Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy
Abstract
1. Introduction
1.1. Alphavirus Vectors
1.2. Alphavirus Vectors as an Oncolytic-Virus-Mediated Therapy
2. Development of Sindbis Virus as an Effective Vector for Cancer Treatment
2.1. Vector Safety
2.2. Early Investigations: Engineered Targeting
2.3. In Vivo Imaging Studies
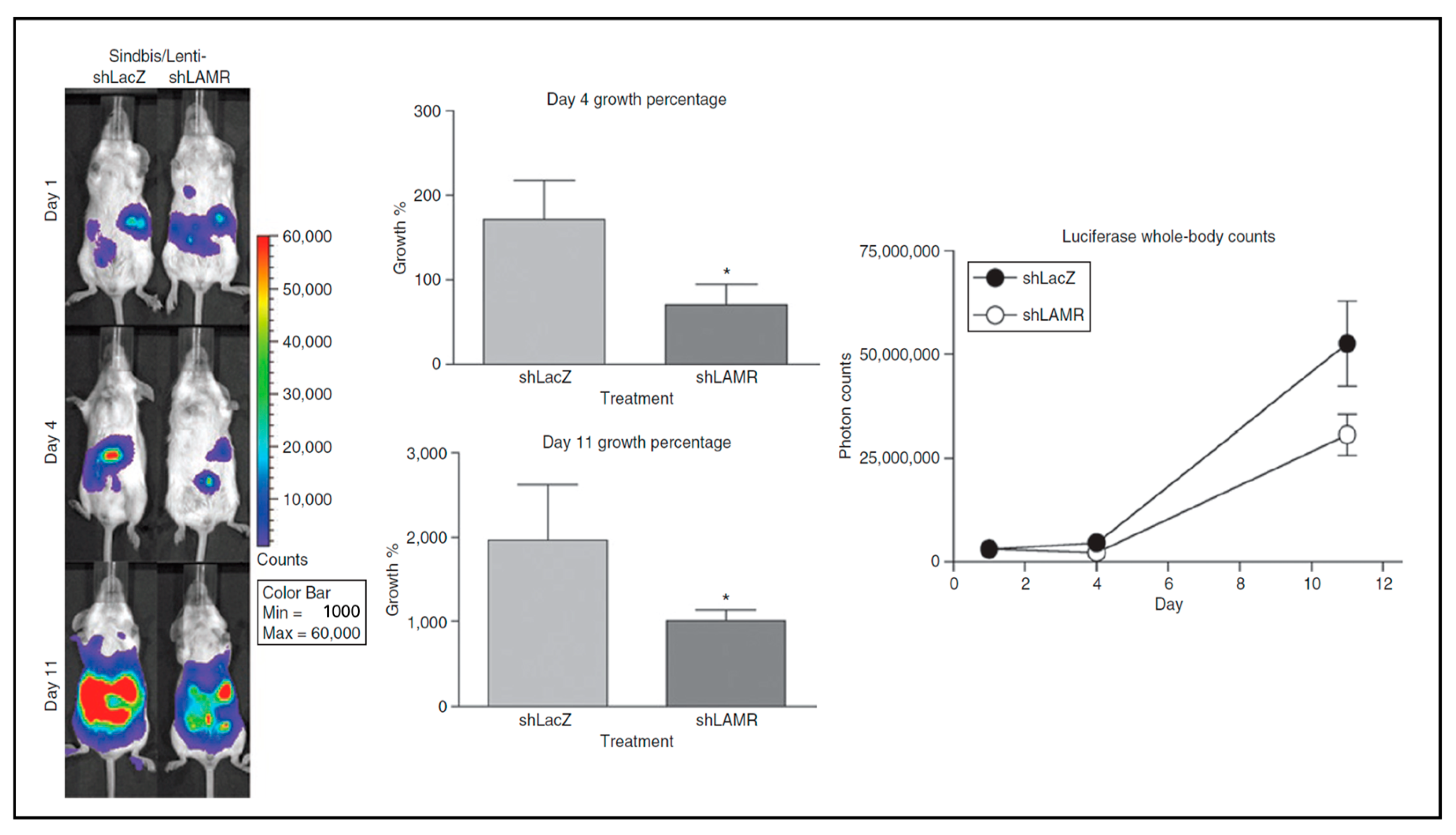
2.4. Sindbis Virus Infection, Tumor Targeting, and the 67 kDa Laminin Receptor
2.5. LAMR as a Cancer Therapy Target
2.6. LAMR as a Receptor for Other Viruses
2.7. Additional Alphavirus Receptors
3. The Delivery of Anti-Tumor Agents
3.1. Cytokines
3.2. Tumor-Associated Antigens
The Modification of TAAs
4. Combination Therapy with Immunomodulatory Proteins
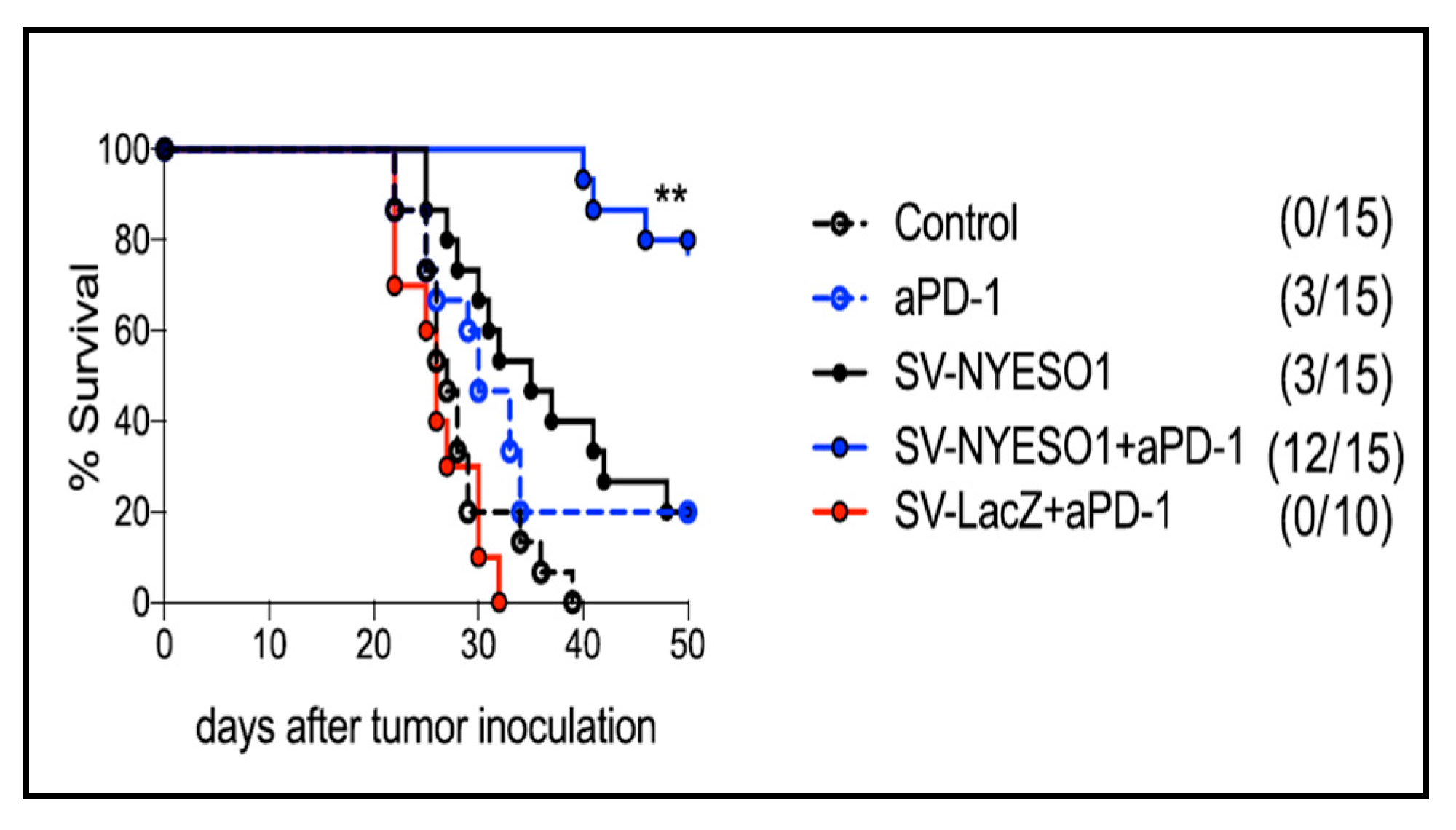
4.1. SV-NYESO1 and PD-1 Antibody
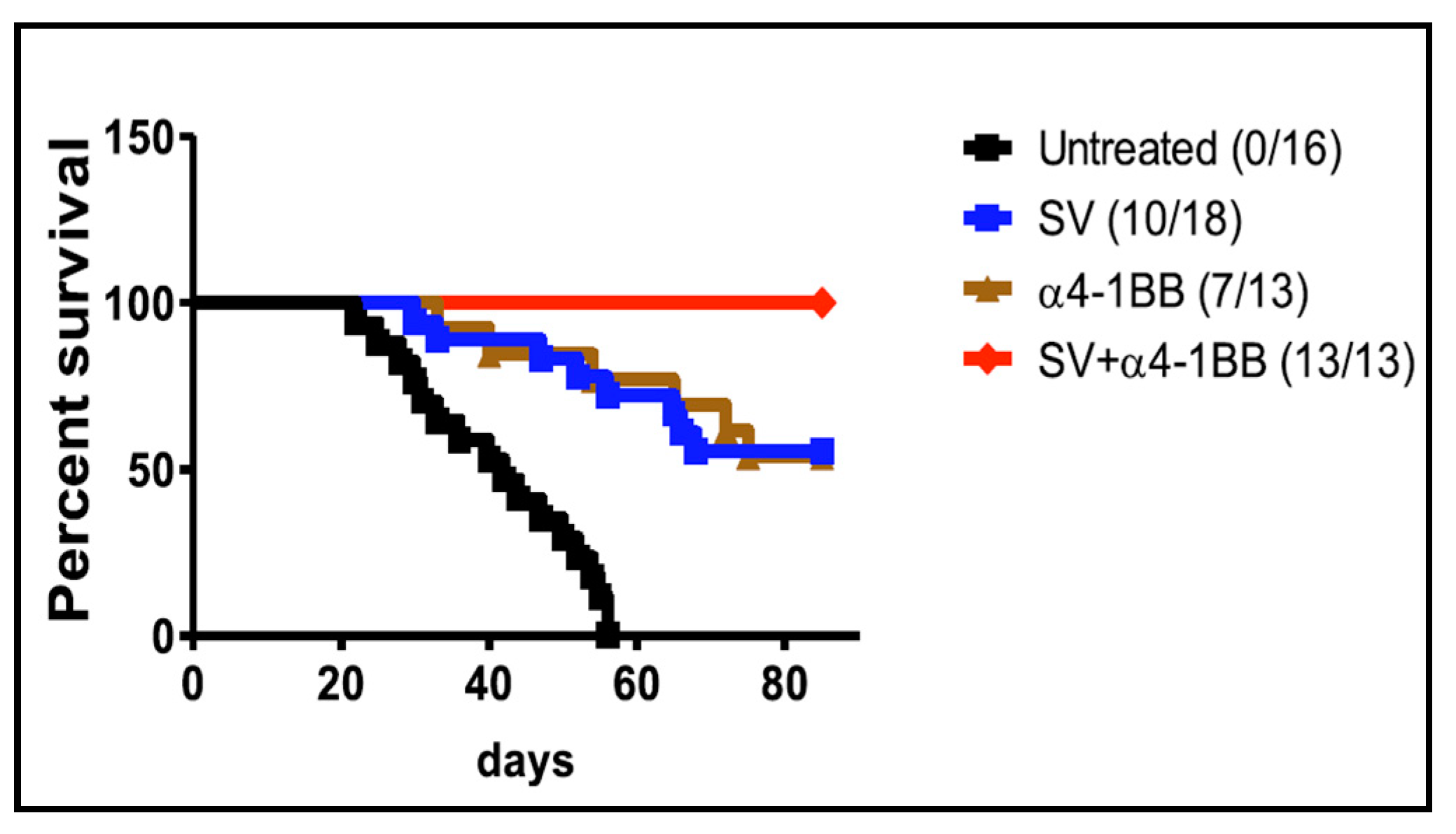
4.2. SV Vector and 4-1BB Agonistic Antibody
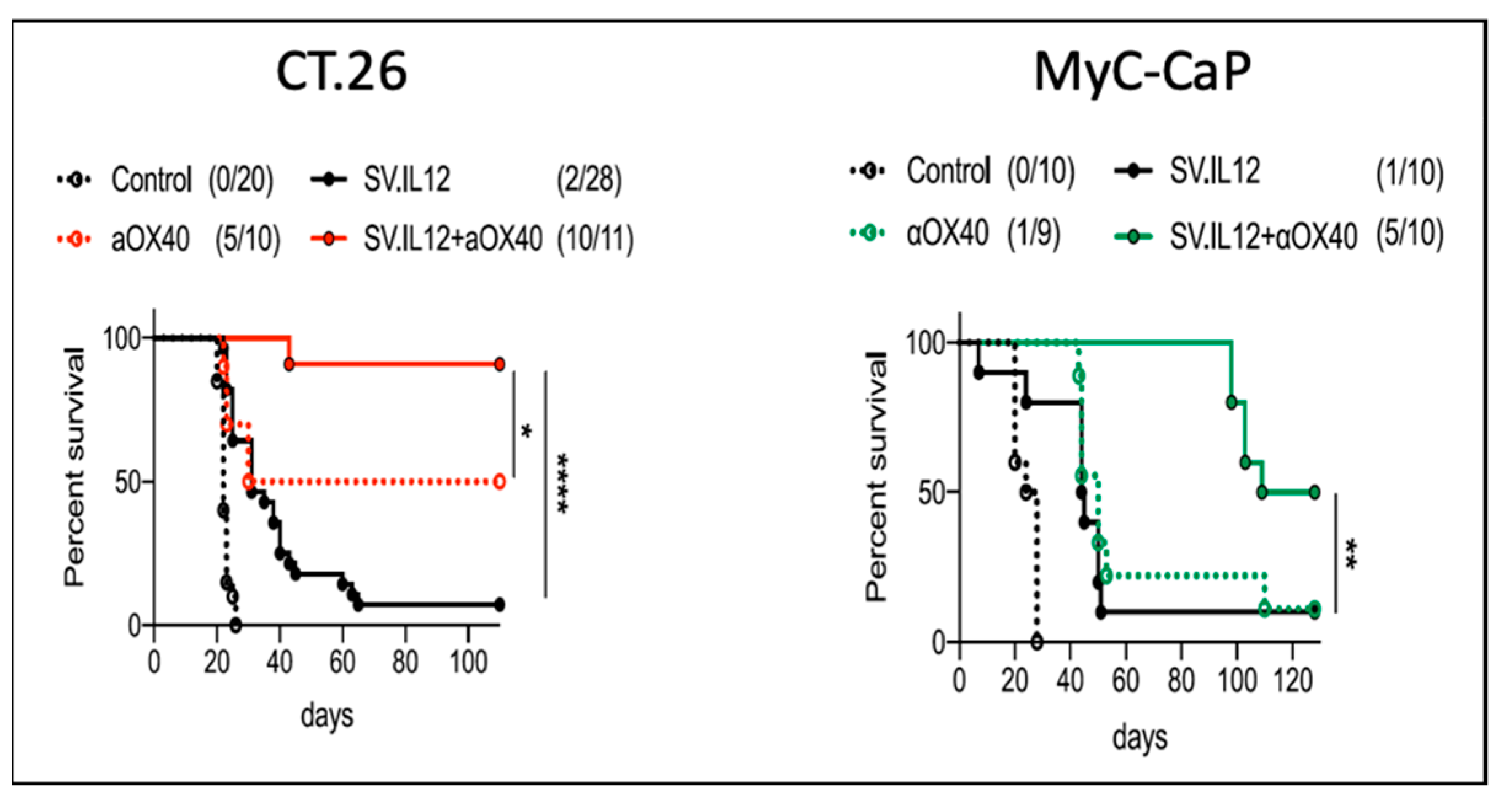
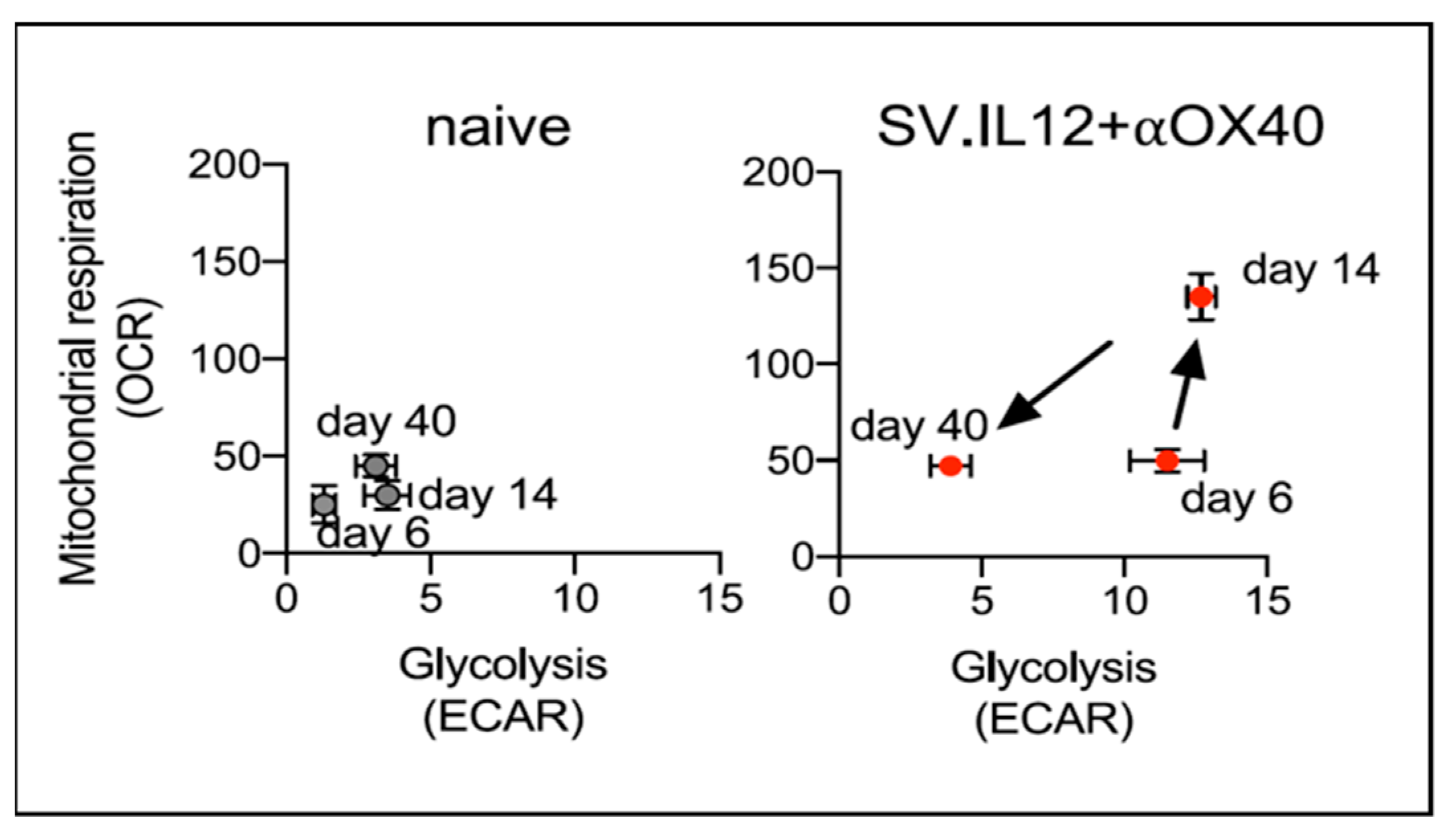
4.3. SV.IL12 and Anti-OX40 Antibody
4.4. SV Vector Expressing αOX40
4.5. Alphavirus Combined with Immunotherapy
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurtado, A.; Tseng, J.C.; Meruelo, D. Gene therapy that safely targets and kills tumor cells throughout the body. Rejuvenation Res. 2006, 9, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Opp, S.; Hurtado, A.; Pampeno, C.; Lin, Z.; Meruelo, D. Potent and Targeted Sindbis Virus Platform for Immunotherapy of Ovarian Cancer. Cells 2022, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Scherwitzl, I.; Hurtado, A.; Pierce, C.M.; Vogt, S.; Pampeno, C.; Meruelo, D. Systemically Administered Sindbis Virus in Combination with Immune Checkpoint Blockade Induces Curative Anti-tumor Immunity. Mol. Ther. Oncolytics 2018, 9, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Scherwitzl, I.; Opp, S.; Hurtado, A.M.; Pampeno, C.; Loomis, C.; Kannan, K.; Yu, M.; Meruelo, D. Sindbis Virus with Anti-OX40 Overcomes the Immunosuppressive Tumor Microenvironment of Low-Immunogenic Tumors. Mol. Ther. Oncolytics 2020, 17, 431–447. [Google Scholar] [CrossRef]
- Tseng, J.C.; Hurtado, A.; Yee, H.; Levin, B.; Boivin, C.; Benet, M.; Blank, S.V.; Pellicer, A.; Meruelo, D. Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res. 2004, 64, 6684–6692. [Google Scholar] [CrossRef]
- Tseng, J.C.; Levin, B.; Hurtado, A.; Yee, H.; Perez de Castro, I.; Jimenez, M.; Shamamian, P.; Jin, R.; Novick, R.P.; Pellicer, A.; et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat. Biotechnol. 2004, 22, 70–77. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New World and Old World Alphaviruses Have Evolved to Exploit Different Components of Stress Granules, FXR and G3BP Proteins, for Assembly of Viral Replication Complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef]
- Taylor, R.M.; Hurlbut, H.S. The isolation of Coxsackie-like viruses from mosquitoes. J. Egypt. Med. Assoc. 1953, 36, 489–494. [Google Scholar]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef]
- Zimmerman, O.; Holmes, A.C.; Kafai, N.M.; Adams, L.J.; Diamond, M.S. Entry receptors—The gateway to alphavirus infection. J. Clin. Investig. 2023, 133, e165307. [Google Scholar] [CrossRef]
- Bredenbeek, P.J.; Frolov, I.; Rice, C.M.; Schlesinger, S. Sindbis virus expression vectors: Packaging of RNA replicons by using defective helper RNAs. J. Virol. 1993, 67, 6439–6446. [Google Scholar] [CrossRef]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.I.; Gorchakov, R.; Pereboeva, L.; Atasheva, S.; Frolov, I. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J. Virol. 2010, 84, 11679–11695. [Google Scholar] [CrossRef] [PubMed]
- Froshauer, S.; Kartenbeck, J.; Helenius, A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J. Cell Biol. 1988, 107, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Kujala, P.; Ikaheimonen, A.; Ehsani, N.; Vihinen, H.; Auvinen, P.; Kaariainen, L. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 2001, 75, 3873–3884. [Google Scholar] [CrossRef]
- Frolov, I.; Hoffman, T.A.; Prágai, B.M.; Dryga, S.A.; Huang, H.V.; Schlesinger, S.; Rice, C.M. Alphavirus-based expression vectors: Strategies and applications. Proc. Natl. Acad. Sci. USA 1996, 93, 11371–11377. [Google Scholar] [CrossRef]
- Strauss, J.H.; Wang, K.S.; Schmaljohn, A.L.; Kuhn, R.J.; Strauss, E.G. Host-cell receptors for Sindbis virus. Arch. Virol. Suppl. 1994, 9, 473–484. [Google Scholar] [CrossRef]
- Scaglione, A.; Opp, S.; Hurtado, A.; Lin, Z.; Pampeno, C.; Noval, M.G.; Thannickal, S.A.; Stapleford, K.A.; Meruelo, D. Combination of a Sindbis-SARS-CoV-2 Spike Vaccine and alphaOX40 Antibody Elicits Protective Immunity Against SARS-CoV-2 Induced Disease and Potentiates Long-Term SARS-CoV-2-Specific Humoral and T-Cell Immunity. Front. Immunol. 2021, 12, 719077. [Google Scholar] [CrossRef]
- Lauer, U.M.; Beil, J. Oncolytic viruses: Challenges and considerations in an evolving clinical landscape. Future Oncol. 2022, 18, 2713–2732. [Google Scholar] [CrossRef]
- Ehrlich, M.; Bacharach, E. Oncolytic Virotherapy: The Cancer Cell Side. Cancers 2021, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Groeneveldt, C.; van den Ende, J.; van Montfoort, N. Preexisting immunity: Barrier or bridge to effective oncolytic virus therapy? Cytokine Growth Factor Rev. 2023, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Swift, E.A.; Pollard, S.M.; Parker, A.L. Engineering Cancer Selective Virotherapies: Are the Pieces of the Puzzle Falling into Place? Hum. Gene Ther. 2022, 33, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, X.; Yu, J.; Ge, S.; Fan, X. Oncolytic Virotherapy: From Bench to Bedside. Front. Cell Dev. Biol. 2021, 9, 790150. [Google Scholar] [CrossRef]
- Venticinque, L.; Meruelo, D. Sindbis viral vector induced apoptosis requires translational inhibition and signaling through Mcl-1 and Bak. Mol. Cancer 2010, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Frolov, I.; Russell, S.J. Gene therapy for malignant glioma using Sindbis vectors expressing a fusogenic membrane glycoprotein. J. Gene Med. 2004, 6, 1082–1091. [Google Scholar] [CrossRef]
- Huckriede, A.; Bungener, L.; Holtrop, M.; de Vries, J.; Waarts, B.L.; Daemen, T.; Wilschut, J. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: Indications for cross-priming. Vaccine 2004, 22, 1104–1113. [Google Scholar] [CrossRef]
- Granot, T.; Yamanashi, Y.; Meruelo, D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014, 22, 112–122. [Google Scholar] [CrossRef]
- Chen, Z.; Moyana, T.; Saxena, A.; Warrington, R.; Jia, Z.; Xiang, J. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int. J. Cancer 2001, 93, 539–548. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Fournier, P.; Zeng, J.; Schirrmacher, V. Two ways to induce innate immune responses in human PBMCs: Paracrine stimulation of IFN-alpha responses by viral protein or dsRNA. Int. J. Oncol. 2003, 23, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Venticinque, L.; Tseng, J.C.; Meruelo, D. Activation of cytotoxic and regulatory functions of NK cells by Sindbis viral vectors. PLoS ONE 2011, 6, e20598. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.W.; Hwang, L.N.; deVeer, M.J.; Zhou, A.; Silverman, R.H.; Williams, B.R.; Dubensky, T.W.; Ying, H.; Restifo, N.P. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 2003, 9, 33–39. [Google Scholar] [CrossRef]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Smith, D.; Bot, S.; Dellamary, L.; Bloom, A.; Bot, A. Noncoding RNA danger motifs bridge innate and adaptive immunity and are potent adjuvants for vaccination. J. Clin. Investig. 2002, 110, 1175–1184. [Google Scholar] [CrossRef]
- Choi, Y.; Chang, J. Viral vectors for vaccine applications. Clin. Exp. Vaccine Res. 2013, 2, 97–105. [Google Scholar] [CrossRef]
- Gardner, J.P.; Frolov, I.; Perri, S.; Ji, Y.; MacKichan, M.L.; zur Megede, J.; Chen, M.; Belli, B.A.; Driver, D.A.; Sherrill, S.; et al. Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 2000, 74, 11849–11857. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.H.; Johnston, R.E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 2000, 74, 914–922. [Google Scholar] [CrossRef]
- Osada, T.; Morse, M.A.; Hobeika, A.; Lyerly, H.K. Novel recombinant alphaviral and adenoviral vectors for cancer immunotherapy. Semin. Oncol. 2012, 39, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: Expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 1997, 239, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, Y.; Vajdy, M.; Lian, Y.; Perri, S.; Greer, C.E.; Legg, H.S.; Galli, G.; Saletti, G.; Otten, G.R.; Rappuoli, R.; et al. Lack of interference with immunogenicity of a chimeric alphavirus replicon particle-based influenza vaccine by preexisting antivector immunity. Clin. Vaccine Immunol. 2012, 19, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Brummer-Korvenkontio, M.; Vapalahti, O.; Kuusisto, P.; Saikku, P.; Manni, T.; Koskela, P.; Nygren, T.; Brummer-Korvenkontio, H.; Vaheri, A. Epidemiology of Sindbis virus infections in Finland 1981-96: Possible factors explaining a peculiar disease pattern. Epidemiol. Infect. 2002, 129, 335–345. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Levine, B. Sindbis virus vector system for functional analysis of apoptosis regulators. Methods Enzymol. 2000, 322, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Manni, T.; Kurkela, S.; Vaheri, A.; Vapalahti, O. Diagnostics of Pogosta disease: Antigenic properties and evaluation of Sindbis virus IgM and IgG enzyme immunoassays. Vector Borne Zoonotic Dis. 2008, 8, 303–311. [Google Scholar] [CrossRef]
- Dubuisson, J.; Rice, C.M. Sindbis virus attachment: Isolation and characterization of mutants with impaired binding to vertebrate cells. J. Virol. 1993, 67, 3363–3374. [Google Scholar] [CrossRef]
- Ohno, K.; Sawai, K.; Iijima, Y.; Levin, B.; Meruelo, D. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 1997, 15, 763–767. [Google Scholar] [CrossRef]
- Surolia, A. Interaction of Protein-a with the Domains of Fc Fragment. Trends Biochem. Sci. 1982, 7, 318–319. [Google Scholar] [CrossRef]
- Sawai, K.; Meruelo, D. Cell-specific transfection of choriocarcinoma cells by using Sindbis virus hCG expressing chimeric vector. Biochem. Biophys. Res. Commun. 1998, 248, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Tseng, J.C.; Boivin, C.; Levin, B.; Yee, H.; Pampeno, C.; Meruelo, D. Identification of amino acids of Sindbis virus E2 protein involved in targeting tumor metastases in vivo. Mol. Ther. 2005, 12, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.C.; Levin, B.; Hirano, T.; Yee, H.; Pampeno, C.; Meruelo, D. In vivo antitumor activity of Sindbis viral vectors. J. Natl. Cancer Inst. 2002, 94, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.C.; Zheng, Y.; Yee, H.; Levy, D.E.; Meruelo, D. Restricted tissue tropism and acquired resistance to Sindbis viral vector expression in the absence of innate and adaptive immunity. Gene Ther. 2007, 14, 1166–1174. [Google Scholar] [CrossRef]
- Tseng, J.C.; Granot, T.; DiGiacomo, V.; Levin, B.; Meruelo, D. Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther. 2010, 17, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Stelter, L.; Tseng, J.C.; Torosjan, A.; Levin, B.; Longo, V.A.; Pillarsetty, N.; Zanzonico, P.; Meruelo, D.; Larson, S.M. Tumor-specific targeting with modified Sindbis viral vectors: Evaluation with optical imaging and positron emission tomography in vivo. Mol. Imaging Biol. 2013, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Lewis, A.D.; Ehsan, M.N.; Sikic, B.I. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991, 51, 5181–5187. [Google Scholar]
- Bhaumik, S.; Gambhir, S.S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad. Sci. USA 2002, 99, 377–382. [Google Scholar] [CrossRef]
- Wang, K.S.; Kuhn, R.J.; Strauss, E.G.; Ou, S.; Strauss, J.H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 1992, 66, 4992–5001. [Google Scholar] [CrossRef]
- Liotta, L.A.; Rao, C.N.; Wewer, U.M. Biochemical interactions of tumor cells with the basement membrane. Annu. Rev. Biochem. 1986, 55, 1037–1057. [Google Scholar] [CrossRef]
- DiGiacomo, V.; Meruelo, D. Looking into laminin receptor: Critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol. Rev. Camb. Philos. Soc. 2016, 91, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Martignone, S.; Menard, S.; Bufalino, R.; Cascinelli, N.; Pellegrini, R.; Tagliabue, E.; Andreola, S.; Rilke, F.; Colnaghi, M.I. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J. Natl. Cancer Inst. 1993, 85, 398–402. [Google Scholar] [CrossRef]
- Viacava, P.; Naccarato, A.G.; Collecchi, P.; Ménard, S.; Castronovo, V.; Bevilacqua, G. The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J. Pathol. 1997, 182, 36–44. [Google Scholar] [CrossRef]
- Menard, S.; Tagliabue, E.; Colnaghi, M.I. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res. Treat. 1998, 52, 137–145. [Google Scholar] [CrossRef]
- Sanjuan, X.; Fernandez, P.L.; Miquel, R.; Munoz, J.; Castronovo, V.; Menard, S.; Palacin, A.; Cardesa, A.; Campo, E. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J. Pathol. 1996, 179, 376–380. [Google Scholar] [CrossRef]
- Cioce, V.; Castronovo, V.; Shmookler, B.M.; Garbisa, S.; Grigioni, W.F.; Liotta, L.A.; Sobel, M.E. Increased expression of the laminin receptor in human colon cancer. J. Natl. Cancer Inst. 1991, 83, 29–36. [Google Scholar] [CrossRef]
- de Manzoni, G.; Guglielmi, A.; Verlato, G.; Tomezzoli, A.; Pelosi, G.; Schiavon, I.; Cordiano, C. Prognostic significance of 67-kDa laminin receptor expression in advanced gastric cancer. Oncology 1998, 55, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Pastorino, U. Re: Killing of laminin receptor-positive human lung cancers by tumor-infiltrating lymphocytes bearing gamma delta + T-cell receptors. J. Natl. Cancer Inst. 1996, 88, 1241–1242. [Google Scholar] [CrossRef][Green Version]
- Pelosi, G.; Pasini, F.; Bresaola, E.; Bogina, G.; Pederzoli, P.; Biolo, S.; Menard, S.; Zamboni, G. High-affinity monomeric 67-kD laminin receptors and prognosis in pancreatic endocrine tumours. J. Pathol. 1997, 183, 62–69. [Google Scholar] [CrossRef]
- van den Brule, F.A.; Buicu, C.; Berchuck, A.; Bast, R.C.; Deprez, M.; Liu, F.T.; Cooper, D.N.; Pieters, C.; Sobel, M.E.; Castronovo, V. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum. Pathol. 1996, 27, 1185–1191. [Google Scholar] [CrossRef]
- van den Brule, F.A.; Berchuck, A.; Bast, R.C.; Liu, F.T.; Gillet, C.; Sobel, M.E.; Castronovo, V. Differential expression of the 67-kD laminin receptor and 31-kD human laminin-binding protein in human ovarian carcinomas. Eur. J. Cancer 1994, 30A, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- van den Brule, F.A.; Castronovo, V.; Menard, S.; Giavazzi, R.; Marzola, M.; Belotti, D.; Taraboletti, G. Expression of the 67 kD laminin receptor in human ovarian carcinomas as defined by a monoclonal antibody, MLuC5. Eur. J. Cancer 1996, 32A, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Liebman, J.M.; Burbelo, P.D.; Yamada, Y.; Fridman, R.; Kleinman, H.K. Altered expression of basement-membrane components and collagenases in ascitic xenografts of OVCAR-3 ovarian cancer cells. Int. J. Cancer 1993, 55, 102–109. [Google Scholar] [CrossRef]
- Song, T.; Choi, C.H.; Cho, Y.J.; Sung, C.O.; Song, S.Y.; Kim, T.J.; Bae, D.S.; Lee, J.W.; Kim, B.G. Expression of 67-kDa laminin receptor was associated with tumor progression and poor prognosis in epithelial ovarian cancer. Gynecol. Oncol. 2012, 125, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Basolo, F.; Pollina, L.; Pacini, F.; Fontanini, G.; Ménard, S.; Castronovo, V.; Bevilacqua, G. Expression of the Mr 67,000 laminin receptor is an adverse prognostic indicator in human thyroid cancer: An immunohistochemical study. Clin. Cancer Res. 1996, 2, 1777–1780. [Google Scholar] [PubMed]
- Ozaki, I.; Yamamoto, K.; Mizuta, T.; Kajihara, S.; Fukushima, N.; Setoguchi, Y.; Morito, F.; Sakai, T. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 1998, 43, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Hand, P.H.; Thor, A.; Schlom, J.; Rao, C.N.; Liotta, L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985, 45, 2713–2719. [Google Scholar]
- Hayman, E.G.; Engvall, E.; Ruoslahti, E. Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J. Cell Biol. 1981, 88, 352–357. [Google Scholar] [CrossRef]
- Liotta, L.A. Tumor invasion and metastases: Role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am. J. Pathol. 1984, 117, 339–348. [Google Scholar]
- Ardini, E.; Sporchia, B.; Pollegioni, L.; Modugno, M.; Ghirelli, C.; Castiglioni, F.; Tagliabue, E.; Ménard, S. Identification of a novel function for 67-kDa laminin receptor: Increase in laminin degradation rate and release of motility fragments. Cancer Res. 2002, 62, 1321–1325. [Google Scholar]
- Zuber, C.; Knackmuss, S.; Zemora, G.; Reusch, U.; Vlasova, E.; Diehl, D.; Mick, V.; Hoffmann, K.; Nikles, D.; Fröhlich, T.; et al. Invasion of tumorigenic HT1080 cells is impeded by blocking or downregulating the 37-kDa/67-kDa laminin receptor. J. Mol. Biol. 2008, 378, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Givant-Horwitz, V.; Davidson, B.; Reich, R. Laminin-induced signaling in tumor cells: The role of the M(r) 67,000 laminin receptor. Cancer Res. 2004, 64, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Berno, V.; Porrini, D.; Castiglioni, F.; Campiglio, M.; Casalini, P.; Pupa, S.M.; Balsari, A.; Ménard, S.; Tagliabue, E. The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocr. Relat. Cancer 2005, 12, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Tseng, J.C.; Zheng, Y.; Meruelo, D. Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol. Ther. 2010, 18, 63–74. [Google Scholar] [CrossRef]
- Scheiman, J.; Jamieson, K.V.; Ziello, J.; Tseng, J.C.; Meruelo, D. Extraribosomal functions associated with the C terminus of the 37/67 kDa laminin receptor are required for maintaining cell viability. Cell Death Dis. 2010, 1, e42. [Google Scholar] [CrossRef]
- Blum, J.L.; Zeigler, M.E.; Wicha, M.S. Regulation of mammary differentiation by the extracellular matrix. Environ. Health Perspect. 1989, 80, 71–83. [Google Scholar] [CrossRef]
- Fujimura, Y.; Umeda, D.; Kiyohara, Y.; Sunada, Y.; Yamada, K.; Tachibana, H. The involvement of the 67 kDa laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochem. Biophys. Res. Commun. 2006, 348, 524–531. [Google Scholar] [CrossRef]
- Venticinque, L.; Jamieson, K.V.; Meruelo, D. Interactions between laminin receptor and the cytoskeleton during translation and cell motility. PLoS ONE 2011, 6, e15895. [Google Scholar] [CrossRef]
- Jamieson, K.V.; Hubbard, S.R.; Meruelo, D. Structure-guided identification of a laminin binding site on the laminin receptor precursor. J. Mol. Biol. 2011, 405, 24–32. [Google Scholar] [CrossRef]
- Naidoo, K.; Malindisa, S.T.; Otgaar, T.C.; Bernert, M.; Da Costa Dias, B.; Ferreira, E.; Reusch, U.; Knackmuss, S.; Little, M.; Weiss, S.F.; et al. Knock-Down of the 37kDa/67kDa Laminin Receptor LRP/LR Impedes Telomerase Activity. PLoS ONE 2015, 10, e0141618. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Zhao, P.; Yao, L.; Jin, H.; Liang, S.; Wang, Y.; Zhang, D.; Pang, Y.; Shi, Y.; et al. Hypoxia promotes metastasis in human gastric cancer by up-regulating the 67-kDa laminin receptor. Cancer Sci. 2010, 101, 1653–1660. [Google Scholar] [CrossRef]
- Khumalo, T.; Ferreira, E.; Jovanovic, K.; Veale, R.B.; Weiss, S.F. Knockdown of LRP/LR Induces Apoptosis in Breast and Oesophageal Cancer Cells. PLoS ONE 2015, 10, e0139584. [Google Scholar] [CrossRef] [PubMed]
- Munien, C.; Rebelo, T.M.; Ferreira, E.; Weiss, S.F. IgG1-iS18 impedes the adhesive and invasive potential of early and late stage malignant melanoma cells. Exp. Cell Res. 2017, 351, 135–141. [Google Scholar] [CrossRef]
- Rebelo, T.M.; Chetty, C.J.; Ferreira, E.; Weiss, S.F. Anti-LRP/LR-specific antibody IgG1-iS18 impedes adhesion and invasion of pancreatic cancer and neuroblastoma cells. BMC Cancer 2016, 16, 917. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, A.L.; Schwarzenberger, P.O. Oncofetal antigen/immature laminin receptor protein in pregnancy and cancer. Cell Mol. Biol. Lett. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Coggin, J.H., Jr.; Barsoum, A.L.; Rohrer, J.W. 37 kiloDalton oncofetal antigen protein and immature laminin receptor protein are identical, universal T-cell inducing immunogens on primary rodent and human cancers. Anticancer Res. 1999, 19, 5535–5542. [Google Scholar]
- Holtl, L.; Zelle-Rieser, C.; Gander, H.; Papesh, C.; Ramoner, R.; Bartsch, G.; Rogatsch, H.; Barsoum, A.L.; Coggin, J.H., Jr.; Thurnher, M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin. Cancer Res. 2002, 8, 3369–3376. [Google Scholar]
- Friedrichs, B.; Siegel, S.; Kloess, M.; Barsoum, A.; Coggin, J., Jr.; Rohrer, J.; Jakob, I.; Tiemann, M.; Heidorn, K.; Schulte, C.; et al. Humoral immune responses against the immature laminin receptor protein show prognostic significance in patients with chronic lymphocytic leukemia. J. Immunol. 2008, 180, 6374–6384. [Google Scholar] [CrossRef]
- Siegel, S.; Wagner, A.; Kabelitz, D.; Marget, M.; Coggin, J., Jr.; Barsoum, A.; Rohrer, J.; Schmitz, N.; Zeis, M. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 2003, 102, 4416–4423. [Google Scholar] [CrossRef]
- Ludwig, G.V.; Kondig, J.P.; Smith, J.F. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 1996, 70, 5592–5599. [Google Scholar] [CrossRef]
- Thepparit, C.; Smith, D.R. Serotype-specific entry of dengue virus into liver cells: Identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol. 2004, 78, 12647–12656. [Google Scholar] [CrossRef]
- Tio, P.H.; Jong, W.W.; Cardosa, M.J. Two dimensional VOPBA reveals laminin receptor (LAMR1) interaction with dengue virus serotypes 1, 2 and 3. Virol. J. 2005, 2, 25. [Google Scholar] [CrossRef]
- Chen, J.; He, W.R.; Shen, L.; Dong, H.; Yu, J.; Wang, X.; Yu, S.; Li, Y.; Li, S.; Luo, Y.; et al. The laminin receptor is a cellular attachment receptor for classical Swine Fever virus. J. Virol. 2015, 89, 4894–4906. [Google Scholar] [CrossRef]
- Bogachek, M.V.; Protopopova, E.V.; Loktev, V.B.; Zaitsev, B.N.; Favre, M.; Sekatskii, S.K.; Dietler, G. Immunochemical and single molecule force spectroscopy studies of specific interaction between the laminin binding protein and the West Nile virus surface glycoprotein E domain II. J. Mol. Recognit. 2008, 21, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Grimm, D.; Pandey, K.; Yant, S.R.; Xu, H.; Kay, M.A. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006, 80, 9831–9836. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.M.; Martinez, E.A.; Bogaert, L.; Kast, W.M. Investigation of the Optimal Prime Boost Spacing Regimen for a Cancer Therapeutic Vaccine Targeting Human Papillomavirus. Cancers 2022, 14, 4339. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Israel, R.; Scher, H.I.; Morris, S. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef]
- Crosby, E.J.; Gwin, W.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.; Kim, S.; Rogatko, A.; et al. Vaccine-Induced Memory CD8+ T Cells Provide Clinical Benefit in HER2 Expressing Breast Cancer: A Mouse to Human Translational Study. Clin. Cancer Res. 2019, 25, 2725–2736. [Google Scholar] [CrossRef]
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J. Clin. Invest. 2010, 120, 3234–3241. [Google Scholar] [CrossRef]
- Bower, J.J.; Song, L.; Bastola, P.; Hirsch, M.L. Harnessing the Natural Biology of Adeno-Associated Virus to Enhance the Efficacy of Cancer Gene Therapy. Viruses 2021, 13, 1205. [Google Scholar] [CrossRef] [PubMed]
- Suzme, R.; Tseng, J.C.; Levin, B.; Ibrahim, S.; Meruelo, D.; Pellicer, A. Sindbis viral vectors target hematopoietic malignant cells. Cancer Gene Ther. 2012, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Backovic, M.; Rey, F.A. Virus entry: Old viruses, new receptors. Curr. Opin. Virol. 2012, 2, 4–13. [Google Scholar] [CrossRef]
- Huang, P.Y.; Guo, J.H.; Hwang, L.H. Oncolytic Sindbis virus targets tumors defective in the interferon response and induces significant bystander antitumor immunity in vivo. Mol. Ther. 2012, 20, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.P.; Hanna, S.L.; Spiridigliozzi, A.; Wannissorn, N.; Beiting, D.P.; Ross, S.R.; Hardy, R.W.; Bambina, S.A.; Heise, M.T.; Cherry, S. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell Host Microbe 2011, 10, 97–104. [Google Scholar] [CrossRef]
- De Caluwe, L.; Coppens, S.; Vereecken, K.; Daled, S.; Dhaenens, M.; Van Ostade, X.; Deforce, D.; Arien, K.K.; Bartholomeeusen, K. The CD147 Protein Complex Is Involved in Entry of Chikungunya Virus and Related Alphaviruses in Human Cells. Front. Microbiol. 2021, 12, 615165. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Zhang, X.; Dai, Z.; Zhi-Peng, W.; Yu, J.; Peng, Y.; Wu, W.; Zhang, N.; Luo, P.; et al. Large-Scale Single-Cell and Bulk Sequencing Analyses Reveal the Prognostic Value and Immune Aspects of CD147 in Pan-Cancer. Front. Immunol. 2022, 13, 810471. [Google Scholar] [CrossRef] [PubMed]
- Basore, K.; Ma, H.; Kafai, N.M.; Mackin, S.; Kim, A.S.; Nelson, C.A.; Diamond, M.S.; Fremont, D.H. Structure of Venezuelan equine encephalitis virus in complex with the LDLRAD3 receptor. Nature 2021, 598, 672–676. [Google Scholar] [CrossRef]
- Clark, L.E.; Clark, S.A.; Lin, C.; Liu, J.; Coscia, A.; Nabel, K.G.; Yang, P.; Neel, D.V.; Lee, H.; Brusic, V.; et al. VLDLR and ApoER2 are receptors for multiple alphaviruses. Nature 2022, 602, 475–480. [Google Scholar] [CrossRef]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Guan, Y.; Xiao, R.; Cai, J.; Chen, W.; Zheng, M.; Sun, K.; Chen, C.; Huang, G.; et al. Seven Genes Associated With Lymphatic Metastasis in Thyroid Cancer That Is Linked to Tumor Immune Cell Infiltration. Front. Oncol. 2021, 11, 756246. [Google Scholar] [CrossRef]
- Song, D.; Jia, X.; Liu, X.; Hu, L.; Lin, K.; Xiao, T.; Qiao, Y.; Zhang, J.; Dan, J.; Wong, C.; et al. Identification of the receptor of oncolytic virus M1 as a therapeutic predictor for multiple solid tumors. Signal Transduct. Target. Ther. 2022, 7, 100. [Google Scholar] [CrossRef]
- Tan, L.; Fu, D.; Liu, F.; Liu, J.; Zhang, Y.; Li, X.; Gao, J.; Tao, K.; Wang, G.; Wang, L.; et al. MXRA8 is an immune-relative prognostic biomarker associated with metastasis and CD8+ T cell infiltration in colorectal cancer. Front. Oncol. 2022, 12, 1094612. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphaviruses in Cancer Therapy. Front. Mol. Biosci. 2022, 9, 864781. [Google Scholar] [CrossRef]
- Morse, M.A.; Crosby, E.J.; Force, J.; Osada, T.; Hobeika, A.C.; Hartman, Z.C.; Berglund, P.; Smith, J.; Lyerly, H.K. Clinical trials of self-replicating RNA-based cancer vaccines. Cancer Gene Ther. 2023, 30, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Quetglas, J.I.; Ruiz-Guillen, M.; Aranda, A.; Casales, E.; Bezunartea, J.; Smerdou, C. Alphavirus vectors for cancer therapy. Virus Res. 2010, 153, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Riezebos-Brilman, A.; Regts, J.; Chen, M.; Wilschut, J.; Daemen, T. Augmentation of alphavirus vector-induced human papilloma virus-specific immune and anti-tumour responses by co-expression of interleukin-12. Vaccine 2009, 27, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pilipich, N.; Lasarte-Cía, A.; Lozano, T.; Martín-Otal, C.; Lasarte, J.J.; Smerdou, C. Intratumoral electroporation of a self-amplifying RNA expressing IL-12 induces antitumor effects in mouse models of cancer. Mol. Ther. Nucleic Acids 2022, 29, 387–399. [Google Scholar] [CrossRef]
- Asselin-Paturel, C.; Lassau, N.; Guinebretière, J.M.; Zhang, J.; Gay, F.; Bex, F.; Hallez, S.; Leclere, J.; Peronneau, P.; Mami-Chouaib, F.; et al. Transfer of the murine interleukin-12 gene in vivo by a Semliki Forest virus vector induces B16 tumor regression through inhibition of tumor blood vessel formation monitored by Doppler ultrasonography. Gene Ther. 1999, 6, 606–615. [Google Scholar] [CrossRef]
- Lyons, J.A.; Sheahan, B.J.; Galbraith, S.E.; Mehra, R.; Atkins, G.J.; Fleeton, M.N. Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther. 2007, 14, 503–513. [Google Scholar] [CrossRef]
- Zappasodi, R.; Merghoub, T. Alphavirus-based vaccines in melanoma: Rationale and potential improvements in immunotherapeutic combinations. Immunotherapy 2015, 7, 981–997. [Google Scholar] [CrossRef]
- Näslund, T.I.; Uyttenhove, C.; Nordström, E.K.; Colau, D.; Warnier, G.; Jondal, M.; Van den Eynde, B.J.; Liljeström, P. Comparative prime-boost vaccinations using Semliki Forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory CTL response against the P815 tumor. J. Immunol. 2007, 178, 6761–6769. [Google Scholar] [CrossRef]
- Leslie, M.C.; Zhao, Y.J.; Lachman, L.B.; Hwu, P.; Wu, G.J.; Bar-Eli, M. Immunization against MUC18/MCAM, a novel antigen that drives melanoma invasion and metastasis. Gene Ther. 2007, 14, 316–323. [Google Scholar] [CrossRef]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E.; Olmsted, R.; Houghton, A.N.; Wolchok, J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010, 5, e12670. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.P.; Maughan, M.F.; Lachman, L.B. Alphavirus replicon particles containing the gene for HER2/neu inhibit breast cancer growth and tumorigenesis. Breast Cancer Res. 2005, 7, R145–R155. [Google Scholar] [CrossRef]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A.; et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2007, 13, 3999–4008. [Google Scholar] [CrossRef]
- Garcia-Hernandez Mde, L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: A candidate antigen for treating prostate cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef]
- Yamanaka, R.; Zullo, S.A.; Ramsey, J.; Onodera, M.; Tanaka, R.; Blaese, M.; Xanthopoulos, K.G. Induction of therapeutic antitumor antiangiogenesis by intratumoral injection of genetically engineered endostatin-producing Semliki Forest virus. Cancer Gene Ther. 2001, 8, 796–802. [Google Scholar] [CrossRef]
- Singh, A.; Koutsoumpli, G.; van de Wall, S.; Daemen, T. An alphavirus-based therapeutic cancer vaccine: From design to clinical trial. Cancer Immunol. Immunother. 2019, 68, 849–859. [Google Scholar] [CrossRef]
- Komdeur, F.L.; Singh, A.; van de Wall, S.; Meulenberg, J.J.M.; Boerma, A.; Hoogeboom, B.N.; Paijens, S.T.; Oyarce, C.; de Bruyn, M.; Schuuring, E.; et al. First-in-Human Phase I Clinical Trial of an SFV-Based RNA Replicon Cancer Vaccine against HPV-Induced Cancers. Mol. Ther. 2021, 29, 611–625. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Chai, C.Y.; Hsu, K.F.; He, L.; Rice, C.M.; Ling, M.; Wu, T.C. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene. J. Immunol. 2001, 166, 6218–6226. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Chang, T.C.; Shaw, S.W.; Cheng, P.J.; Huang, C.T.; Chao, A.; Soong, Y.K.; Lai, C.H. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine 2006, 24, 6199–6207. [Google Scholar] [CrossRef]
- Cheng, W.F.; Lee, C.N.; Su, Y.N.; Chai, C.Y.; Chang, M.C.; Polo, J.M.; Hung, C.F.; Wu, T.C.; Hsieh, C.Y.; Chen, C.A. Sindbis virus replicon particles encoding calreticulin linked to a tumor antigen generate long-term tumor-specific immunity. Cancer Gene Ther. 2006, 13, 873–885. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Hsu, K.F.; Chai, C.Y.; He, L.; Polo, J.M.; Slater, L.A.; Ling, M.; Wu, T.C. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum. Gene Ther. 2002, 13, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Gnjatic, S.; Nishikawa, H.; Jungbluth, A.A.; Güre, A.O.; Ritter, G.; Jäger, E.; Knuth, A.; Chen, Y.T.; Old, L.J. NY-ESO-1: Review of an Immunogenic Tumor Antigen. Adv. Cancer Res. 2006, 95, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Houot, R.; Goldstein, M.J.; Kohrt, H.E.; Myklebust, J.H.; Alizadeh, A.A.; Lin, J.T.; Irish, J.M.; Torchia, J.A.; Kolstad, A.; Chen, L.; et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009, 114, 3431–3438. [Google Scholar] [CrossRef]
- Yu, M.; Scherwitzl, I.; Opp, S.; Tsirigos, A.; Meruelo, D. Molecular and metabolic pathways mediating curative treatment of a non-Hodgkin B cell lymphoma by Sindbis viral vectors and anti-4-1BB monoclonal antibody. J. Immunother. Cancer 2019, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Aspeslagh, S.; Postel-Vinay, S.; Rusakiewicz, S.; Soria, J.C.; Zitvogel, L.; Marabelle, A. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer 2016, 52, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, X.; Lan, P.; Li, J.; Dou, Y.; Chen, W.; Ishii, N.; Chen, S.; Xia, B.; Chen, K.; et al. OX40 Costimulation Inhibits Foxp3 Expression and Treg Induction via BATF3-Dependent and Independent Mechanisms. Cell Rep. 2018, 24, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zander, R.A.; Obeng-Adjei, N.; Guthmiller, J.J.; Kulu, D.I.; Li, J.; Ongoiba, A.; Traore, B.; Crompton, P.D.; Butler, N.S. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe 2015, 17, 628–641. [Google Scholar] [CrossRef]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Kruse, B.; Buzzai, A.C.; Shridhar, N.; Braun, A.D.; Gellert, S.; Knauth, K.; Pozniak, J.; Peters, J.; Dittmann, P.; Mengoni, M.; et al. CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours. Nature 2023, 618, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Quetglas, J.I.; Labiano, S.; Aznar, M.A.; Bolanos, E.; Azpilikueta, A.; Rodriguez, I.; Casales, E.; Sanchez-Paulete, A.R.; Segura, V.; Smerdou, C.; et al. Virotherapy with a Semliki Forest Virus-Based Vector Encoding IL12 Synergizes with PD-1/PD-L1 Blockade. Cancer Immunol. Res. 2015, 3, 449–454. [Google Scholar] [CrossRef]
- Ballesteros-Briones, M.C.; Martisova, E.; Casales, E.; Silva-Pilipich, N.; Buñuales, M.; Galindo, J.; Mancheño, U.; Gorraiz, M.; Lasarte, J.J.; Kochan, G.; et al. Short-Term Local Expression of a PD-L1 Blocking Antibody from a Self-Replicating RNA Vector Induces Potent Antitumor Responses. Mol. Ther. 2019, 27, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Zappasodi, R.; Yang, A.; Budhu, S.; Malandro, N.; Hirschhorn-Cymerman, D.; Tiwari, S.; Maughan, M.F.; Olmsted, R.; Wolchok, J.D.; et al. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: Therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol. Res. 2014, 2, 448–458. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphaviruses in Immunotherapy and Anticancer Therapy. Biomedicines 2022, 10, 2263 . [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Lin, Y.; Liang, J.K.; Zhong, W.W.; Li, K.; Huang, W.T.; Wang, D.J.; Yan, G.M.; Zhu, W.B.; et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018, 9, 274. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 2021, 40, 4783–4795. [Google Scholar] [CrossRef]
- Design, I. Phase 1 Study of Intradermal LV305 in Patients with Locally Advanced, Relapsed or Metastatic Cancer Expressing NY-ESO-1. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02122861 (accessed on 15 September 2023).
- Yigit, R. Immune Modulating Effects and Safety of Vvax001, a Therapeutic Semliki Forest Virus Based Cancer Vaccine, in Patients with a History of (Pre) Malignant Cervical Lesions. Available online: https://clinicaltrials.gov/study/NCT03141463 (accessed on 15 September 2023).
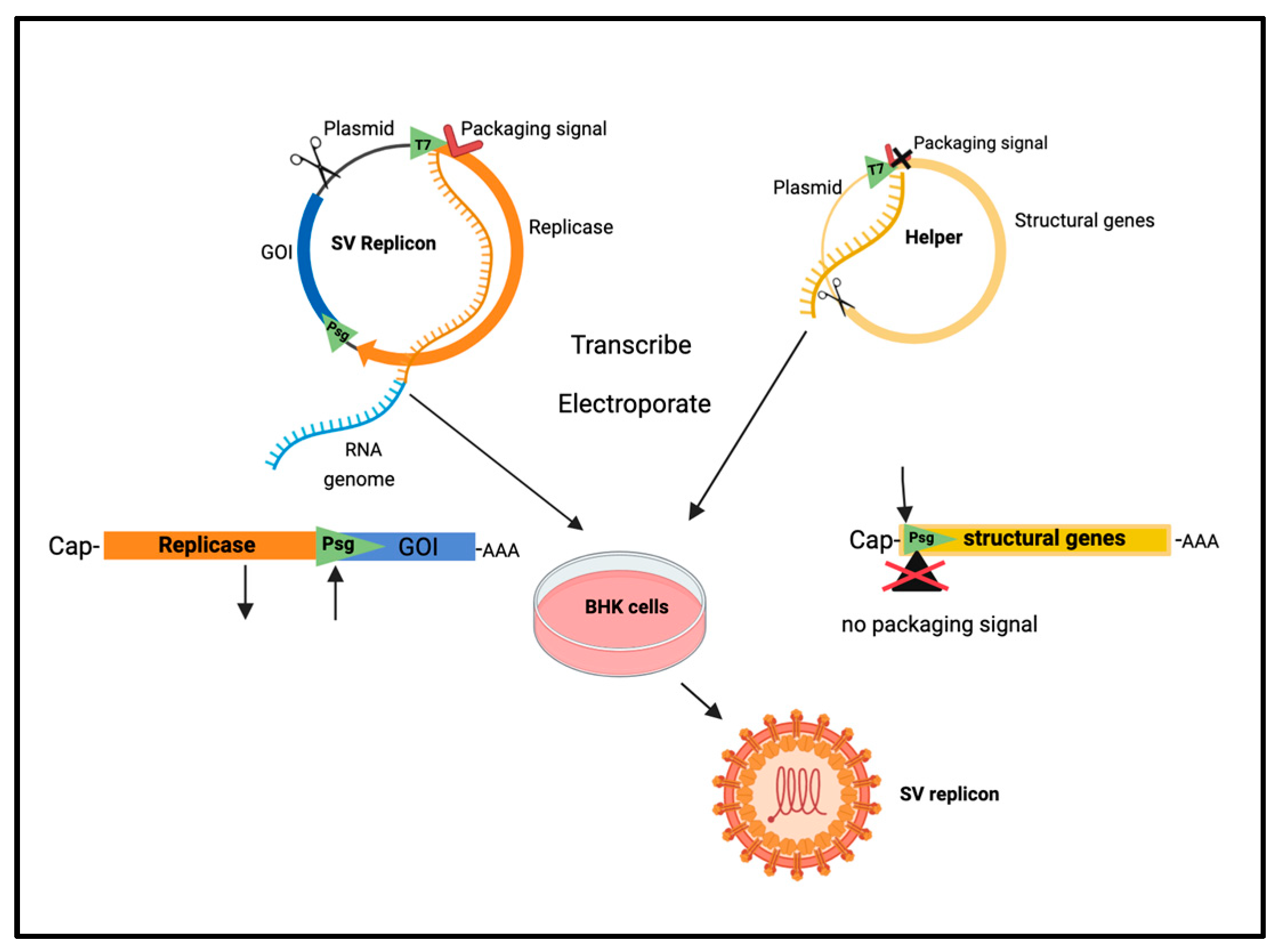
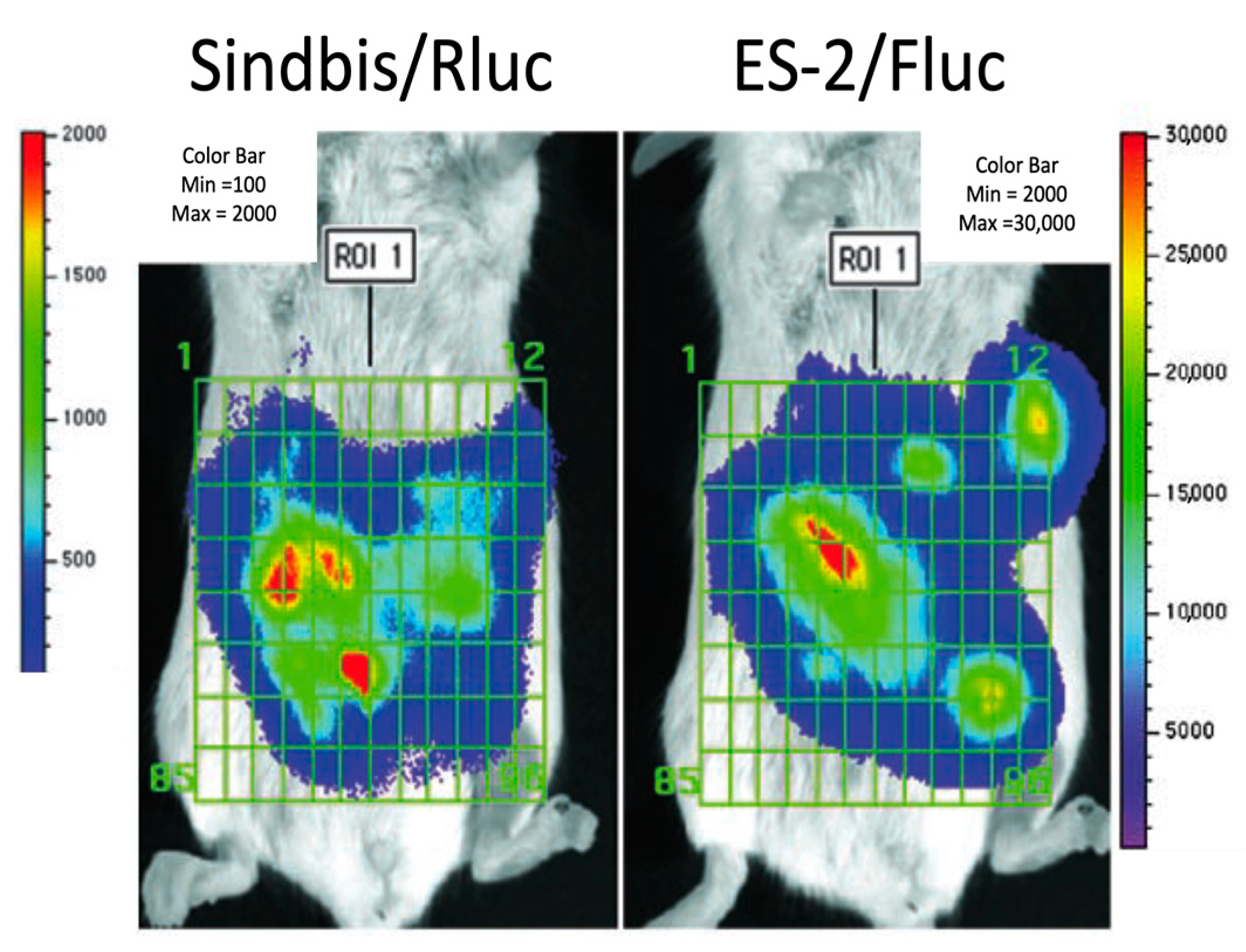
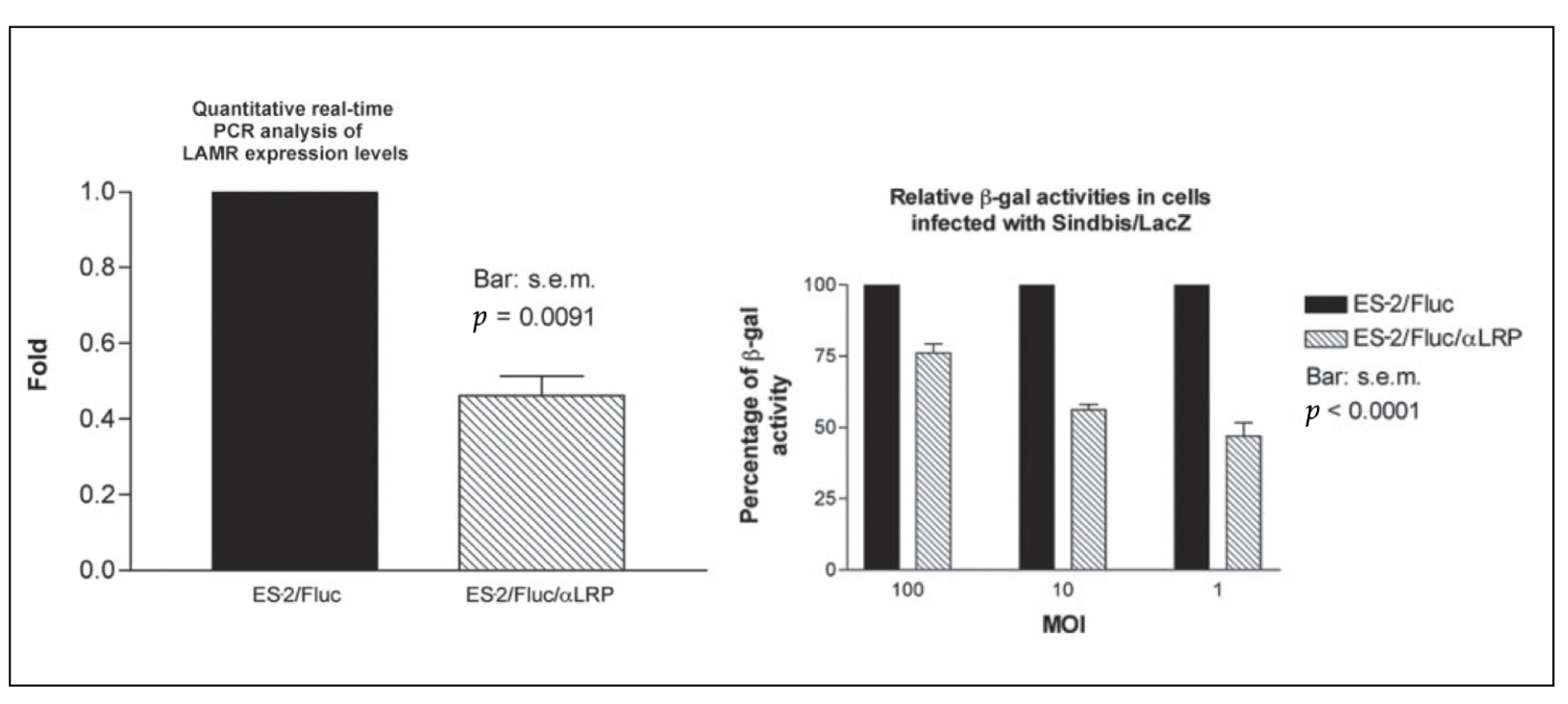
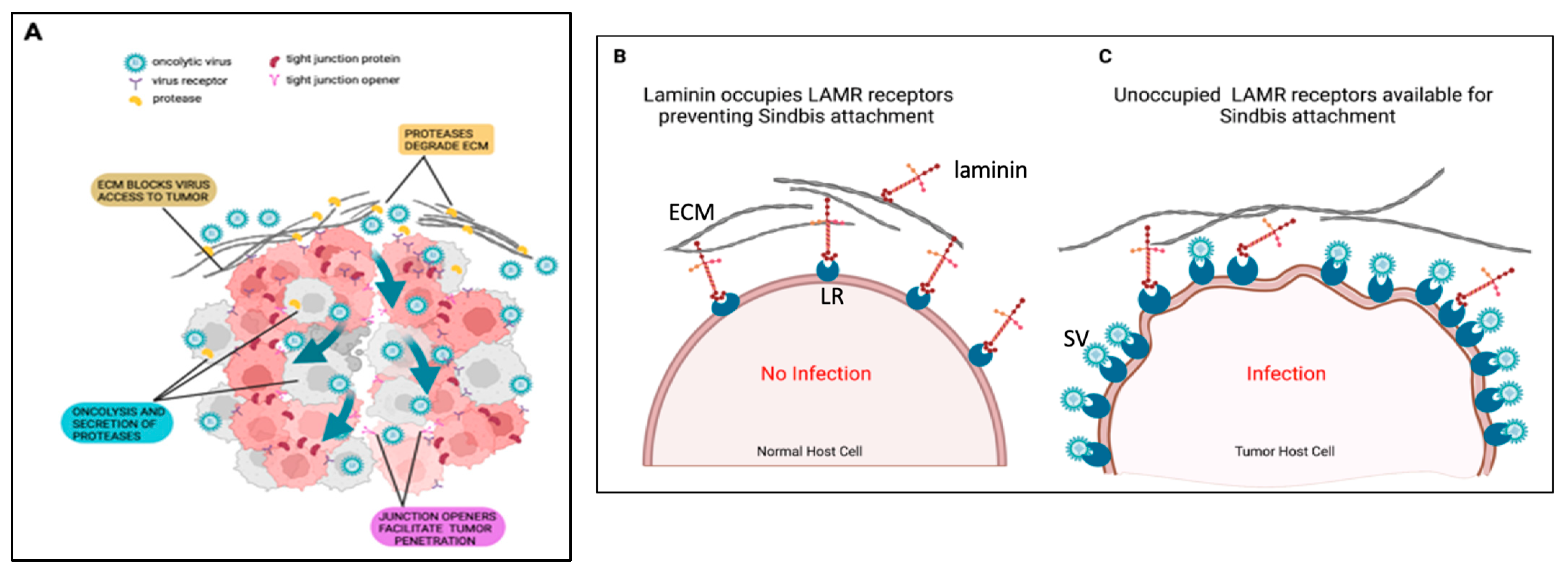

| Vector/Agent | Delivery | Cancer | Immune Response | Clinical Outcome | |
|---|---|---|---|---|---|
| SV-NY-ESO-1 + PD-1 (VPR) | i.p. | colon | T cell activation TILs | tumor growth inhibition | [3] |
| SV + 4-1BB (VRP) | i.p. | B cell Lymphoma | T cell activation | tumor growth inhibition, protection against rechallenge | [147] |
| SV-IL12 (VPR) SV-IL12 + OX40 | i.p. | colon prostate | T cell activation, metabolic reprogramming TILs | tumor elimination | [4] |
| SV-IL12 + OX40 (VPR) | i.p. | ovarian | T cell activation, metabolic reprogramming TILs | tumor elimination, protection against rechallenge | [2] |
| VEE-HPV-E6/E7 (VRP) | i.m. | cervical | CD8 T cells, IFNγ T memory cells | protection against rechallenge | [107] |
| SFV-PDL-1 (VPR) | i.t. | colon | T cells, IFNγ,TILs | >40% tumor regression | [154] |
| M1 | i.v. | bladder | Lower Ki-67 signals in tumor | tumor growth inhibition, increased survival | [157] |
| M1 + doxorubicin | i.v. | breast | ND | tumor growth inhibition | [158] |
| Vector/Agent | Delivery | Cancer | Immune Response | Clinical Outcome | |
|---|---|---|---|---|---|
| Phase I VEE(VPR)-CEA | i.m. | Stage III colon | T cells, IFNγ CEA | Improved OS in IFNγ (+) patients | [125] |
| Phase I Vvax001 SFV-E6/E7 | i.m. | cervical | T cells, IFNγ Th1 response | ND | [159] |
| Phase I LV305 LV-NY-ESO-1 (SV PV) | i.d. | metastatic NY-ESO-1 (+) tumor | T cell response in 85% patients | tumor growth inhibition improved OS | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pampeno, C.; Hurtado, A.; Opp, S.; Meruelo, D. Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy. Int. J. Mol. Sci. 2023, 24, 14948. https://doi.org/10.3390/ijms241914948
Pampeno C, Hurtado A, Opp S, Meruelo D. Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy. International Journal of Molecular Sciences. 2023; 24(19):14948. https://doi.org/10.3390/ijms241914948
Chicago/Turabian StylePampeno, Christine, Alicia Hurtado, Silvana Opp, and Daniel Meruelo. 2023. "Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy" International Journal of Molecular Sciences 24, no. 19: 14948. https://doi.org/10.3390/ijms241914948
APA StylePampeno, C., Hurtado, A., Opp, S., & Meruelo, D. (2023). Channeling the Natural Properties of Sindbis Alphavirus for Targeted Tumor Therapy. International Journal of Molecular Sciences, 24(19), 14948. https://doi.org/10.3390/ijms241914948





