Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. Characteristics of Donors
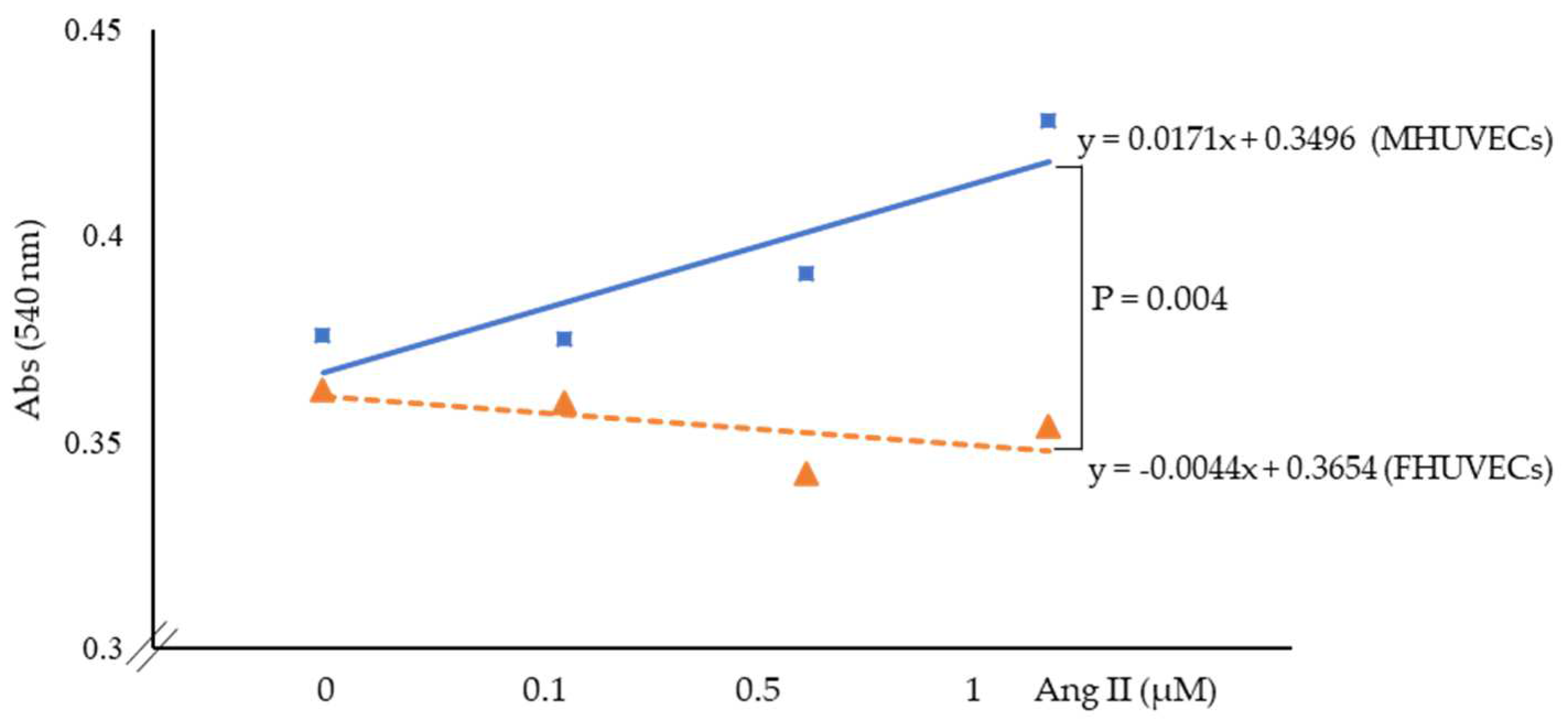
2.2. LDH Release and MDA Levels
2.3. Viability
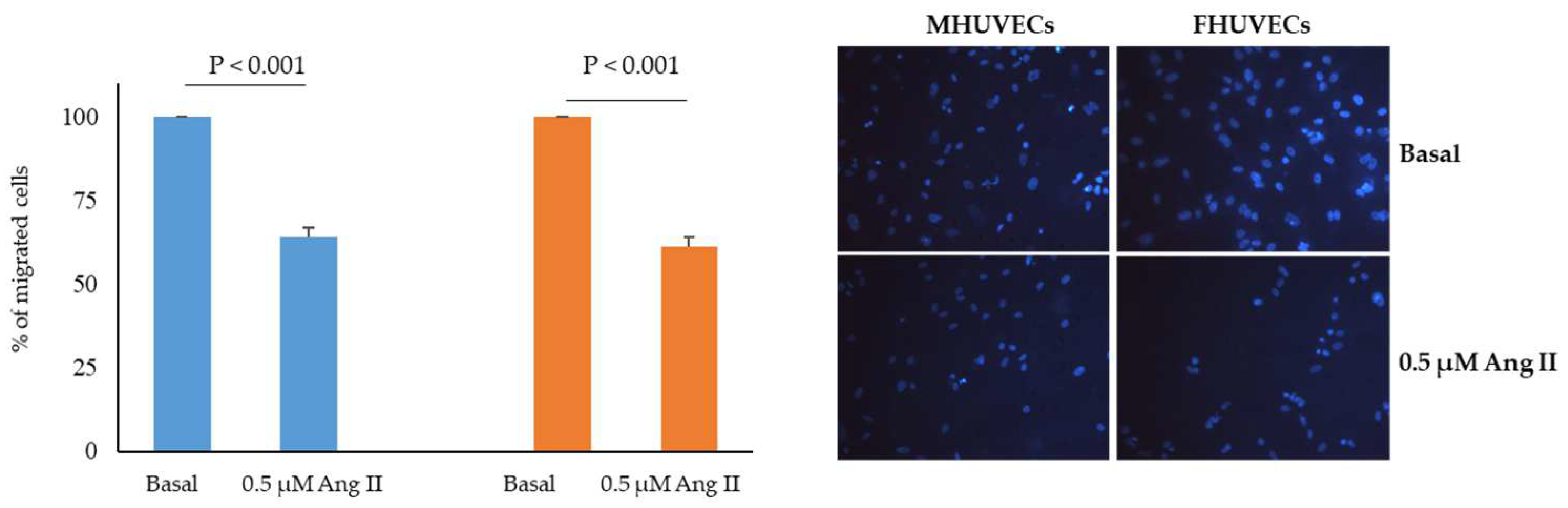
2.4. Effect of Ang II on HUVECs Migration
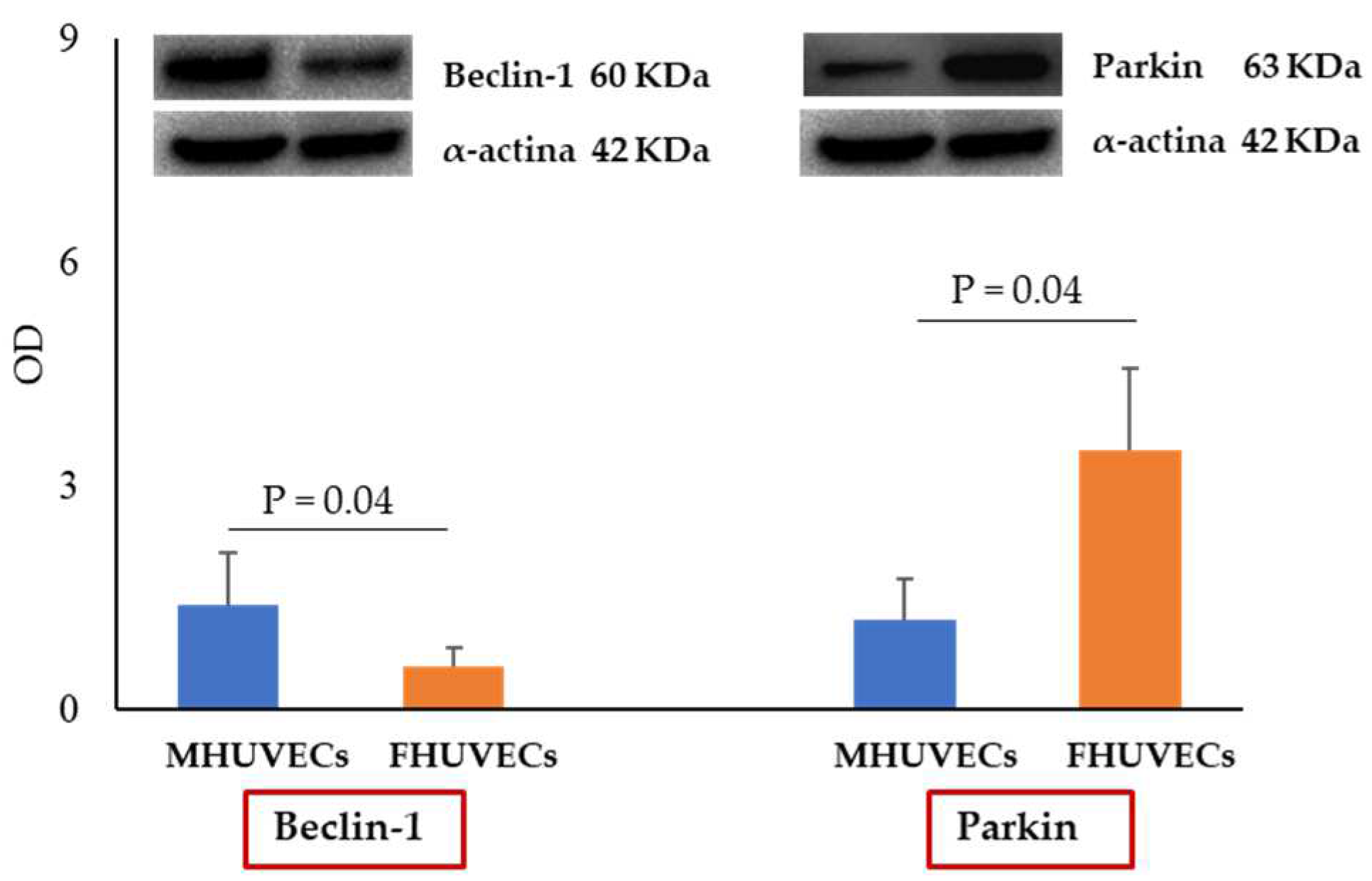
2.5. Effect of Ang II on Macroautophagy and Mitophagy
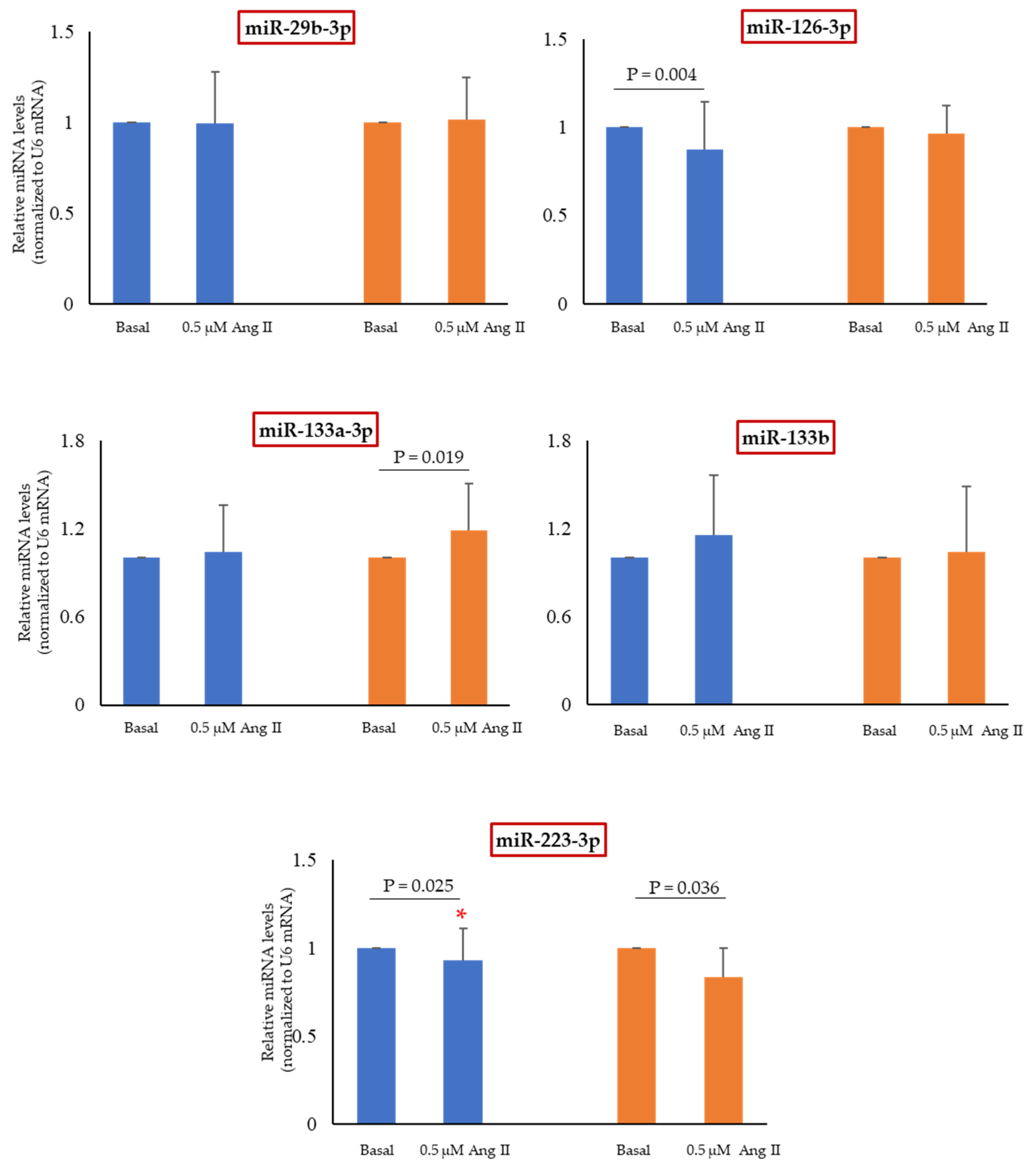
2.6. Effect of Ang II on miRNAs
3. Discussion
4. Materials and Methods
4.1. Donors
4.2. Experimental Model
4.3. Cell Isolation and Characterization
4.4. Cell Viability
4.5. LDH Assay
4.6. Migration Assay
4.7. MDA Determination
4.8. Western Blotting
4.9. RNA Isolation, Reverse Transcription (RT)-Quantitative (q)PCR Analysis
4.10. RNA Isolation, miRNAs Reverse Transcription, and Quantitative (q)PCR Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Nwia, S.M.; Leite, A.P.O.; Li, X.C.; Zhuo, J.L. Sex Differences in the Renin-Angiotensin-Aldosterone System and Its Roles in Hypertension, Cardiovascular, and Kidney Diseases. Front. Cardiovasc. Med. 2023, 10, 1198090. [Google Scholar] [CrossRef]
- Campesi, I.; Occhioni, S.; Tonolo, G.; Cherchi, S.; Basili, S.; Carru, C.; Zinellu, A.; Franconi, F. Ageing/Menopausal Status in Healthy Women and Ageing in Healthy Men Differently Affect Cardiometabolic Parameters. Int. J. Med. Sci. 2016, 13, 124–132. [Google Scholar] [CrossRef]
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-Gender-Related Therapeutic Approaches for Cardiovascular Complications Associated with Diabetes. Pharmacol. Res. 2017, 119, 195–207. [Google Scholar] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial Dysfunction and Cardiovascular Disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary Microvascular Dysfunction and Future Risk of Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Noel Bairey Merz, C.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E. Female Cardiovascular Biology and Resilience in the Setting of Physiological and Pathological Stress. Redox Biol. 2023, 63, 102747. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial Dysfunction and Vascular Disease—A 30th Anniversary Update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Franconi, F.; Rosano, G.; Basili, S.; Montella, A.; Campesi, I. Human Cells Involved in Atherosclerosis Have a Sex. Int. J. Cardiol. 2017, 228, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Modulation of Endothelial Function by TMAO, a Gut Microbiota-Derived Metabolite. Int. J. Mol. Sci. 2023, 24, 5806. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Montella, A.; Sotgiu, G.; Dore, S.; Carru, C.; Zinellu, A.; Palermo, M.; Franconi, F. Combined Oral Contraceptives Modify the Effect of Smoking on Inflammatory Cellular Indexes and Endothelial Function in Healthy Subjects. Eur. J. Pharmacol. 2021, 891, 173762. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Araldi, E.; Suárez, Y. MicroRNAs as Pharmacological Targets in Endothelial Cell Function and Dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef]
- Weiss, D.; Kools, J.J.; Taylor, W.R. Angiotensin II-Induced Hypertension Accelerates the Development of Atherosclerosis in ApoE-Deficient Mice. Circulation 2001, 103, 448–454. [Google Scholar] [CrossRef]
- Nickenig, G. Central Role of the AT(1)-Receptor in Atherosclerosis. J. Hum. Hypertens. 2002, 16 (Suppl. S3), S26–S33. [Google Scholar] [CrossRef]
- Satoh, C.; Fukuda, N.; Hu, W.Y.; Nakayama, M.; Kishioka, H.; Kanmatsuse, K. Role of Endogenous Angiotensin II in the Increased Expression of Growth Factors in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. J. Cardiovasc. Pharmacol. 2001, 37, 108–118. [Google Scholar] [CrossRef]
- Bardhan, P.; Yang, T. Sexual Dimorphic Interplays between Gut Microbiota and Antihypertensive Drugs. Curr. Hypertens. Rep. 2023, 25, 163–172. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Denton, K.M. Sex-Specific Differences in Hypertension and Associated Cardiovascular Disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.; Mehay, D.; Arnold, A.C. Sex Differences in Cardiovascular Actions of the Renin-Angiotensin System. Clin. Auton. Res. 2020, 30, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Wang, L.; Klionsky, D.J.; Cheng, H.; Zhou, R. Sex Differences in Autophagy-Mediated Diseases: Toward Precision Medicine. Autophagy 2021, 17, 1065–1076. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, J.; Ran, Z. Emerging Views of Mitophagy in Immunity and Autoimmune Diseases. Autophagy 2020, 16, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human Umbilical Endothelial Cells (HUVECs) Have a Sex: Characterisation of the Phenotype of Male and Female Cells. Biol. Sex Differ. 2014, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Occhioni, S.; Capobianco, G.; Montella, A.; Dessole, S.; Franconi, F. Sex-Specific Pharmacological Modulation of Autophagic Process in Human Umbilical Artery Smooth Muscle Cells. Pharmacol. Res. 2016, 113, 166–174. [Google Scholar] [CrossRef]
- Lista, P.; Straface, E.; Brunelleschi, S.; Franconi, F.; Malorni, W. On the Role of Autophagy in Human Diseases: A Gender Perspective. J. Cell. Mol. Med. 2011, 15, 1443–1457. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, A.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial Autophagy: Molecular Mechanisms and Implications for Cardiovascular Disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, K.K. Sex-Specific Differences in Mitochondrial Function and Its Role in Health Disparities. In Principles of Gender-Specific Medicine: Sex and Gender-Specific Biology in the Postgenomic Era; Academic Press: Cambridge, MA, USA, 2023; pp. 129–144. [Google Scholar]
- Um, J.H.; Yun, J. Emerging Role of Mitophagy in Human Diseases and Physiology. BMB Rep. 2017, 50, 299–307. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Wu, W.K.; Kirichenko, T.V.; Orekhov, A.N. Autophagy and Mitophagy as Essential Components of Atherosclerosis. Cells 2021, 10, 443. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C. MicroRNAs: An Emerging Player in Autophagy. ScienceOpen Res. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Klinge, C.M. MiRNAs Regulated by Estrogens, Tamoxifen, and Endocrine Disruptors and Their Downstream Gene Targets. Mol. Cell Endocrinol. 2015, 418, 273. [Google Scholar] [CrossRef] [PubMed]
- Mughal, W.; Nguyen, L.; Pustylnik, S.; Da Silva Rosa, S.C.; Piotrowski, S.; Chapman, D.; Du, M.; Alli, N.S.; Grigull, J.; Halayko, A.J.; et al. A Conserved MADS-Box Phosphorylation Motif Regulates Differentiation and Mitochondrial Function in Skeletal, Cardiac, and Smooth Muscle Cells. Cell Death Dis. 2015, 6, e1944. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Gulfem Ozturk, D.; Kocak, M. Autophagy-Regulating MicroRNAs and Cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef]
- Caria, A.C.I.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; de Freitas Souza, B.S. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Watanabe, K.; Narumi, T.; Watanabe, T.; Otaki, Y.; Takahashi, T.; Aono, T.; Goto, J.; Toshima, T.; Sugai, T.; Wanezaki, M.; et al. The Association between MicroRNA-21 and Hypertension-Induced Cardiac Remodeling. PLoS ONE 2020, 15, e0226053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Xu, R.; Yu, H.M.; Chang, Q.; Zhong, J.C. The ACE2/Apelin Signaling, MicroRNAs, and Hypertension. Int. J. Hypertens. 2015, 2015, 896861. [Google Scholar] [CrossRef]
- Marques, F.Z.; Campain, A.E.; Tomaszewski, M.; Zukowska-Szczechowska, E.; Yang, Y.H.J.; Charchar, F.J.; Morris, B.J. Gene Expression Profiling Reveals Renin MRNA Overexpression in Human Hypertensive Kidneys and a Role for MicroRNAs. Hypertension 2011, 58, 1093–1098. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex Differences in Endothelial Function Important to Vascular Health and Overall Cardiovascular Disease Risk across the Lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Lorenz, M.; Blaschke, B.; Benn, A.; Hammer, E.; Witt, E.; Kirwan, J.; Fritsche-Guenther, R.; Gloaguen, Y.; Bartsch, C.; Vietzke, A.; et al. Sex-Specific Metabolic and Functional Differences in Human Umbilical Vein Endothelial Cells from Twin Pairs. Atherosclerosis 2019, 291, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lingappan, K. Differential Sex-Specific Effects of Oxygen Toxicity in Human Umbilical Vein Endothelial Cells. Biochem. Biophys. Res. Commun. 2017, 486, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mudrovcic, N.; Arefin, S.; Van Craenenbroeck, A.H.; Kublickiene, K. Endothelial Maintenance in Health and Disease: Importance of Sex Differences. Pharmacol. Res. 2017, 119, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.G.; Banfi, C.; Brioschi, M.; Lattuada, D.; Vicentini, L.M. Sex-Dependent Differences in the Secretome of Human Endothelial Cells. Biol. Sex Differ. 2020, 12, 7. [Google Scholar] [CrossRef]
- Campesi, I.; Brunetti, A.; Capobianco, G.; Galistu, A.; Montella, A.; Ieri, F.; Franconi, F. Sex Differences in X-Ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine. Antioxidants 2022, 12, 77. [Google Scholar] [CrossRef]
- Cadeddu, C.; Franconi, F.; Cassisa, L.; Campesi, I.; Pepe, A.; Cugusi, L.; Maffei, S.; Gallina, S.; Sciomer, S.; Mercuro, G. Arterial Hypertension in the Female World: Pathophysiology and Therapy. J. Cardiovasc. Med. 2016, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ro, S.H.; Jang, Y.; Bae, J.; Kim, I.M.; Schaecher, C.; Shomo, Z.D. Autophagy in Adipocyte Browning: Emerging Drug Target for Intervention in Obesity. Front. Physiol. 2019, 10, 22. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Role of Autophagy in Aging: The Good, the Bad, and the Ugly. Aging Cell 2023, 22, e13753. [Google Scholar] [CrossRef]
- Cui, J.; Zhuang, S.; Qi, S.; Li, L.; Zhou, J.; Zhang, W.; Zhao, Y.; Qi, N.; Yin, Y.; Huang, L. Hydrogen Sulfide Facilities Production of Nitric Oxide via the Akt/Endothelial Nitric Oxide Synthases Signaling Pathway to Protect Human Umbilical Vein Endothelial Cells from Injury by Angiotensin II. Mol. Med. Rep. 2017, 16, 6255–6261. [Google Scholar] [CrossRef]
- Hu, H.J.; Jiang, Z.S.; Qiu, J.; Zhou, S.H.; Liu, Q.M. Protective Effects of Hydrogen Sulfide against Angiotensin II-Induced Endoplasmic Reticulum Stress in HUVECs. Mol. Med. Rep. 2017, 15, 2213–2222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, S.; Pan, N.; Xu, M.; Su, Y.; Qiao, K.; Chen, B.; Zheng, B.; Xiao, M.; Liu, Z. ACE Inhibitory Peptide from Skin Collagen Hydrolysate of Takifugu Bimaculatus as Potential for Protecting HUVECs Injury. Mar. Drugs 2021, 19, 655. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, J.; Wang, Q.; Yin, X.; Sun, Z.; Huang, C.; Chen, G.; Zheng, L.; Jiang, D. Ibandronate Promotes Autophagy by Inhibiting Rac1-MTOR Signaling Pathway in Vitro and in Vivo. Cell Death Discov. 2022, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.J.; Jiang, Z.S.; Zhou, S.H.; Liu, Q.M. Hydrogen Sulfide Suppresses Angiotensin II-Stimulated Endothelin-1 Generation and Subsequent Cytotoxicity-Induced Endoplasmic Reticulum Stress in Endothelial Cells via NF-ΚB. Mol. Med. Rep. 2016, 14, 4729–4740. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Su, P.; Li, Y. FGFR2 Modulates the Akt/Nrf2/ARE Signaling Pathway to Improve Angiotensin II-Induced Hypertension-Related Endothelial Dysfunction. Clin. Exp. Hypertens. 2023, 45, 2208777. [Google Scholar] [CrossRef]
- Du, J.; Leng, J.; Zhang, L.; Bai, G.; Yang, D.; Lin, H.; Qin, J. Angiotensin II-Induced Apoptosis of Human Umbilical Vein Endothelial Cells Was Inhibited by Blueberry Anthocyanin Through Bax- and Caspase 3-Dependent Pathways. Med. Sci. Monit. 2016, 22, 3223–3228. [Google Scholar] [CrossRef]
- Li, D.X.; Chen, W.; Jiang, Y.L.; Ni, J.Q.; Lu, L. Antioxidant Protein Peroxiredoxin 6 Suppresses the Vascular Inflammation, Oxidative Stress and Endothelial Dysfunction in Angiotensin II-Induced Endotheliocyte. Gen. Physiol. Biophys. 2020, 39, 545–555. [Google Scholar] [CrossRef]
- Li, M.; Liu, X. Pitavastatin Maintains MAPK7 Expression and Alleviates Angiotensin II-Induced Vascular Endothelial Cell Inflammation and Injury. Exp. Ther. Med. 2022, 23, 11055. [Google Scholar] [CrossRef]
- Song, J.; Huang, S.; Wang, K.; Li, W.; Pao, L.; Chen, F.; Zhao, X. Long Non-Coding RNA MEG3 Attenuates the Angiotensin II-Induced Injury of Human Umbilical Vein Endothelial Cells by Interacting With P53. Front. Genet. 2019, 10, 78. [Google Scholar] [CrossRef]
- Wang, Q.; Lao, M.; Xu, Z.; Ding, M.; Guo, S.; Li, L. Caveolin-1 Modulates Hypertensive Vascular Remodeling via Regulation of the Notch Pathway. Mol. Med. Rep. 2020, 22, 4320–4328. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, J.; Liu, B. Bazedoxifene Activates the Angiotensin II-Induced HUVEC Hypertension Model by Targeting SIRT1. Exp. Ther. Med. 2022, 23, 11043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Jiang, L. Sulforaphane Attenuates Angiotensin II-Induced Human Umbilical Vein Endothelial Cell Injury by Modulating ROS-Mediated Mitochondrial Signaling. Hum. Exp. Toxicol. 2020, 39, 734–747. [Google Scholar] [CrossRef]
- Long Non-Coding RNA-ATB Attenuates the Angiotensin II-Induced Injury of Vascular Endothelial Cell. Available online: https://www.researchgate.net/publication/342477757_Long_Non-Coding_RNA-ATB_Attenuates_the_Angiotensin_II-Induced_Injury_of_Vascular_Endothelial_Cell (accessed on 2 October 2023).
- Li, M.; Liu, X.; He, Y.; Zheng, Q.; Wang, M.; Wu, Y.; Zhang, Y.; Wang, C. Celastrol Attenuates Angiotensin II Mediated Human Umbilical Vein Endothelial Cells Damage through Activation of Nrf2/ERK1/2/Nox2 Signal Pathway. Eur. J. Pharmacol. 2017, 797, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Sun, Y.; Lu, J. Knockdown of Long Noncoding RNA (LncRNA) AK094457 Relieved Angiotensin II Induced Vascular Endothelial Cell Injury. Med. Sci. Monit. 2020, 26, e919854-1. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic Control of Mitochondrial Function and Biogenesis. J. Cell Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef]
- Charoensin, S.; Eroglu, E.; Opelt, M.; Bischof, H.; Madreiter-Sokolowski, C.T.; Kirsch, A.; Depaoli, M.R.; Frank, S.; Schrammel, A.; Mayer, B.; et al. Intact Mitochondrial Ca2+ Uniport Is Essential for Agonist-Induced Activation of Endothelial Nitric Oxide Synthase (ENOS). Free Radic. Biol. Med. 2017, 102, 248–259. [Google Scholar] [CrossRef]
- Straface, E.; Vona, R.; Campesi, I.; Franconi, F. Mitochondria Can Orchestrate Sex Differences in Cell Fate of Vascular Smooth Muscle Cells from Rats. Biol. Sex Differ. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, H.; Guo, D.; Li, X.; Zhao, X.; Li, W.; Bai, X. From Autophagy to Senescence and Apoptosis in Angiotensin II-Treated Vascular Endothelial Cells. APMIS 2014, 122, 985–992. [Google Scholar] [CrossRef]
- Choubey, V.; Cagalinec, M.; Liiv, J.; Safiulina, D.; Hickey, M.A.; Kuum, M.; Liiv, M.; Anwar, T.; Eskelinen, E.L.; Kaasik, A. BECN1 Is Involved in the Initiation of Mitophagy: It Facilitates PARK2 Translocation to Mitochondria. Autophagy 2014, 10, 1105–1119. [Google Scholar] [CrossRef]
- Huang, J.; Huang, C.; Luo, Y.; Liu, S.; Chen, X. Role of MiR-30a in Cardiomyocyte Autophagy Induced by Angiotensin II. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1–5. [Google Scholar] [CrossRef]
- Seong, S.B.; Ha, D.S.; Min, S.Y.; Ha, T.S. Autophagy Precedes Apoptosis in Angiotensin II-Induced Podocyte Injury. Cell Physiol. Biochem. 2019, 53, 747–759. [Google Scholar] [CrossRef]
- Gallagher, E.R.; Holzbaur, E.L.F. The Selective Autophagy Adaptor P62/SQSTM1 Forms Phase Condensates Regulated by HSP27 That Facilitate the Clearance of Damaged Lysosomes via Lysophagy. Cell Rep. 2023, 42, 112037. [Google Scholar] [CrossRef]
- Peng, S.Z.; Chen, X.H.; Chen, S.J.; Zhang, J.; Wang, C.Y.; Liu, W.R.; Zhang, D.; Su, Y.; Zhang, X.K. Phase Separation of Nur77 Mediates Celastrol-Induced Mitophagy by Promoting the Liquidity of P62/SQSTM1 Condensates. Nat. Commun. 2021, 12, 5989. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aicha, S.; Caporali, A.; Srivastava, P.; Emanueli, C. Role of MicroRNAs in Vascular Remodeling and Repair. In MicroRNA in Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2023; pp. 453–488. [Google Scholar]
- Jansen, F.; Stumpf, T.; Proebsting, S.; Franklin, B.S.; Wenzel, D.; Pfeifer, P.; Flender, A.; Schmitz, T.; Yang, X.; Fleischmann, B.K.; et al. Intercellular Transfer of MiR-126-3p by Endothelial Microparticles Reduces Vascular Smooth Muscle Cell Proliferation and Limits Neointima Formation by Inhibiting LRP6. J. Mol. Cell. Cardiol. 2017, 104, 43–52. [Google Scholar] [CrossRef]
- Ahmed, S.; Kurusamy, S.; David, E.L.S.; Khan, K.; Kalyanakrishnan, K.; Ian-Gobo, M.; Kola, T.M.; Wilkinson, R.N.; Kannappan, V.; Wang, W.; et al. Aberrant Expression of MiR-133a in Endothelial Cells Inhibits Angiogenesis by Reducing pro-Angiogenic but Increasing Anti-Angiogenic Gene Expression. Sci. Rep. 2022, 12, 14730. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Ortega, A.; Flores-Chova, A.; Sanchez-Garcia, B.; Garcia-Garcia, A.B.; Chaves, F.J.; Martin-Escudero, J.C.; Forner, M.J.; Redon, J.; Cortes, R. High MiR-126-3p Levels Associated with Cardiovascular Events in a General Population. Eur. J. Intern. Med. 2023, 113, 49–56. [Google Scholar] [CrossRef]
- Liu, B.; Lan, M.; Wei, H.; Zhang, D.; Liu, J.; Teng, J. Downregulated MicroRNA-133a Induces HUVECs Injury: Potential Role of the (pro) Renin Receptor in Angiotensin II-dependent Hypertension. Mol. Med. Rep. 2019, 20, 2796–2804. [Google Scholar] [CrossRef]
- Shi, L.; Fisslthaler, B.; Zippel, N.; Frömel, T.; Hu, J.; Elgheznawy, A.; Heide, H.; Popp, R.; Fleming, I. MicroRNA-223 Antagonizes Angiogenesis by Targeting Β1 Integrin and Preventing Growth Factor Signaling in Endothelial Cells. Circ. Res. 2013, 113, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, F.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. MiR-223: An Inflammatory OncomiR Enters the Cardiovascular Field. Biochim. Biophys. Acta 2014, 1842, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ceinos, J.; Rangel-Zuñiga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcalá-Diaz, J.F.; Guzmán-Ruiz, R.; López-Miranda, J.; Malagón, M.M. MiR-223-3p as a Potential Biomarker and Player for Adipose Tissue Dysfunction Preceding Type 2 Diabetes Onset. Mol. Ther. Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef]
- Hasegawa, T.; Lewis, H.; Esquela-Kerscher, A. The Role of Noncoding RNAs in Prostate Cancer. In Translating MicroRNAs to the Clinic; Academic Press: Cambridge, MA, USA, 2017; pp. 329–369. [Google Scholar]
- Tang, T.T.; Wang, B.Q. Inhibition of MicroRNA-346 Inhibits Myocardial Inflammation and Apoptosis after Myocardial Infarction via Targeting NFIB. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11747–11751. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.G.; Chen, B.Y.; Sun, R.H.; Mou, X.Z.; Han, F.; Li, Q.; Huang, H.J.; Liu, J.Q.; Tu, Y.X. MiR-133b Downregulation Reduces Vulnerable Plaque Formation in Mice with AS through Inhibiting Macrophage Immune Responses. Mol. Ther. Nucleic Acids 2019, 16, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Di Nicola, P.; Varalda, A.; Occhi, L.; Giuliani, F.; Coscia, A. Neonatal Growth Charts. J. Matern. Fetal Neonatal Med. 2012, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Elengoe, A.; Hamdan, S. Evaluation of Hyperthermia Effect on Cell Viability Using Crystal Violet Staining, LDH and Trypan Blue Assays. Adv. Environ. Biol. 2014, 8, 744–747. [Google Scholar]
- Campesi, I.; Straface, E.; Occhioni, S.; Montella, A.; Franconi, F. Protein Oxidation Seems to Be Linked to Constitutive Autophagy: A Sex Study. Life Sci. 2013, 93, 145–152. [Google Scholar] [CrossRef]


| Male Neonates (n = 14) | Female Neonates (n = 13) | p | |
|---|---|---|---|
| Age of mothers (years) | 34.5 ± 4.7 | 35.1± 4.1 | NS |
| Body weight of mothers (start) (kg) | 56.6 ± 9.4 | 56.4 ± 6.4 | NS |
| Body weight of mothers (end) (kg) | 66.0 ± 10.2 | 68.4 ± 7.7 | NS |
| Body mass index (start) (kg/m2) | 21.4 ± 3.5 | 21.4 ± 2.1 | NS |
| Body mass index (end) (kg/m2) | 24.9 ± 4.3 | 26.0 ± 2.6 | NS |
| Gestational age (weeks) | 38.7 ± 1.5 | 38.7 ± 1.7 | NS |
| Weight of newborns (kg) | 3.3 ± 0.5 | 3.2 ± 0.4 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franconi, F.; Capobianco, G.; Diana, G.; Lodde, V.; De Donno, A.; Idda, M.L.; Montella, A.; Campesi, I. Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 14929. https://doi.org/10.3390/ijms241914929
Franconi F, Capobianco G, Diana G, Lodde V, De Donno A, Idda ML, Montella A, Campesi I. Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(19):14929. https://doi.org/10.3390/ijms241914929
Chicago/Turabian StyleFranconi, Flavia, Giampiero Capobianco, Giuseppe Diana, Valeria Lodde, Alberto De Donno, Maria Laura Idda, Andrea Montella, and Ilaria Campesi. 2023. "Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells" International Journal of Molecular Sciences 24, no. 19: 14929. https://doi.org/10.3390/ijms241914929
APA StyleFranconi, F., Capobianco, G., Diana, G., Lodde, V., De Donno, A., Idda, M. L., Montella, A., & Campesi, I. (2023). Sex Influence on Autophagy Markers and miRNAs in Basal and Angiotensin II-Treated Human Umbilical Vein Endothelial Cells. International Journal of Molecular Sciences, 24(19), 14929. https://doi.org/10.3390/ijms241914929









