The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
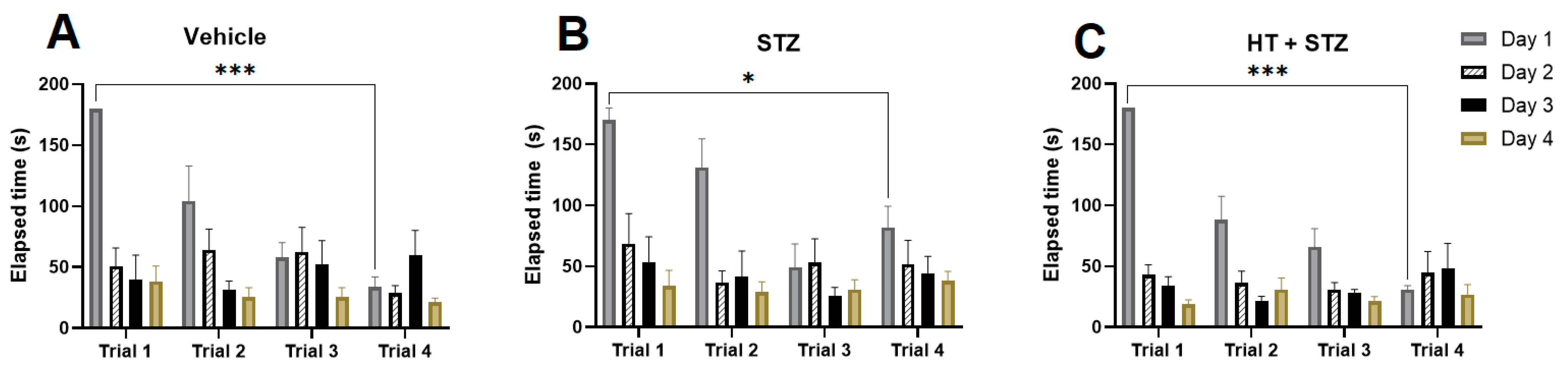
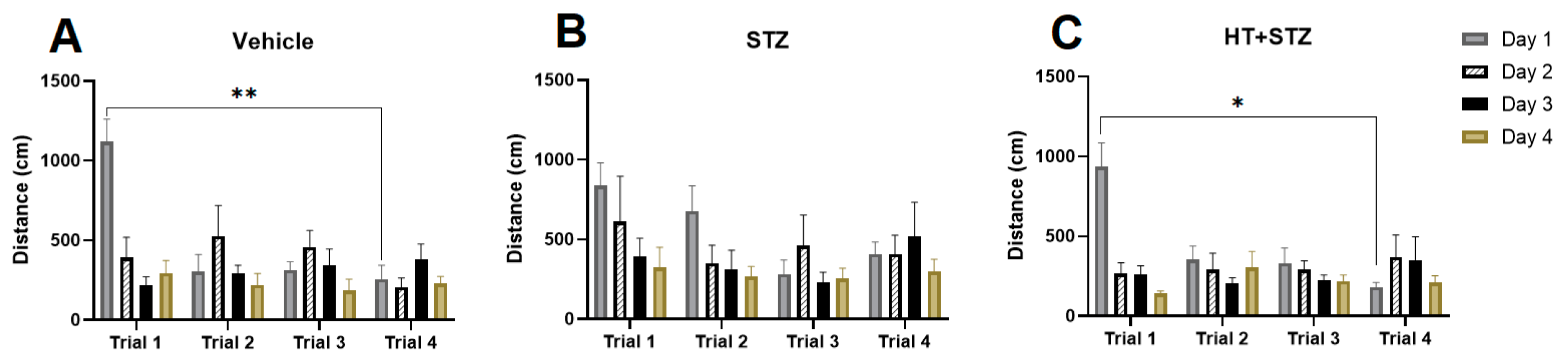
2.1. Barnes Maze Test
2.2. Metal Content in Tissue Samples
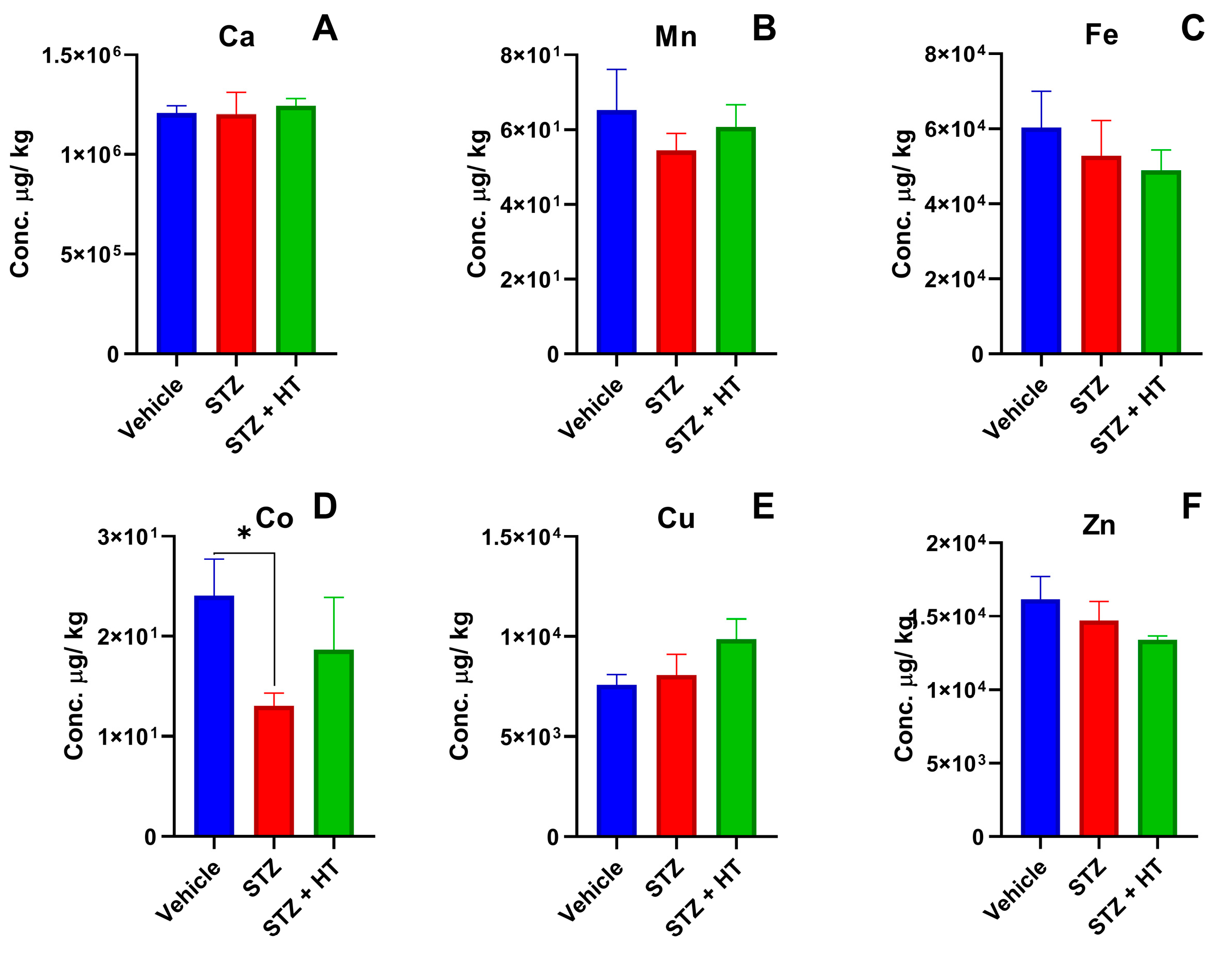
2.2.1. Metallomics Profile of Plasma
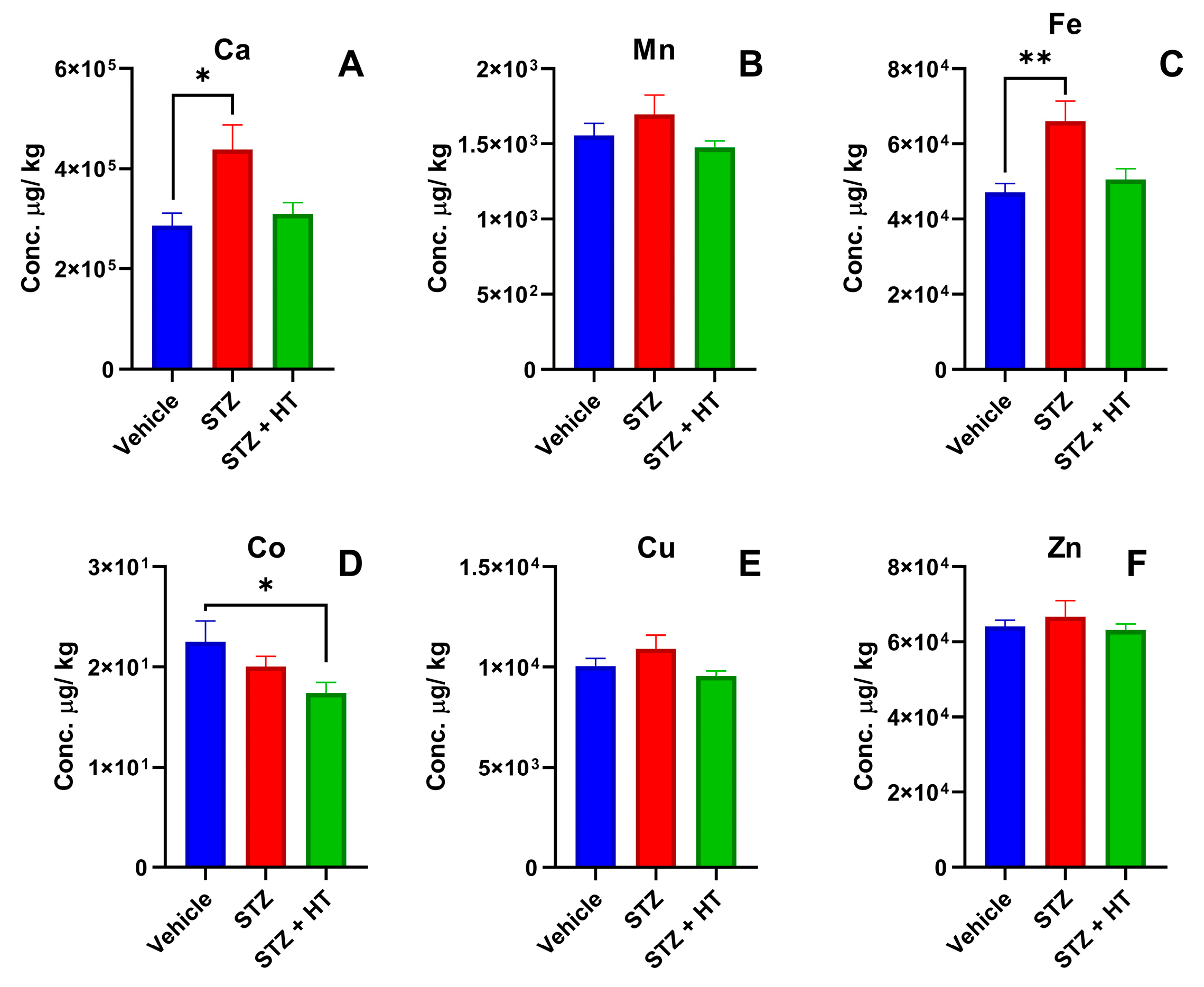
2.2.2. Metallomics Profile of the Frontal Cortex
2.2.3. Metallomics Profile of the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Surgery and Injection Procedure
4.3. Barnes Maze Behavior Test
4.4. Blood Plasma Sampling and Brain Dissection
4.5. Metallomic Profile with ICP-MS
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2019, 4, 810–834. [Google Scholar] [CrossRef]
- Pantiya, P.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Mitochondrial abnormalities in neurodegenerative models and possible interventions: Focus on Alzheimer’s disease, Parkinson’s disease, Huntington’s disease. Mitochondrion 2020, 55, 14–47. [Google Scholar] [CrossRef] [PubMed]
- International AD. Numbers of People with Dementia around the World. Dementia Statistics. 2020, pp. 20–21. Available online: https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide/ (accessed on 3 January 2021).
- Anwal, L. A Comprehensive Review on Alzheimer’s Disease. World J. Pharm. Pharm. Sci. 2021, 10, 1170. [Google Scholar]
- Folwell, J.; Cowan, C.M.; Ubhi, K.K.; Shiabh, H.; Newman, T.A.; Shepherd, D.; Mudher, A. Aβ exacerbates the neuronal dysfunction caused by human tau expression in a Drosophila model of Alzheimer’s disease. Exp. Neurol. 2010, 223, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Shi, R.; Li, L.; Chen, F.; Zhou, Y.; Tung, Y.C.; Liu, F. Pathological Tau from Alzheimer’s Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo. Front. Aging Neurosci. 2019, 11, 34. [Google Scholar] [CrossRef]
- D’Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef]
- Von Arnim, C.A.F.; Gola, U.; Biesalski, H.K. More than the sum of its parts? Nutrition in Alzheimer’s disease. Nutrition 2010, 26, 694–700. [Google Scholar] [CrossRef]
- Costanzo, P.; Oliverio, M.; Maiuolo, J.; Bonacci, S.; De Luca, G.; Masullo, M. Novel Hydroxytyrosol-Donepezil Hybrids as Potential Antioxidant and Neuroprotective Agents. Front. Chem. 2021, 9, 741444. [Google Scholar] [CrossRef]
- Karkovi, A.; Barbari, M. Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Deiana, M.; Jenner, A.; Halliwell, B.; Kaur, H.; Banni, S. Effect of Hydroxytyrosol Found in Extra Virgin Olive Oil on Oxidative DNA Damage and on Low-Density Lipoprotein Oxidation. J. Agric. Food Chem. 1998, 46, 5181–5187. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Santos, V.; Mangericão, H.; Gordon, M.H. Effects of copper on the antioxidant activity of olive polyphenols in bulk oil and oil-in-water emulsions. J. Agric. Food Chem. 2006, 54, 3738–3743. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.D.; López-Villodres, J.A.; Arrebola, M.M.; Martín-Aurioles, E.; Fernández-Prior, Á.; Bermúdez-Oria, A. Nephroprotective effect of the virgin olive oil polyphenol hydroxytyrosol in type 1-like experimental diabetes mellitus: Relationships with its antioxidant effect. Antioxidants 2021, 10, 1783. [Google Scholar] [CrossRef]
- Costantini, F.; Di Sano, C.; Barbieri, G. The hydroxytyrosol induces the death for apoptosis of human melanoma cells. Int. J. Mol. Sci. 2020, 21, 8074. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C. The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef]
- Visioli, F.; Rodríguez-Pérez, M.; Gómez-Torres, Ó.; Pintado-Losa, C.; Burgos-Ramos, E. Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer’s disease. Nutr. Neurosci. 2022, 25, 990–1000. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Motzko-Soares, A.C.P.; Vizin, R.C.L.; Martins, T.M.S.; Hungaro, A.R.O.; Sato, J.R.; Almeida, M.C.; Carrettiero, D.C. Thermoregulatory profile of neurodegeneration-induced dementia of the Alzheimer’s type using intracerebroventricular streptozotocin in rats. Acta Physiol. 2018, 224, e13084. [Google Scholar] [CrossRef] [PubMed]
- Zappa Villar, M.F.; López Hanotte, J.; Falomir Lockhart, E.; Trípodi, L.S.; Morel, G.R.; Reggiani, P.C. Intracerebroventricular streptozotocin induces impaired Barnes maze spatial memory and reduces astrocyte branching in the CA1 and CA3 hippocampal regions. J. Neural Transm. 2018, 125, 1787–1803. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lin, D.; Cheng, Y.; Jiang, S.; Riaz, M.W.; Fu, N. Amyloid Cascade Hypothesis for the Treatment of Alzheimer’s Disease: Progress and Challenges. Aging Dis. 2022, 13, 1745. [Google Scholar] [CrossRef]
- Mohamed, T.; Shakeri, A.; Rao, P.P.N. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur. J. Med. Chem. 2016, 113, 258–272. [Google Scholar] [CrossRef]
- Sunyer, B.; Patil, S.; Höger, H.; Luber, G. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc. Exch. 2007, 198, 58–68. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Juottonen, K.; Laakso, M.P.; Partanen, K.; Soininen, H. Comparative MR analysis of the entorhinal cortex and hippocampus in diagnosing Alzheimer disease. Am. J. Neuroradiol. 1999, 20, 139–144. [Google Scholar]
- Sun, L.; Wang, X.; Liu, S.; Wang, Q.; Wang, J.; Bennecib, M.; Sengupta, A.; Grundke-Iqbal, I.; Iqbal, K. Bilateral injection of isoproterenol into hippocampus induces Alzheimer-like hyperphosphorylation of tau and spatial memory deficit in rat. FEBS Lett. 2005, 579, 251–258. [Google Scholar] [CrossRef]
- Castro, L.; Moreira, E.G.; Vasconcellos, M.B.A.; Lange, C.N.; Pedron, T.; Batista, B.L. Stability assessment of a bovine kidney reference-material candidate. J. Radioanal. Nucl. Chem. 2018, 317, 1133–1139. [Google Scholar] [CrossRef]
- Tripathi, S.; Varun Kumar, Y.V.B.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 83001. [Google Scholar] [CrossRef]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Kim, M.J.; Jung, G. Serum zinc levels and in vivo beta-amyloid deposition in the human brain. Alzheimer’s Res. Ther. 2021, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yang, H.; Tian, X.; Wang, H.; Zhou, T.; Zhang, S. High manganese, a risk for Alzheimer’s disease: High manganese induces amyloid-β related cognitive impairment. J. Alzheimer’s Dis. 2014, 42, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting copper homeostasis in diabetes mellitus. IUBMB Life 2017, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.L.; Failla, M.L. Urinary Excretion of Zinc, Copper and Iron in the Streptozotocin-Diabetic Rat12. J. Nutr. 1984, 114, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hilário-Souza, E.; Cuillel, M.; Mintz, E.; Charbonnier, P.; Vieyra, A.; Cassio, D. Modulation of hepatic copper-ATPase activity by insulin and glucagon involves protein kinase A (PKA) signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 2086–2097. [Google Scholar] [CrossRef]
- Squitti, R.; Catalli, C.; Gigante, L.; Marianetti, M.; Rosari, M.; Mariani, S.; Bucoss, S.; Mastromoro, G.; Ventriglia, M.; Simonelli, I.; et al. Non-Ceruloplasmin Copper Identifies a Subtype of Alzheimer’s Disease (CuAD): Characterization of the Cognitive Profile and Case of a CuAD Patient Carrying an RGS7 Stop-Loss Variant. Int. J. Mol. Sci. 2023, 24, 6377. [Google Scholar] [CrossRef]
- Marianetti, M.; Pinna, S.; Venuti, A.; Liguri, G. Olive polyphenols and bioavailable glutathione: Promising results in patients diagnosed with mild Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12278. [Google Scholar] [CrossRef]
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G. Copper imbalance in alzheimer’s disease: Meta-analysis of serum, plasma, and brain specimens, and replication study evaluating atp7b gene variants. Biomolecules 2021, 11, 960. [Google Scholar] [CrossRef]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef]
- Chaudhari, V.; Bagwe-Parab, S.; Buttar, H.S.; Gupta, S.; Vora, A.; Kaur, G. Challenges and Opportunities of Metal Chelation Therapy in Trace Metals Overload-Induced Alzheimer’s Disease. Neurotox. Res. 2023, 41, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, Y.-H.; Nam, G.-H.; Choe, S.-H.; Lee, S.-R.; Kim, S.-U.; Kim, J.-S.; Sim, B.-W.; Song, B.-S.; Jeong, K.-J.; et al. Quantitative expression analysis of APP pathway and tau phosphorylation-related genes in the ICV STZ-induced non-human primate model of sporadic Alzheimer’s disease. Int. J. Mol. Sci. 2015, 16, 2386–2402. [Google Scholar] [CrossRef] [PubMed]
- Knezovic, A.; Osmanovic-Barilar, J.; Curlin, M.; Hof, P.R.; Simic, G.; Riederer, P. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J. Neural Transm. 2015, 122, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Hu, S.; Zhang, S.; Zhao, D.; Wang, Y.; Li, H. Hydroxytyrosol Acetate Improves the Cognitive Function of APP/PS1 Transgenic Mice in ERβ-dependent Manner. Mol. Nutr. Food Res. 2021, 65, 2000797. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Luccarini, I.; Ed Dami, T.; Grossi, C.; Rigacci, S.; Stefani, M.; Casamenti, F. Oleuropein aglycone counteracts Aβ42 toxicity in the rat brain. Neurosci. Lett. 2014, 558, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio. Protoc. 2018, 8, e2744. [Google Scholar] [CrossRef]
- Zappa Villar, M.F.; López Hanotte, J.; Pardo, J.; Morel, G.R.; Mazzolini, G.; García, M.G. Mesenchymal Stem Cells Therapy Improved the Streptozotocin-Induced Behavioral and Hippocampal Impairment in Rats. Mol. Neurobiol. 2020, 57, 600–615. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpilowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Lundh, T.; Minthon, L.; Londos, E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Nexo, E.; Hoffmann-Lücke, E. Holotranscobalamin, a marker of vitamin B-12 status: Analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011, 94, 359S–365S. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D. Low vitamin B-12 status in confirmed Alzheimer’s disease as revealed by serum holotranscobalamin. J. Neurol. Neurosurg. Psychiatry 2003, 74, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef]
- Hu, B.; Tong, F.; Xu, L.; Shen, Z.; Yan, L.; Xu, G. Role of Calcium Sensing Receptor in Streptozotocin-Induced Diabetic Rats Exposed to Renal Ischemia Reperfusion Injury. Kidney Blood Press. Res. 2018, 43, 276–286. [Google Scholar] [CrossRef]
- Palmerini, C.A.; Carlini, E.; Saccardi, C.; Servili, M.; Montedoro, G.; Arienti, G. Activity of olive oil phenols on lymphomonocyte cytosolic calcium. J. Nutr. Biochem. 2005, 16, 109–113. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of copper in the onset of Alzheimer’s disease compared to other metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Volitakis, I.; Adlard, P.A.; Duce, J.A.; Masters, C.L.; Cherny, R.A. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Mueller, C.; Oyoyo, U.; Smith, M.A.; Kirsch, W.M. Iron, zinc and copper in the Alzheimer’s disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog. Neurobiol. 2011, 94, 296–306. [Google Scholar] [CrossRef]
- Yeste, N.; Valent, D.; Arroyo, L.; Vázquez-Gómez, M.; García-Contreras, C.; Pumarola, M. Polyphenols and iugr pregnancies: Effects of the antioxidant hydroxytyrosol on brain neurochemistry and development in a porcine model. Antioxidants 2021, 10, 884. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K. Hydroxytyrosol alleviates oxidative stress and neuroinflammation and enhances hippocampal neurotrophic signaling to improve stress-induced depressive behaviors in mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Damulina, A.; Pirpamer, L.; Soellradl, M.; Sackl, M.; Tinauer, C.; Hofer, E. Cross-sectional and longitudinal assessment of brain iron level in Alzheimer disease using 3-T MRI. Radiology 2020, 296, 619–626. [Google Scholar] [CrossRef]
- Raven, E.P.; Lu, P.H.; Tishler, T.A.; Heydari, P.; Bartzokis, G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J. Alzheimer’s Dis. 2013, 37, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Kim, H.S.; Song, J. Iron metabolism in diabetes-induced Alzheimer’s disease: A focus on insulin resistance in the brain. BioMetals 2018, 31, 705–714. [Google Scholar] [CrossRef]
- Pal, A.; Cerchiaro, G.; Rani, I.; Ventriglia, M.; Rongioletti, M.; Longobardi, A. Iron in Alzheimer’s Disease: From Physiology to Disease Disabilities. Biomolecules 2022, 12, 1248. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The rat brain in stereotaxic coordinates (2nd edn). Trends Neurosci. 1997, 10, 439. [Google Scholar]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, L.C.; Tsai, T.H. Measurement of free hydroxytyrosol in microdialysates from blood and brain of anesthetized rats by liquid chromatography with fluorescence detection. J. Chromatogr. A 2009, 1216, 3501–3507. [Google Scholar] [CrossRef]
- Dyomina, A.V.; Smolensky, I.V.; Zaitsev, A.V. Refinement of the Barnes and Morris water maze protocols improves characterization of spatial cognitive deficits in the lithium-pilocarpine rat model of epilepsy. Epilepsy Behav. 2023, 147, 109391. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.,, |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabanez, M.; Santos, I.R.; Ikebara, J.M.; Camargo, M.L.M.; Dos Santos, B.A.; Freire, B.M.; Batista, B.L.; Takada, S.H.; Squitti, R.; Kihara, A.H.; et al. The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14950. https://doi.org/10.3390/ijms241914950
Tabanez M, Santos IR, Ikebara JM, Camargo MLM, Dos Santos BA, Freire BM, Batista BL, Takada SH, Squitti R, Kihara AH, et al. The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(19):14950. https://doi.org/10.3390/ijms241914950
Chicago/Turabian StyleTabanez, Miguel, Ilma R. Santos, Juliane M. Ikebara, Mariana L. M. Camargo, Bianca A. Dos Santos, Bruna M. Freire, Bruno L. Batista, Silvia H. Takada, Rosanna Squitti, Alexandre H. Kihara, and et al. 2023. "The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 19: 14950. https://doi.org/10.3390/ijms241914950
APA StyleTabanez, M., Santos, I. R., Ikebara, J. M., Camargo, M. L. M., Dos Santos, B. A., Freire, B. M., Batista, B. L., Takada, S. H., Squitti, R., Kihara, A. H., & Cerchiaro, G. (2023). The Impact of Hydroxytyrosol on the Metallomic-Profile in an Animal Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 24(19), 14950. https://doi.org/10.3390/ijms241914950






