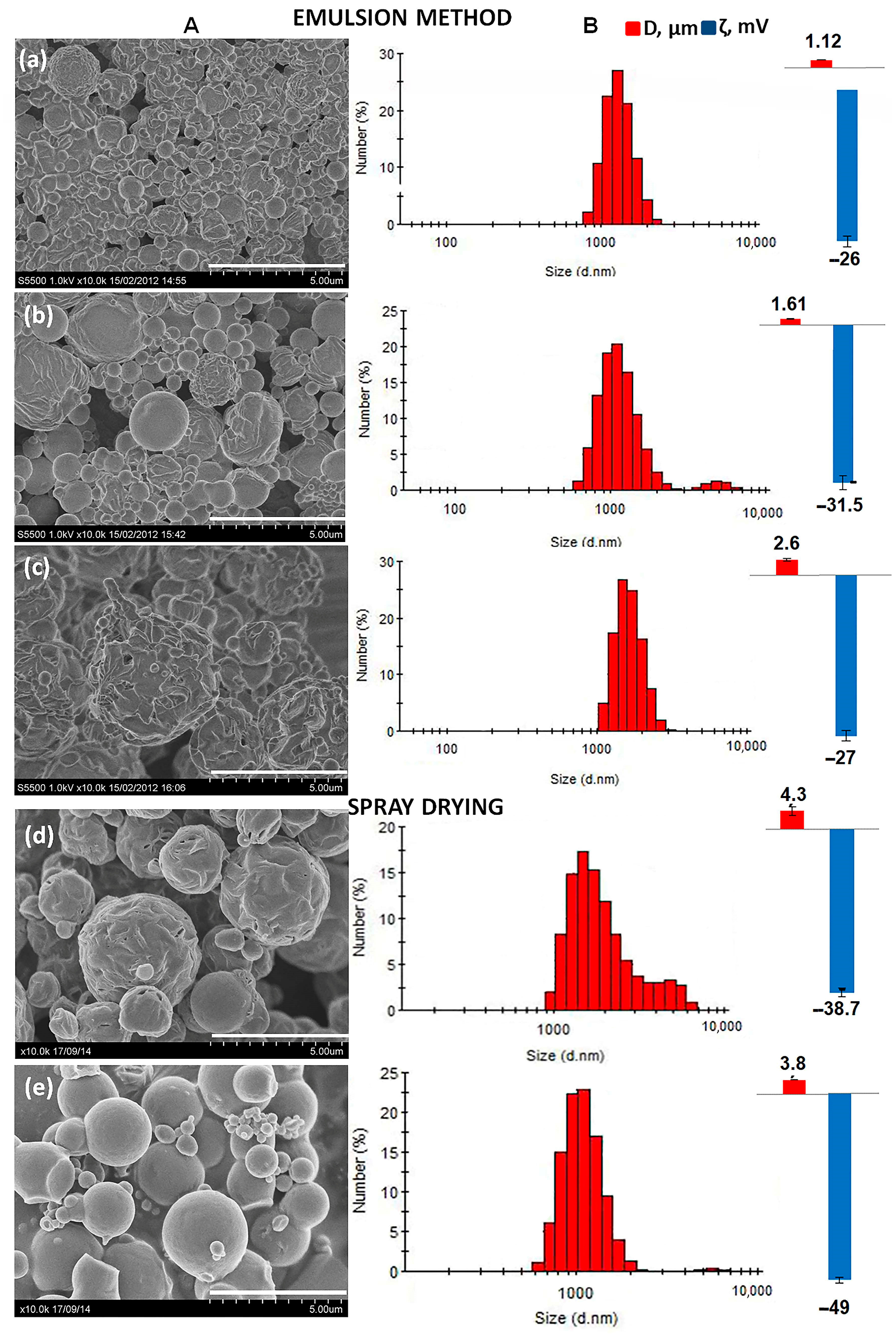
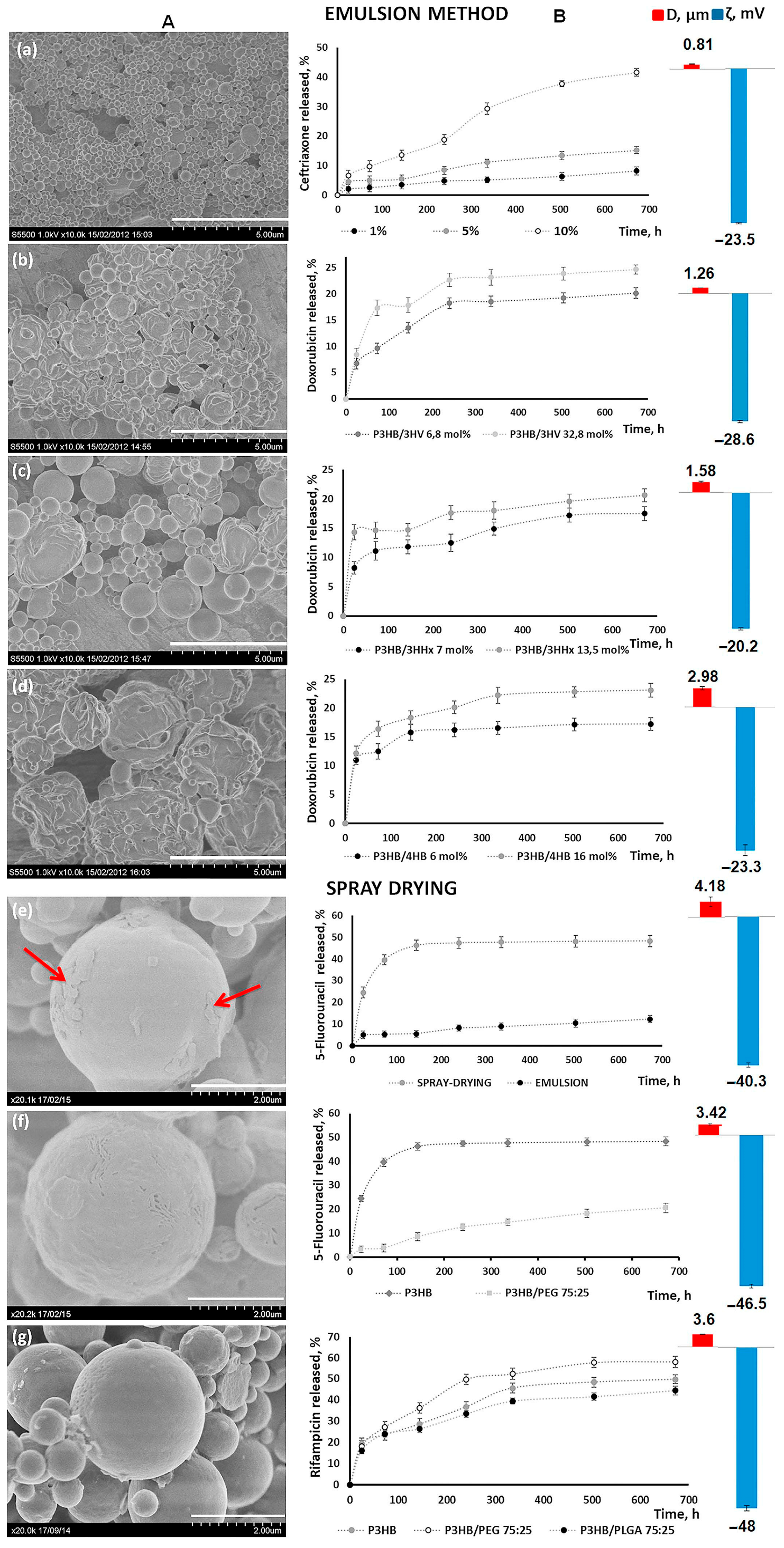
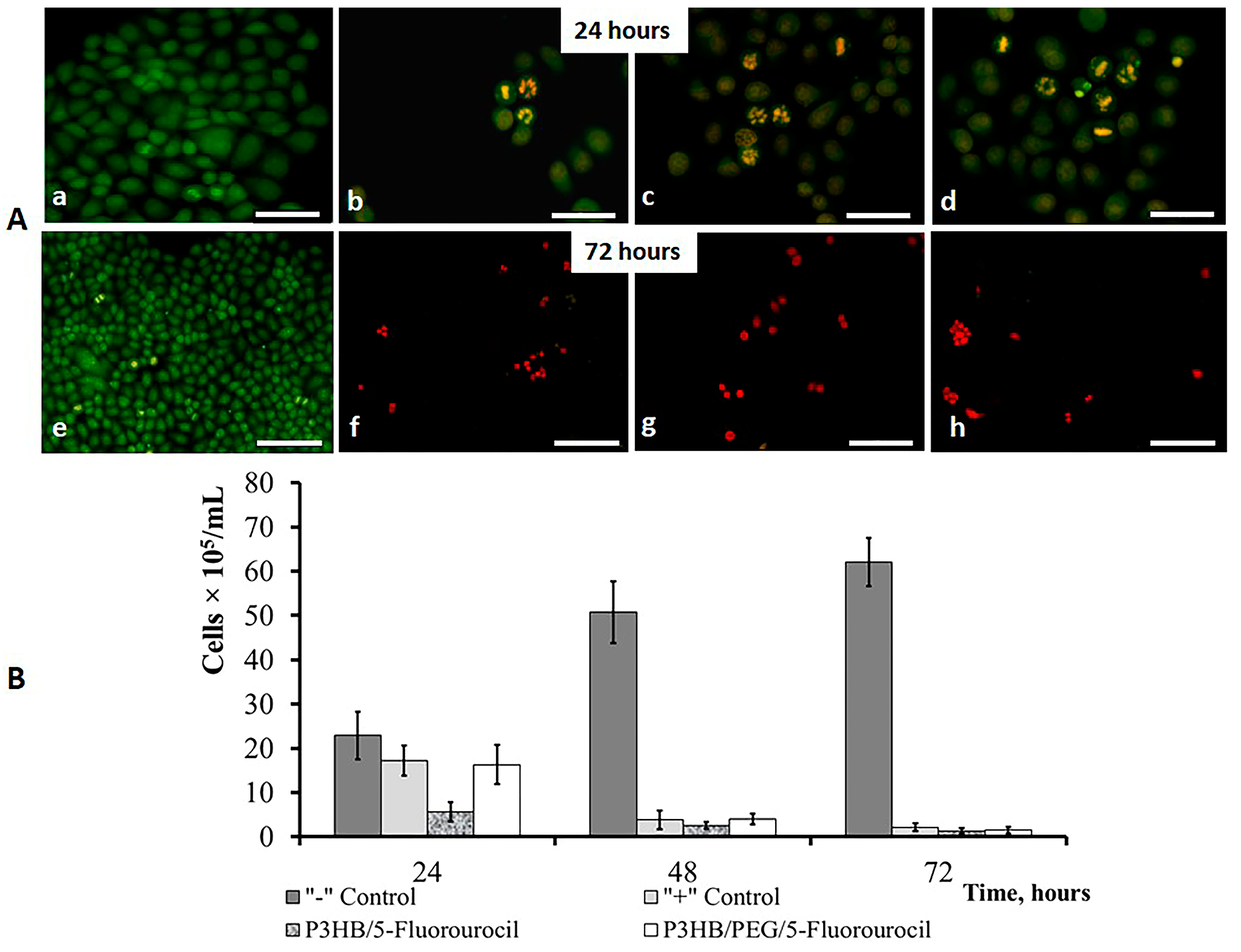
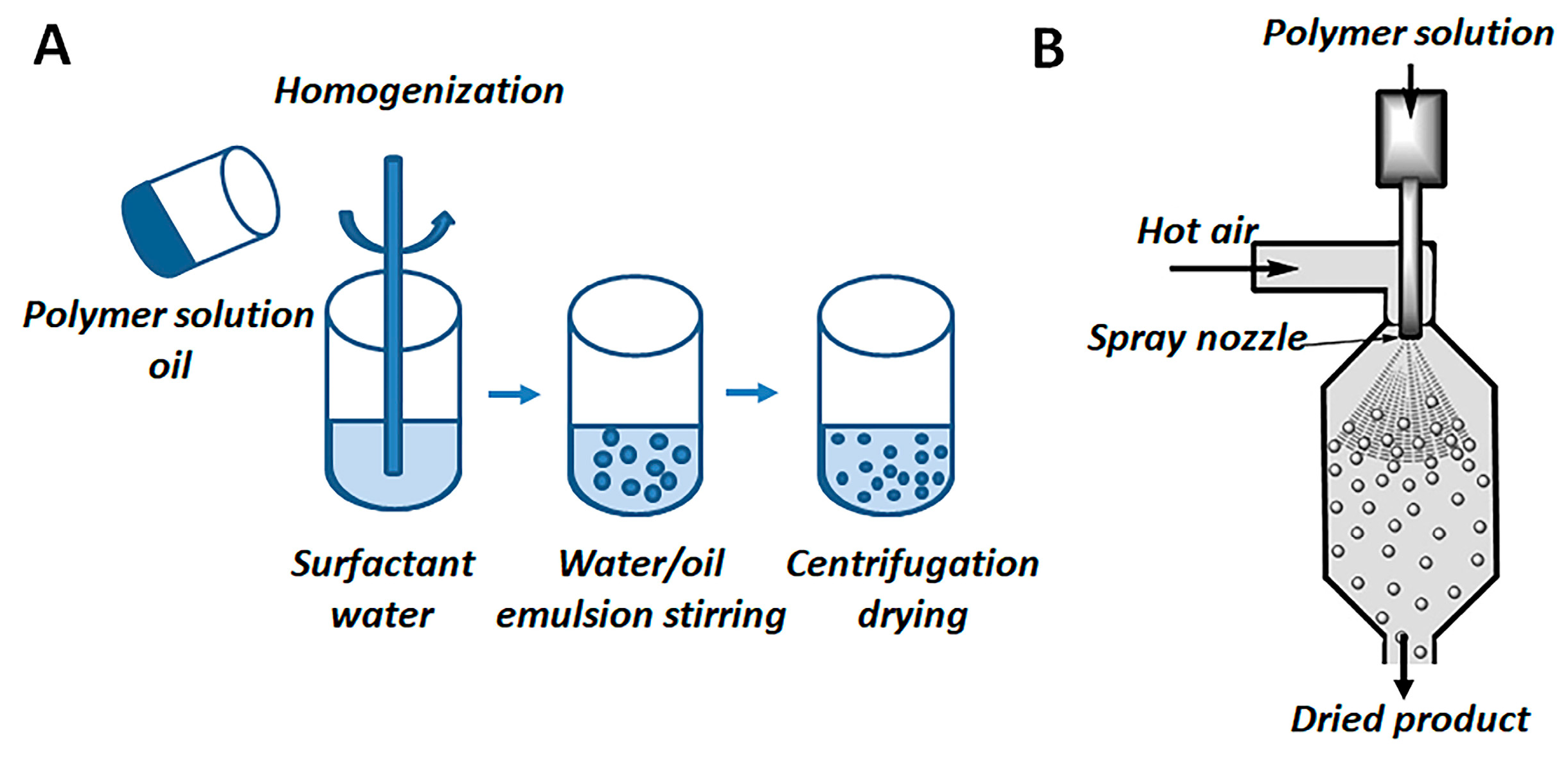
| Concentration of P(3HB), % | Dispersion Technique | Average Diameter, μm | ζ Potential, mV | Yield of Microparticles, % |
| Emulsion method | ||||
| 1.0 | Hh * | 1.2 ± 0.09 | −16.2 ± 0.49 | 73.8 ± 4.6 |
| 2.0 | Hh * | 1.58 ± 0.15 | −15 ± 0.28 | 78.5 ± 3.8 |
| 4.0 | Hh * | 2.32 ± 0.3 | −21.6 ± 0.7 | 78.7 ± 4.3 |
| 2.0 | Us 12 * | 2.5 ± 0.14 | −23 ± 0.31 | 56 ± 4.2 |
| 2.0 | Us 20 * | 1.2 ± 0.08 | −19 ± 0.25 | 61.5 ± 3.5 |
| Spray drying | ||||
| Temperature, °C | Solution Feed Rate, mL/min | Average Diameter, μm | ζ Potential, mV | Yield Microparticles, % |
| 75 | 1.5 | 3.4 ± 0.6 | −45 ± 0.5 | 83.5 |
| 75 | 3.2 | 3.8 ± 0.5 | −44.5 ± 1.1 | 71 |
| 75 | 5.0 | 5.5± 0.5 | −43.2± 1.2 | 85 |
| 85 | 1.5 | 5.7 ± 0.5 | −47.1 ± 0.4 | 78 |
| 85 | 3.2 | 5.6 ± 0.6 | −42.3 ± 0.6 | 90 |
| 85 | 5.0 | 5.2 ± 0.3 | −49 ± 3.4 | 45 |
| 95 | 1.5 | 4.2 ± 0.3 | −41.6 ± 0.8 | 75 |
| 95 | 3.2 | 7.0 ± 0.48 | −47.4 ± 0.4 | 71 |
| 95 | 5.0 | 6.2 ± 0.5 | −50.8 ± 0.5 | 75 |
| PHA Composition, mol.% | Structural Formula | Mw, kDa | Mn, kDa | D | Cx, % | Tmelt, °C | Tdegr, °C |
|---|---|---|---|---|---|---|---|
| P(3HB) | |||||||
| 100.0 | 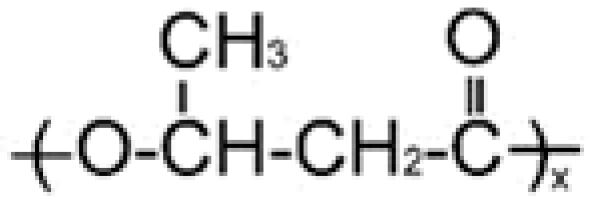 | 1200 | 710 | 1.69 | 76 | 169 | 272 |
| P(3HB/3HV) | |||||||
| 93.2/6.8 | 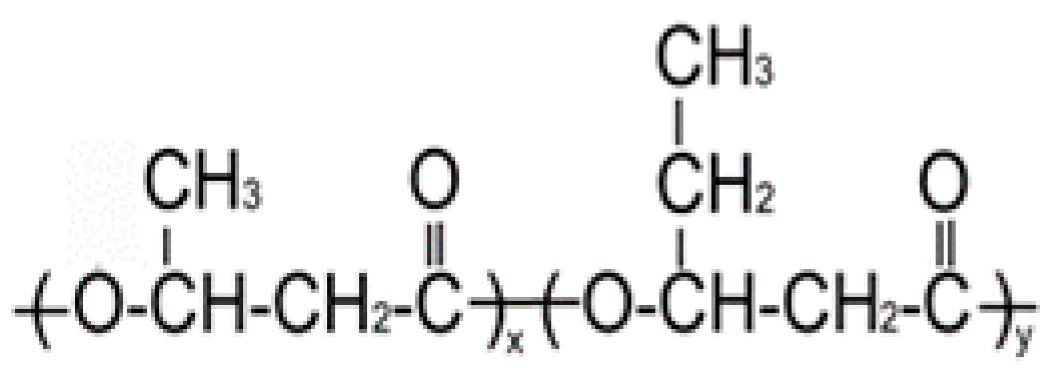 | 890 | 466 | 1.91 | 60 | 162 | 264 |
| 67.2/32.8 | 398 | 115 | 3.46 | 54 | 160 | 259 | |
| P(3HB/4HHx) | |||||||
| 93.0/7.0 |  | 410 | 140 | 2.93 | 56 | 162 | 258 |
| 86.4/13.6 | 390 | 130 | 3.00 | 49 | 160 | 264 | |
| P(3HB/4HB) | |||||||
| 93.9/6.1 | 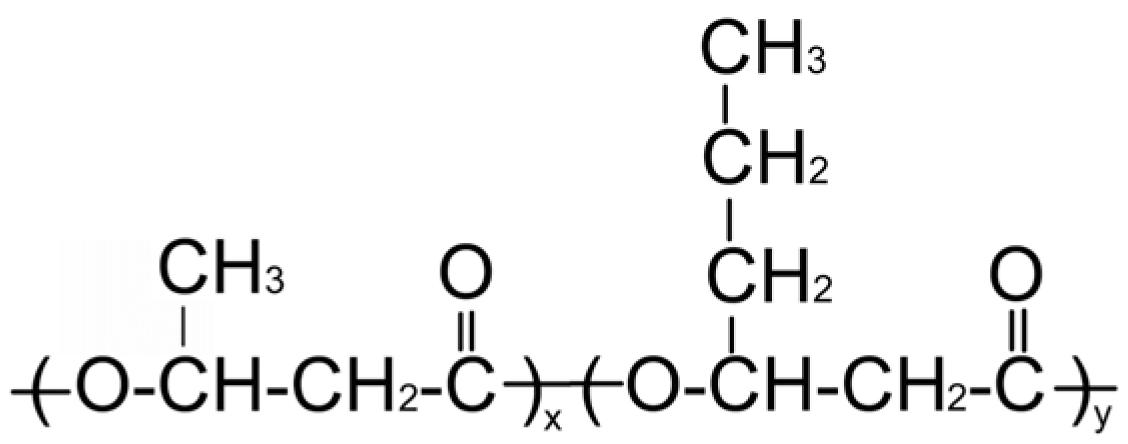 | 416 | 142 | 2.93 | 52 | 160 | 254 |
| 84.0/16.0 | 520 | 168 | 3.10 | 41 | 154 | 260 | |
| Drug | Structural Formula | Mechanism of Action |
|---|---|---|
| Ceftriaxone | 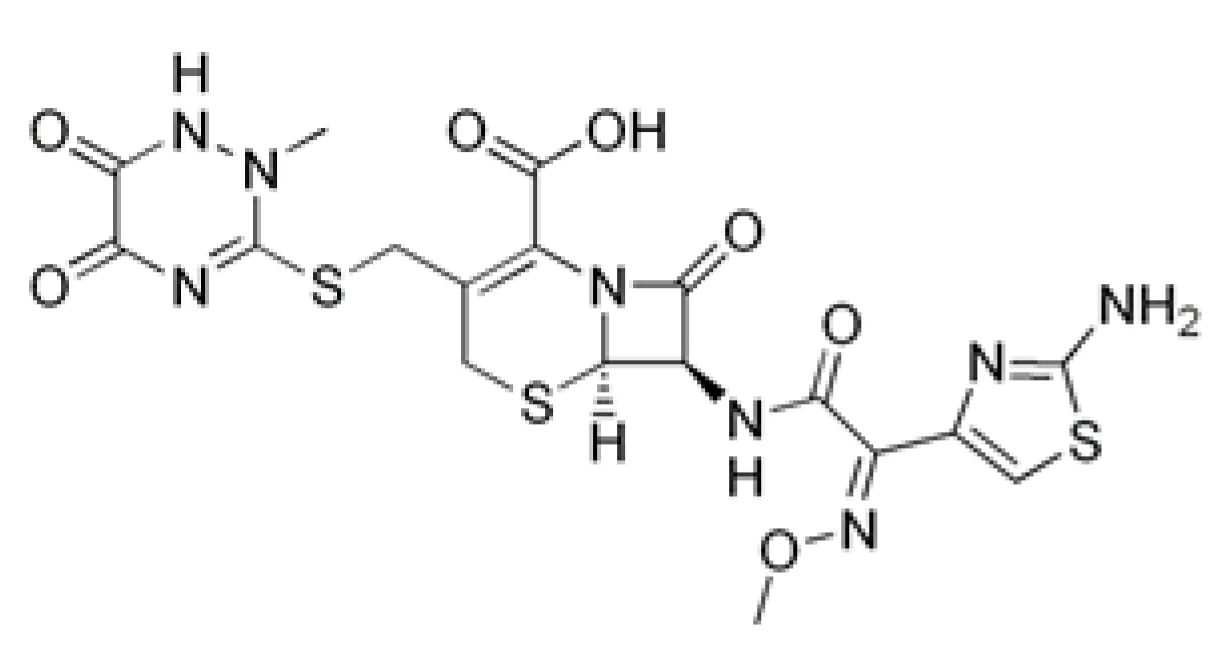 | Inhibition of bacterial cell wall synthesis through acetylation of membrane-bound transpeptidases with further disruption of cell wall peptidoglycan synthesis. |
| Rifampicin |  | Violates RNA synthesis in a bacterial cell: binds to the beta subunit of DNA-dependent RNA polymerase, preventing its attachment to DNA, and inhibits RNA transcription. |
| Doxorubicin | 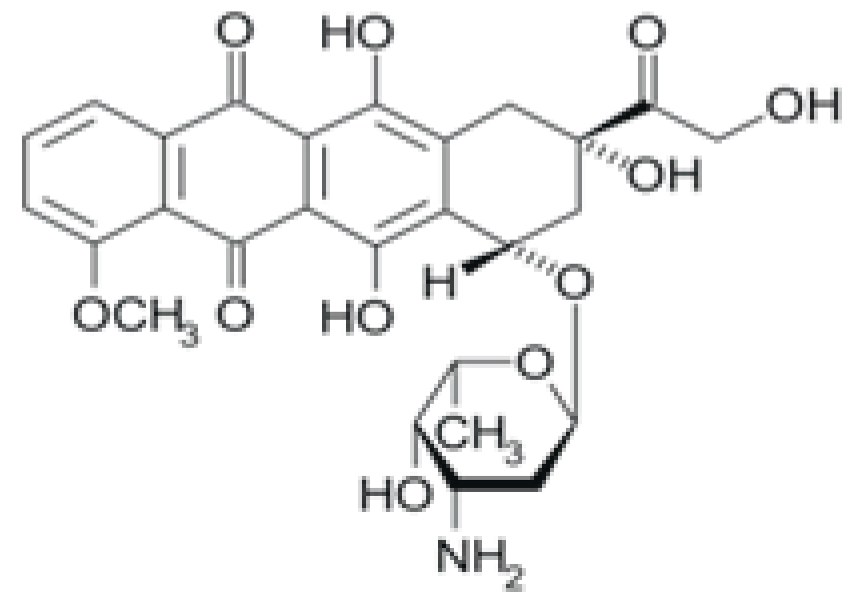 | Suppression of the synthesis of nucleic acids, followed by the formation of free radicals and effect on cell membranes. |
| 5-Fluorourocil | 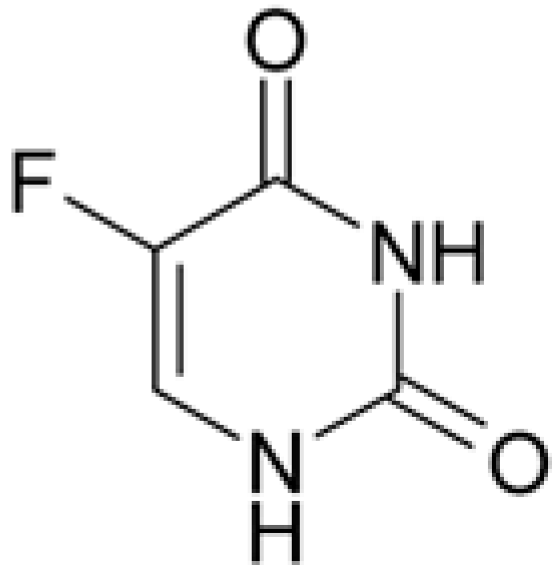 | Inhibits the process of cell division by blocking DNA synthesis (due to inhibition of thymidylate synthetase enzyme activity) and the formation of structurally imperfect RNA (due to the introduction of fluorouracil into its structure). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murueva, A.V.; Shershneva, A.M.; Shishatskaya, E.I.; Volova, T.G. Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs. Int. J. Mol. Sci. 2023, 24, 14983. https://doi.org/10.3390/ijms241914983
Murueva AV, Shershneva AM, Shishatskaya EI, Volova TG. Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs. International Journal of Molecular Sciences. 2023; 24(19):14983. https://doi.org/10.3390/ijms241914983
Chicago/Turabian StyleMurueva, Anastasiya V., Anna M. Shershneva, Ekaterina I. Shishatskaya, and Tatiana G. Volova. 2023. "Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs" International Journal of Molecular Sciences 24, no. 19: 14983. https://doi.org/10.3390/ijms241914983
APA StyleMurueva, A. V., Shershneva, A. M., Shishatskaya, E. I., & Volova, T. G. (2023). Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs. International Journal of Molecular Sciences, 24(19), 14983. https://doi.org/10.3390/ijms241914983







