Insulin Clearance at the Pubertal Transition in Youth with Obesity and Steatosis Liver Disease
Abstract
:1. Introduction
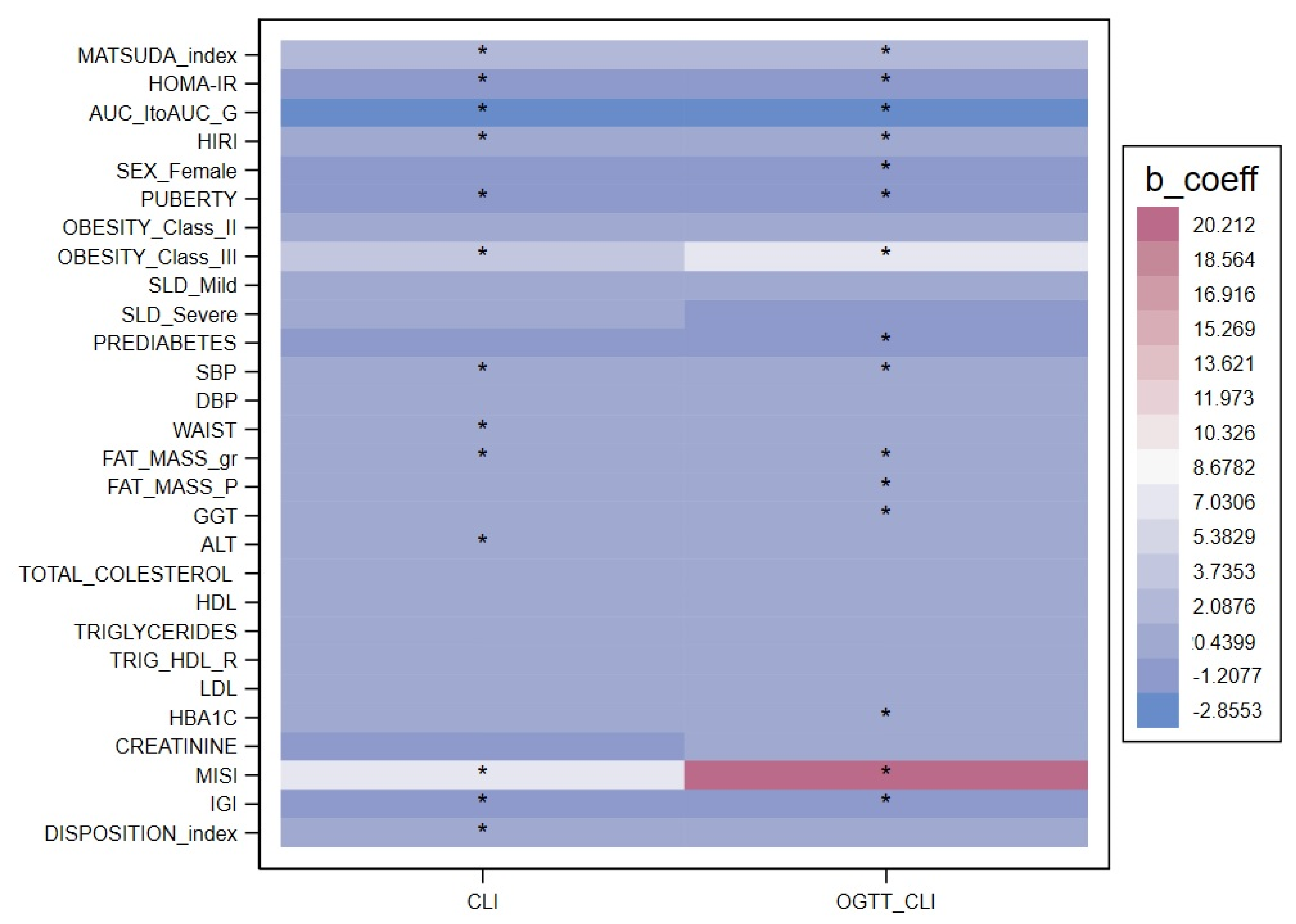
2. Results
3. Discussion
3.1. The Role of Puberty
3.2. The Role of SLD
3.3. Obesity, Prediabetes and Other Metabolic Abnormalities
3.4. Strengths and Limitations
3.5. Conclusions
4. Materials and Methods
4.1. Study Design and Sample Size
4.2. Anthropometric Measurements and Biochemical Assays
4.3. Case Definition and Calculations
4.4. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrannini, E.; Wahren, J.; Faber, O.K.; Felig, P.; Binder, C.; DeFronzo, R.A. Splanchnic and Renal Metabolism of Insulin in Human Subjects: A Dose-Response Study. Am. J. Physiol.-Endocrinol. Metab. 1983, 244, E517–E527. [Google Scholar] [CrossRef]
- Polidori, D.C.; Bergman, R.N.; Chung, S.T.; Sumner, A.E. Hepatic and Extrahepatic Insulin Clearance Are Differentially Regulated: Results From a Novel Model-Based Analysis of Intravenous Glucose Tolerance Data. Diabetes 2016, 65, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Piccinini, F.; Kabir, M.; Kolka, C.M.; Ader, M. Hypothesis: Role of Reduced Hepatic Insulin Clearance in the Pathogenesis of Type 2 Diabetes. Diabetes 2019, 68, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Natali, A.; Arslanian, S.; Mari, A.; Ferrannini, E. Identification, Pathophysiology, and Clinical Implications of Primary Insulin Hypersecretion in Nondiabetic Adults and Adolescents. JCI Insight 2018, 3, e124912. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.A.; Saad, R.; Lewy, V.; Danadian, K.; Janosky, J. Hyperinsulinemia in African-American Children. Diabetes 2002, 51, 3014–3019. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Given, B.D.; Hirsch, L.; Shapiro, E.T.; Tillil, H.; Beebe, C.; Galloway, J.A.; Frank, B.H.; Karrison, T.; Van Cauter, E. Quantitative Study of Insulin Secretion and Clearance in Normal and Obese Subjects. J. Clin. Invest. 1988, 81, 435–441. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.D.; Burgert, T.S.; Taksali, S.E.; Tamborlane, W.V.; Caprio, S. Ethnic Differences in Beta Cell Adaptation to Insulin Resistance in Obese Children and Adolescents. Diabetologia 2006, 49, 571–579. [Google Scholar] [CrossRef]
- Gower, B.A.; Granger, W.M.; Franklin, F.; Shewchuk, R.M.; Goran, M.I. Contribution of Insulin Secretion and Clearance to Glucose-Induced Insulin Concentration in African-American and Caucasian Children. J. Clin. Endocrinol. Metab. 2002, 87, 2218–2224. [Google Scholar] [CrossRef]
- Bar-Tana, J. Insulin Resistance, Secretion and Clearance –Taming the Three Effector Encounter of Type 2 Diabetes. Front. Endocrinol. 2021, 12, 741114. [Google Scholar] [CrossRef]
- Tricò, D.; Galderisi, A.; Mari, A.; Polidori, D.; Galuppo, B.; Pierpont, B.; Samuels, S.; Santoro, N.; Caprio, S. Intrahepatic Fat, Irrespective of Ethnicity, Is Associated with Reduced Endogenous Insulin Clearance and Hepatic Insulin Resistance in Obese Youths: A Cross-sectional and Longitudinal Study from the Y Ale P Ediatric NAFLD Cohort. Diabetes Obes. Metab. 2020, 22, 1628–1638. [Google Scholar] [CrossRef]
- Galderisi, A.; Polidori, D.; Weiss, R.; Giannini, C.; Pierpont, B.; Tricò, D.; Caprio, S. Lower Insulin Clearance Parallels a Reduced Insulin Sensitivity in Obese Youths and Is Associated With a Decline in β-Cell Function Over Time. Diabetes 2019, 68, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dufour, S.; Taksali, S.E.; Tamborlane, W.V.; Petersen, K.F.; Bonadonna, R.C.; Boselli, L.; Barbetta, G.; Allen, K.; Rife, F.; et al. Prediabetes in Obese Youth: A Syndrome of Impaired Glucose Tolerance, Severe Insulin Resistance, and Altered Myocellular and Abdominal Fat Partitioning. Lancet 2003, 362, 951–957. [Google Scholar] [CrossRef]
- Weiss, R.; Santoro, N.; Giannini, C.; Galderisi, A.; Umano, G.R.; Caprio, S. Prediabetes in Youths: Mechanisms and Biomarkers. Lancet Child Adolesc. Health 2017, 1, 240–248. [Google Scholar] [CrossRef] [PubMed]
- The RISE Consortium; Ehrmann, D.A.; Temple, K.A.; Rue, A.; Barengolts, E.; Mokhlesi, B.; Van Cauter, E.; Sam, S.; Miller, M.A.; Kahn, S.E.; et al. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care 2018, 41, 1696–1706. [Google Scholar] [CrossRef]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal Study of Physiologic Insulin Resistance and Metabolic Changes of Puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Bergman, R.N. The Measurement of Insulin Clearance. Diabetes Care. 2020, 43, 2296–2302. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Abdul Ghani, M.; DeFronzo, R.A. Adaptation of Insulin Clearance to Metabolic Demand Is a Key Determinant of Glucose Tolerance. Diabetes 2021, 70, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.E.; Cao, C.; Mittendorfer, B. Insulin Clearance in Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 596. [Google Scholar] [CrossRef]
- Najjar, S.M.; Caprio, S.; Gastaldelli, A. Insulin Clearance in Health and Disease. Annu. Rev. Physiol. 2023, 85, 363–381. [Google Scholar] [CrossRef]
- RISE Consortium. Metabolic Contrasts between Youth Adults with Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care 2018, 41, 1707–1716. [Google Scholar] [CrossRef]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Caprio, S.; Perry, R.; Kursawe, R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology 2017, 152, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Cali, A.M.G.; Weiss, R.; Santoro, N.; Pierpont, B.; Northrup, V.; Caprio, S. Central Role of Fatty Liver in the Pathogenesis of Insulin Resistance in Obese Adolescents. Diabetes Care 2010, 33, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Koutny, F.; Weghuber, D.; Bollow, E.; Greber-Platzer, S.; Hartmann, K.; Körner, A.; Reinehr, T.; Roebl, M.; Simic-Schleicher, G.; Wabitsch, M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children with Obesity and Increased Transaminases in European German-speaking Countries. Analysis of the APV Initiative. Pediatr. Obes. 2020, 15, e12601. [Google Scholar] [CrossRef] [PubMed]
- Rudovich, N.; Pivovarova, O.; Fisher, E.; Fischer-Rosinsky, A.; Spranger, J.; Möhlig, M.; Schulze, M.B.; Boeing, H.; Pfeiffer, A.F.H. Polymorphisms within Insulin-Degrading Enzyme (IDE) Gene Determine Insulin Metabolism and Risk of Type 2 Diabetes. J. Mol. Med. 2009, 87, 1145–1151. [Google Scholar] [CrossRef]
- Bril, F.; Lomonaco, R.; Orsak, B.; Ortiz-Lopez, C.; Webb, A.; Tio, F.; Hecht, J.; Cusi, K. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 2178–2187. [Google Scholar] [CrossRef]
- Kotronen, A.; Juurinen, L.; Tiikkainen, M.; Vehkavaara, S.; Yki-Järvinen, H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef]
- Lee, W.H.; Najjar, S.M.; Kahn, C.R.; Hinds, T.D., Jr. Hepatic insulin receptor: New views on the mechanisms of liver disease. Metabolism 2023, 145, 155607. [Google Scholar] [CrossRef]
- Pivovarova, O.; Bernigau, W.; Bobbert, T.; Isken, F.; Möhlig, M.; Spranger, J.; Weickert, M.O.; Osterhoff, M.; Pfeiffer, A.F.H.; Rudovich, N. Hepatic Insulin Clearance Is Closely Related to Metabolic Syndrome Components. Diabetes Care 2013, 36, 3779–3785. [Google Scholar] [CrossRef]
- Bergman, R.N.; Kabir, M.; Ader, M. The Physiology of Insulin Clearance. Int. J. Mol. Sci. 2022, 23, 1826. [Google Scholar] [CrossRef]
- Tricò, D.; Galderisi, A.; Van Name, M.A.; Caprio, S.; Samuels, S.; Li, Z.; Galuppo, B.T.; Savoye, M.; Mari, A.; Feldstein, A.E.; et al. A low n-6 to n-3 polyunsaturated fatty acid ratio diet improves hyperinsulinaemia by restoring insulin clearance in obese youth. Diabetes Obes. Metab. 2022, 24, 1267–1276. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Dunn, W.; Norman, G.J.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C.B. SAFETY Study: Alanine Aminotransferase Cutoff Values Are Set Too High for Reliable Detection of Pediatric Chronic Liver Disease. Gastroenterology 2010, 138, 1357–1364.e2. [Google Scholar] [CrossRef] [PubMed]
- Pedicelli, S.; Fintini, D.; Ravà, L.; Inzaghi, E.; Deodati, A.; Spreghini, M.R.; Bizzarri, C.; Mariani, M.; Cianfarani, S.; Cappa, M.; et al. Prevalence of Prediabetes in Children and Adolescents by Class of Obesity. Pediatr. Obes. 2022, 17, e12900. [Google Scholar] [CrossRef]
- Ravà, L.; Fintini, D.; Mariani, M.; Deodati, A.; Inzaghi, E.; Pedicelli, S.; Bizzarri, C.; Cappa, M.; Cianfarani, S.; Manco, M. High 1-h glucose in youths with obesity as marker of prediabetes and cardiovascular risk. J. Endocrinol. Invest. 2022, 19. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Whitehouse, R.H. Clinical Longitudinal Standards for Height, Weight, Height Velocity, Weight Velocity, and Stages of Puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin Sensitivity Indices Obtained from Oral Glucose Tolerance Testing: Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and Liver Insulin Resistance Indexes Derived From the Oral Glucose Tolerance Test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.W.; Clark, P.M.; Hales, C.N.; Osmond, C. Understanding Oral Glucose Tolerance: Comparison of Glucose or Insulin Measurements During the Oral Glucose Tolerance Test with Specific Measurements of Insulin Resistance and Insulin Secretion. Diabet. Med. 1994, 11, 286–292. [Google Scholar] [CrossRef]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Juan-Lin; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]

| Characteristics | Median (IQR) or N (%) | |
|---|---|---|
| Sex (male/female) | 490 (50.4%)/483 (49.6%) | |
| Age (years) | 12.0 (10.0–13.8) | |
| BMI (Kg/m2) | 29.4 (26.8–32.8) | |
| BMI z-score (SDS) | 2.3 (2.0–2.6) | |
| Waist circumference (cm) | 97.0 (89.5–105.5) | |
| Systolic blood pressure (mmHg) | 117 (110–125) | |
| Diastolic blood pressure (mmHg) | 66 (60–73) | |
| Fat mass (Kg) | 27.9 (22.5–33.7) | |
| Fat mass (%) | 40.8 (37.8–43.8) | |
| Prepubertal_Tanner | Stage I | 343 (35.2%) |
| Puberty | 630 (64.8%) | |
| Early puberty_Tanner | Stage II | 40 (6.3%) |
| Stage III | 170 (27.0%) | |
| Stage IV | 208 (33.1%) | |
| Stage V | 212 (33.6%) | |
| ALT (IU/L) | 28 ± 14 | |
| GGT (IU/L) | 16 ± 11 | |
| Total cholesterol (mg/dL) | 156 (139–175) | |
| HDL (mg/dL) | 45 (39–52) | |
| LDL (mg/dL) | 92 (76–107) | |
| Triglycerides (mg/dL) | 80 (59–112) | |
| Triglyceride-to-HDL-cholesterol ratio | 1.8 (1.2–2.7) | |
| HbA1c (mmol/L) | 34 (32–36) | |
| Fasting glucose (mmol/L) | 4.6 (4.3–4.9) | |
| Fasting insulin (pmol/L) | 17 0 (11.5–23.7) | |
| Fasting C-peptide (ng/mL) | 1.9 (1.4–2.5) | |
| 2 h glucose (mmol/L) | 6.1 (5.4–6.9) | |
| HOMA IR | 3.5 (2.5–5.0) | |
| HIRI | 716 (397–1118) | |
| Insulinogenic index (pmol/µmol) | 2.1 (1.3–3.4) | |
| Matsuda index (µmol/kg/pM) | 2.6 (1.8–3.9) | |
| MISI (µmol/kg/pM) | 0.08 (0.04–0.16) | |
| ClI (nmol/pmol) | 0.11 (0.09–0.14) | |
| OGTT ClI (nmol/pmol) | 0.07 (0.06–0.10) | |
| AUC insulin to AUC glucose (pmol/mmol) | 0.85 (0.57–1.26) | |
| Disposition index | 5.4 (3.4–8.2) | |
| N | HOMA-IR | Matsuda Index (µmol/kg/ pmol) | IGI (pmol/µmol) | DI | AUCI/AUCG | Fasting ClI (nmol/pmol × 10−2) | OGTT ClI (nmol/pmol × 10−2) | |
|---|---|---|---|---|---|---|---|---|
| Males | 490 | 3.31 (2.14–4.62) | 2.81 (1.90–4.41) | 1.90 (1.16–3.18) | 5.38 (3.45–8.23) | 0.78 (0.51–1.19) | 0.11 (0.09–0.15) | 0.08 (0.06–0.10) |
| Females | 483 | 3.64 (2.52–5.49) | 2.40 (1.69–3.55) | 2.29 (1.37–3.72) | 5.29 (3.27–8.14) | 0.91 (0.66–1.35) | 0.11 (0.09–0.14) | 0.07 (0.06–0.09) |
| p-value | 0.027 | 0.011 | 0.005 | 0.902 | 0.006 | 0.232 | 0.024 | |
| Prepuberty | 335 | 2.51 (1.52–3.72) | 3.29 (2.16–5.14) | 1.62 (0.93–2.91) | 5.42 (3.62–8.56) | 0.71 (0.47–1.05) | 0.12 (0.09–0.16) | 0.08 (0.06–0.11) |
| Puberty | 615 | 3.90 (2.84–5.73) | 2.33 (1.59–3.39) | 2.32 (1.50–3.73) | 5.30 (3.32–8.08) | 0.92 (0.63–1.37) | 0.11 (0.08–0.14) | 0.07 (0.05–0.09) |
| p-value | <0.001 | <0.001 | <0.001 | 0.655 | <0.001 | 0.001 | 0.004 | |
| Obesity class I | 262 | 3.19 (2.09–4.39) | 2.92 (1.98–4.26) | 2.00 (1.15–3.15) | 5.24 (3.49–7.89) | 0.79 (0.56–1.16) | 0.11 (0.09–0.14) | 0.07 (0.05–0.10) |
| Obesity class II | 694 | 3.57 (2.36–5.11) | 2.54 (1.69–3.73) | 2.15 1.32–3.49) | 5.38 (3.32–8.21) | 0.86 (0.58–1.29) | 0.11 (0.09–0.14) | 0.07 (0.06–0.09) |
| Obesity class III | 17 | 2.42 (1.69–3.24) | 4.72 (2.18–7.60) | 1.72 (0.40–2.09) | 5.28 (4.17–12.45) | 0.41 (0.33–0.70) | 0.15 (0.11–0.16) | 0.14 (0.08–0.17) |
| p-value | class II vs. I 0.022 class III vs. I 0.270 | class II vs. I 0.052 class III vs. I 0.006 | class II vs. I 0.378 class III vs. I 0.632 | class II vs. I 0.717 class III vs. I 0.975 | class II vs. I 0.197 class III vs. I 0.034 | class II vs. I 0.994 class III vs. I 0.001 | class II vs. I 0.938 class III vs. I <0.001 | |
| No SLD | 418 | 3.99 (2.94–5.74) | 2.14 (1.52–3.16) | 2.94 (1.61–4.19) | 5.91 (3.59–8.52) | 1.09 (0.78–1.56) | 0.09 (0.08–0.12) | 0.06 (0.04–0.07) |
| Mild SLD | 511 | 3.74 (2.54–5.44) | 2.45 (1.65–3.69) | 2.64 (1.59–3.92) | 5.98 (3.76–9.01) | 1.01 (0.65–1.45) | 0.10 (0.08–0.13) | 0.05 (0.04–0.07) |
| Severe SLD | 44 | 4.22 (2.35–6.39) | 3.40 (1.78–6.49) | 2.21 (0.03–3.45) | 5.66 (0.16–15.22) | 0.69 (0.33–1.50) | 0.10 (0.08–0.17) | 0.09 (0.04–0.14) |
| p-value | Mild vs. No 0.296 Severe vs. No 0.223 | Mild vs. No 0.151 Severe vs. SLD 0.023 | Mild vs. No 0.186 Severe vs. No 0.318 | Mild vs. No 0.955 Severe vs. No 0.876 | Mild vs. No 0.339 Severe vs. No 0.220 | Mild vs. No 0.195 Severe vs. No 0.664 | Mild vs. No 0.471 Severe vs. No <0.001 | |
| Normoglycemia | 906 | 3.35 (2.24–4.71) | 2.69 (1.79–4.00) | 2.09 (1.25–3.36) | 5.40 (3.45–8.27) | 0.84 (0.55–1.26) | 0.11 (0.09–0.15) | 0.07 (0.06–0.10) |
| Prediabetes | 67 | 5.39 (3.62–8.16) | 1.68 (1.16–2.29) | 2.45 (1.40–4.68) | 4.03 (3.14–6.06) | 1.09 (0.79–1.50) | 0.10 (0.08–0.13) | 0.06 (0.04–0.07) |
| p-value | <0.001 | 0.005 | 0.250 | 0.075 | 0.016 | 0.081 | 0.022 |
| Fasting ClI (nmol/pmol × 10−2) | p-Value | OGTT ClI (nmol/pmol × 10−2) | p-Value | |
|---|---|---|---|---|
| Sex (M/F) | −0.38 (−1.02; 0.25) | 0.232 | −0.69 (−1.3; −0.09) | 0.024 |
| Age (years) | −0.18 (−0.29; −0.08) | 0.001 | −0.14 (−0.24; −0.04) | 0.006 |
| Puberty | −1.14 (−1.80; −0.48) | 0.001 | −0.86 (−1.44; −0.28) | 0.004 |
| Systolic blood pressure (mmHg) | −0.06 (−0.09; −0.03) | <0.001 | −0.06 (−0.08; −0.03) | <0.001 |
| Diastolic blood pressure (mmHg) | −0.01 (−0.04; 0.03) | 0.754 | −0.02 (−0.06; 0.01) | 0.161 |
| Waist circumference (cm) | −0.04 (−0.07; −0.01) | 0.006 | −0.02 (−0.06; 0.01) | 0.215 |
| Fat mass (g) | 0.00 (0.00–0.00) | 0.005 | 0.00 (0.00–0.00) | <0.001 |
| Fat mass (%) | −0.05 (−0.14; 0.05) | 0.344 | −0.07 (−0.13; −0.005) | 0.035 |
| Obesity class II Obesity class III | 0.00 (−0.73; 0.72) 4.21 (1.75; 6.67) | 0.994 0.001 | 0.03 (−0.69; 0.75) 6.31 (3.12; 9.49) | 0.938 <0.001 |
| Mild SLD Severe SLD | 0.46 (−0.24; 1.15) 0.37 (−1.29; 2.03) | 0.195 0.664 | −0.34 (−1.28; 0.59) 8.27 (4.47; 12.07) | 0.471 <0.001 |
| GGT (IU/L) | −0.02 (−0.06; 0.01) | 0.212 | −0.04 (−0.07; −0.06) | 0.022 |
| Total cholesterol (mg/dL) | −0.01 (−0.02; 0.01) | 0.321 | −0.00 (0.01; 0.01) | 0.678 |
| HDL (mg/dL) | 0.00 (−0.03; 0.02) | 0.865 | 0.01 (−0.01; 0.03) | 0.418 |
| LDL (mg/dL) | 0.00 (−0.02; 0.01) | 0.531 | −0.00 (−0.01; 0.01) | 0.787 |
| Triglycerides (mg/dL) | −0.00 (−0.01; 0.00) | 0.460 | −0.00 (−0.01; 0.00) | 0.124 |
| TGD/HDL | −0.01 (−0.05; 0.03) | 0.750 | −0.01(−0.04; 0.01) | 0.333 |
| Creatinine (mg/dL) | −0.73 (−4.32; −2.9) | 0.692 | 0.58 (−3.43; 4.58) | 0.776 |
| HbA1c (mmol/L) | −0.06 (−0.12; 0.01) | 0.093 | −0.07 (−0.11; −0.04) | <0.001 |
| HOMA IR | −0.79 (−0.88; −0.72) | <0.001 | −0.42 (−0.49; −0.33) | <0.001 |
| HIRI | 0.00 (0.00; −0.00) | <0.001 | 0.00 (0.00; −0.00)) | <0.001 |
| Matsuda index (µmol/kg/pM) | 1.59 (1.45; 1.73) | <0.001 | 1.28 (1.18; 1.39) | <0.001 |
| IGI (pmol/µmol) | −0.46 (−0.57; −0.36) | <0.001 | −0.54 (−0.61; −0.47) | <0.001 |
| MISI (µmol/kg/pM) | 7.16 (5.17; 9.16) | <0.001 | 21.0 (17.70; 24.37) | <0.001 |
| AUC insulin/glucose | −3.30 (−4.0; −2.6) | <0.001 | −3.67 (−4.07; −3.28) | <0.001 |
| Disposition index | 0.13 (0.08; 0.19) | <0.001 | −0.02 (−0.06; −0.02) | 0.297 |
| Any prediabetes | 1.10 (−2.34; 0.14) | 0.081 | −1.69 (−3.14; −0.25) | 0.022 |
| Fasting ClI | p-Value | |
|---|---|---|
| Insulinogenic index (pmol/µmol) | −0.43 (−0.69; −0.17) | <0.001 |
| HDL (mg/dL) | −0.04 (−0.07; −0.02) | <0.001 |
| Disposition index | 0.19 (0.08; 0.30) | <0.001 |
| MISI (µmol/kg/pM) | −8.61 (−12.89; −4.32) | <0.001 |
| Matsuda index (µmol/kg/pM) | 1.55 (1.35; 1.74) | <0.001 |
| OGTT-derived ClI | ||
| HbA1c (mmol/L) | −0.039 (−0.058; −0.020) | <0.001 |
| Matsuda index (µmol/kg/pM) | 0.490 (0.266; 0.714) | <0.001 |
| AUC insulin/glucose | −1.172 (−1.645; −0.698) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschi, R.; Fintini, D.; Ravà, L.; Mariani, M.; Aureli, A.; Inzaghi, E.; Pedicelli, S.; Deodati, A.; Bizzarri, C.; Cappa, M.; et al. Insulin Clearance at the Pubertal Transition in Youth with Obesity and Steatosis Liver Disease. Int. J. Mol. Sci. 2023, 24, 14963. https://doi.org/10.3390/ijms241914963
Franceschi R, Fintini D, Ravà L, Mariani M, Aureli A, Inzaghi E, Pedicelli S, Deodati A, Bizzarri C, Cappa M, et al. Insulin Clearance at the Pubertal Transition in Youth with Obesity and Steatosis Liver Disease. International Journal of Molecular Sciences. 2023; 24(19):14963. https://doi.org/10.3390/ijms241914963
Chicago/Turabian StyleFranceschi, Roberto, Danilo Fintini, Lucilla Ravà, Michela Mariani, Alessia Aureli, Elena Inzaghi, Stefania Pedicelli, Annalisa Deodati, Carla Bizzarri, Marco Cappa, and et al. 2023. "Insulin Clearance at the Pubertal Transition in Youth with Obesity and Steatosis Liver Disease" International Journal of Molecular Sciences 24, no. 19: 14963. https://doi.org/10.3390/ijms241914963
APA StyleFranceschi, R., Fintini, D., Ravà, L., Mariani, M., Aureli, A., Inzaghi, E., Pedicelli, S., Deodati, A., Bizzarri, C., Cappa, M., Cianfarani, S., & Manco, M. (2023). Insulin Clearance at the Pubertal Transition in Youth with Obesity and Steatosis Liver Disease. International Journal of Molecular Sciences, 24(19), 14963. https://doi.org/10.3390/ijms241914963








