Regenerative Therapies for Basal Thumb Arthritis—A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
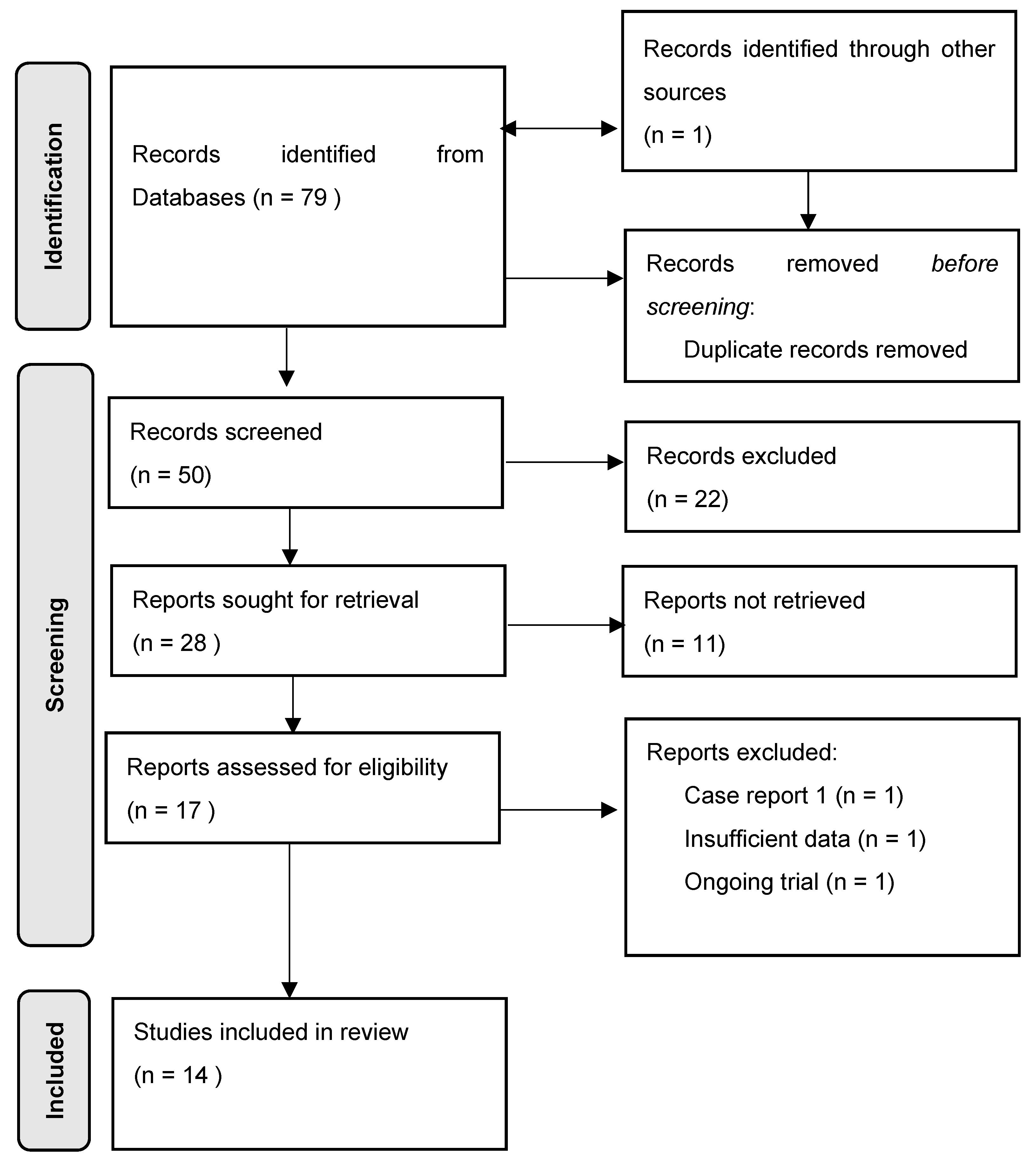
3. Results
3.1. Outcome
3.1.1. Fat Grafting
3.1.2. PRP
3.1.3. PRP and Fat Grafting
3.1.4. Low-Level Laser Treatment
3.1.5. Autologous Chondrocyte Transplantation
3.2. Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonetti, M.A.; Rovere, G.; Fulchignoni, C.; De Santis, V.; Ziranu, A.; Maccauro, G.; Pataia, E. Autologous fat transplantation for the treatment of trapeziometacarpal join osteoarthritis. Orthop. Rev. 2020, 12, 58–60. [Google Scholar] [CrossRef]
- Winter, R.; Hasiba-Pappas, S.K.; Tuca, A.C.; Zrim, R.; Nischwitz, S.; Popp, D.; Lumenta, D.B.; Girsch, W.; Kamolz, L.P. Autologous Fat and Platelet-Rich Plasma Injections in Trapeziometacarpal Osteoarthritis: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2023, 151, 119–131. [Google Scholar] [CrossRef]
- Barron, O.A.; Glickel, S.Z.; Eaton, R.G. Basal joint arthritis of the thumb. JAAOS—J. Am. Acad. Orthop. Surg. 2000, 8, 314–323. [Google Scholar] [CrossRef]
- Kim, J.R.; Yoo, J.J.; Kim, H.A. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Bühler, M.; Chapple, C.M.; Stebbings, S.; Pōtiki-Bryant, K.; David Baxter, G. Impact of Thumb Carpometacarpal Joint Osteoarthritis: A Pragmatic Qualitative Study. Arthritis Care Res. 2021, 73, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Raven, E.E.J.; Kerkhoffs, G.M.M.J.; Rutten, S.; Marsman, A.J.W.; Marti, R.K.; Albers, G.H.R. Long term results of surgical intervention for osteoarthritis of the trapeziometacarpal joint: Comparison of resection arthroplasty, trapeziectomy with tendon interposition and trapezio-metacarpal arthrodesis. Int. Orthop. 2007, 31, 547–554. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- An, C.; Cheng, Y.; Yuan, Q.; Li, J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann. Biomed. Eng. 2010, 38, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; De Munter, W.; Grevers, L.C.; Jeanson, J.; Noël, D.; Casteilla, L.; Jorgensen, C.; et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Payne, R.; Rizk, E.; Koduru, S.V.; Hazard, S.W. Adipose-derived stem cells in peripheral nerve regeneration. Curr. Surg. Rep. 2017, 5, 5. [Google Scholar] [CrossRef]
- Smith, O.J.; Jell, G.; Mosahebi, A. The use of fat grafting and platelet-rich plasma for wound healing: A review of the current evidence. Int. Wound J. 2019, 16, 275–285. [Google Scholar] [CrossRef]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic potential of multipotential cells from human adipose tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Rennekampff, H.O.; Groddeck, R.; Allert, S. Autologous fat transfer for thumb carpometacarpal joint osteoarthritis: A prospective study. Plast. Reconstr. Surg. 2017, 140, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Tajiri, T.; Ninomiya, Y.; Awazu, K. Chondrogenic mRNA expression in prechondrogenic cells after blue laser irradiation. J. Photochem. Photobiol. B Biol. 2010, 98, 211–215. [Google Scholar] [CrossRef]
- Soriano, F.; Campana, V.; Moya, M.; Gavotto, A.; Simes, J.; Soriano, M.; Soriano, R.; Spitale, L.; Palma, J. Photobiomodulation of pain and inflammation in microcrystalline arthropathies: Experimental and clinical results. Photomed. Laser Surg. 2006, 24, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Stausholm, M.B.; Naterstad, I.F.; Joensen, J.; Lopes-Martins, R.Á.B.; Sæbø, H.; Lund, H.; Fersum, K.V.; Bjordal, J.M. Efficacy of low-level laser therapy on pain and disability in knee osteoarthritis: Systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 2019, 9, e031142. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys. Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef]
- Erne, H.C.; Cerny, M.K.; Ehrl, D.; Bauer, A.T.; Schmauss, V.; Moog, P.; Broer, P.N.; Loew, S.; Schmauss, D. Autologous Fat Injection versus Lundborg Resection Arthroplasty for the Treatment of Trapeziometacarpal Joint Osteoarthritis. Plast. Reconstr. Surg. 2018, 141, 119–124. [Google Scholar] [CrossRef]
- Froschauer, S.M.; Holzbauer, M.; Wenny, R.; Schmidt, M.; Huemer, G.M.; Kwasny, O.; Duscher, D. Autologous fat transplantation for thumb carpometacarpal joint osteoarthritis (Liparthroplasty): A case series with two years of follow-up. J. Clin. Med. 2021, 10, 113. [Google Scholar] [CrossRef]
- Haas, E.M.; Volkmer, E.; Giunta, R.E.; Haas, E.M. Pilotstudie über die Wirkung und den Nutzen von autologen Fettgewebstransplantaten bei Rhizarthrose verglichen mit einer Kortisoninjektion—3 Monatsergebnisse Pilot study on the effects and benefits of autologous fat grafting in osteoarthritis of the CMC. Handchir. Mikrochir. Plast. Chir. 2017, 49, 288–296. [Google Scholar]
- Haas, E.M.; Eisele, A.; Arnoldi, A.; Paolini, M.; Ehrl, D.; Volkmer, E.; Giunta, R.E. One-Year Outcomes of Intraarticular Fat Transplantation for Thumb Carpometacarpal Joint Osteoarthritis: Case Review of 99 Joints. Plast. Reconstr. Surg. 2020, 145, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Fleischer, O.; Allert, S. Eigenfettinjektion in das Sattelgelenk zur Behandlung der Rhizarthrose—Eine viel versprechende Therapieoption. Handchir. Mikrochir. Plast. Chir. 2014, 46, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, M.V.; Batsilas, I.; Fischer, H.; Dahmann, S.; Happe, C.; Herold, C. A prospective Study about medium-term Results after autologous Fat Transplantation into arthritic CMC-I-joints. Handchir. Mikrochir. Plast. Chir. 2022, 54, 38–43. [Google Scholar] [CrossRef]
- Loibl, M.; Lang, S.; Dendl, L.M.; Nerlich, M.; Angele, P.; Gehmert, S.; Huber, M. Leukocyte-Reduced Platelet-Rich Plasma Treatment of Basal Thumb Arthritis: A Pilot Study. Biomed Res. Int. 2016, 2016, 9262909. [Google Scholar] [CrossRef]
- Malahias, M.A.; Roumeliotis, L.; Nikolaou, V.S.; Chronopoulos, E.; Sourlas, I.; Babis, G.C. Platelet-Rich Plasma versus Corticosteroid Intra-Articular Injections for the Treatment of Trapeziometacarpal Arthritis: A Prospective Randomized Controlled Clinical Trial. Cartilage 2021, 12, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Swärd, E.; Wilcke, M. Effects of intra-articular Platelet-Rich Plasma (PRP) injections on osteoarthritis in the thumb basal joint and scaphoidtrapeziotrapezoidal joint. PLoS ONE 2022, 17, e0264203. [Google Scholar] [CrossRef] [PubMed]
- Abdelsabor Sabaah, H.M.; El Fattah, R.A.; Al Zifzaf, D.; Saad, H. A Comparative Study for Different Types of Thumb Base Osteoarthritis Injections: A Randomized Controlled Interventional Study. Ortop. Traumatol. Rehabil. 2020, 22, 447–454. [Google Scholar] [CrossRef]
- Winter, R.; Tuca, A.-C.; Justich, I.; Tschauner, S.; Friedl, H.; Girsch, W.; Lebo, P.; Zrim, R.; Lumenta, D.B.; Kamolz, L.-P. Minimal invasive treatment of trapeziometacarpal osteoarthritis: Results of a blinded, randomized controlled trial. Plast. Reconstr. Surg. 2023. [Google Scholar] [CrossRef]
- Brosseau, L.; Wells, G.; Marchand, S.; Gaboury, I.; Stokes, B.; Morin, M.; Casimiro, L.; Yonge, K.; Tugwell, P. Randomized controlled trial on low level laser therapy (LLLT) in the treatment of osteoarthritis (OA) of the hand. Lasers Surg. Med. 2005, 36, 210–219. [Google Scholar] [CrossRef]
- Messina, J.C.; Torretta, F.; Randelli, P.S. Autologous chondrocyte transplantation in the treatment of thumb CMC joint osteoarthritis. Hand Surg. Rehabil. 2021, 40, S21–S28. [Google Scholar] [CrossRef]
- Yeğin, E.E.; Yeğin, M.E.; Kosova, B.; Gür, E.; Nuriyev, U. Analysis of Fat Graft Survival and Platelet-Rich Plasma Effects: The Transcriptomic Differences. Cureus 2023, 15, e34380. [Google Scholar] [CrossRef] [PubMed]
- Siegel, K.R.; Clevenger, T.N.; Clegg, D.O.; Proctor, D.A.; Proctor, C.S. Adipose Stem Cells Incorporated in Fibrin Clot Modulate Expression of Growth Factors. Arthrosc.—J. Arthrosc. Relat. Surg. 2018, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, V–160. [Google Scholar] [CrossRef]
- Karimi, M.; Asefnejad, A.; Aflaki, D.; Surendar, A.; Baharifar, H.; Saber-Samandari, S.; Khandan, A.; Khan, A.; Toghraie, D. Fabrication of shapeless scaffolds reinforced with baghdadite-magnetite nanoparticles using a 3D printer and freeze-drying technique. J. Mater. Res. Technol. 2021, 14, 3070–3079. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Medina-Porqueres, I.; Cantero-Tellez, R. Class IV laser therapy for trapeziometacarpal joint osteoarthritis: Study protocol for a randomized placebo-controlled trial. Physiother. Res. Int. 2018, 23, e1706. [Google Scholar] [CrossRef]
| (1) 1 | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|
| AND | |||||
| Carpometacarpal arthritis | LLLT | ||||
| OR | OR | ||||
| Rhizarthrosis | Regenerative therapy | Low level laser treatment | Fat grafting | PRP | |
| OR | OR | OR | OR | OR | |
| Trapeziometacarpal arthritis | Regenerative medicine | Photobio-modulation | Lipofilling | Platelet-rich plasma | |
| OR | OR | OR | |||
| Basal thumb arthritis | Photo therapy | Fat injection | |||
| OR | OR | ||||
| Arthritis of the basal thumb joint | Light therapy |
| Authors | Study Type, Number of Participants | Age (Mean) | Gender | OA Stage (Eaton–Littler) | Follow-Up Period | Control Group | Technique | Injection (N, mL) |
|---|---|---|---|---|---|---|---|---|
| Fat grafting | ||||||||
| Erne et al., 2018 | Comparative Study, n = 21 | 62.1–63.3 | 17 female | III–IV | 18 months (mean) | Resection arthroplasty (12 patients) | Centrifugation | 1.3 mL |
| Froschauer et al., 2021 | Case series, n = 31 | 57.3–57.7 | 27 female | II–III | 6, 24 m | - | Decanting method, mechanical homogenization | 1 mL |
| Haas et al., 2017 | Pilot study, prospective, n = 24 | 63.3 | 17 female | I–III | 2, 6, 12 weeks | Cortisone (12) | Manual homogenization | 1–1.5 mL |
| Haas et al., 2019 | Case review, n = 99 | 61 | 69 female | I–III | 2, 6 w 3, 6, 12 m | - | Mechanical homogenization | 1–2 mL |
| Herold et al., 2014 | Pilot study, n= 5 | 64 | 5 female | II–III | 1, 3 m | - | Coleman technique, centrifugation | 1.5 mL |
| Herold et al., 2017 | Prospective study, n = 50 | 59.9 | 38 female | II–IV | 6, 12 m | - | Coleman technique, centrifugation | 1 mL |
| Meyer-Marcotty et al., 2021 | Prospective study, n = 27 | 59.8 | 22 female | I–III | Mid-term results after 45,3 m (mean) | - | Coleman technique, centrifugation | - |
| PRP + FG | ||||||||
| Winter et al., 2023 | RCT, n = 95 | 64 | 80 female | I–IV | 2 w, 1, 2, 3, 24 m | PRP (24) vs. FG (25) vs. FG + PRP (25) vs. saline (21) | Fat: harvest, put away 10 min to sediment PRP: centrifugation with Endoret kit; PRP activation | FG: 1.5 mL PRP: 1.5 mL PRP + FG: 0.75 mL each |
| PRP | ||||||||
| Loibl et al., 2016 | Pilot study, n = 10 | 56.1 | 8 female | II–IV | 3, 6 m | - | Arthrex double-syringe system, centrifugation | 1–2 mL × 2 (4 w interval) |
| Malahias et al., 2018 | RCT, n = 33 | 62.8–63 | 26 female | I–III | 3, 12 m | Cortisone (17) | 2 consecutive, manual centrifugations; non-activated PRP | 2 mL × 2 (2 w interval) |
| Sabah et al., 2020 | Comparative study, n = 45 | 52.45 | 38 | I–IV | 4, 12 w | HA (15), cortisone (15) | Double centrifugation | 1 mL × 1 |
| Swärd et al., 2022 | Retrospective study, n = 29 (21 CMC) | 63 | 17 female | I–IV | 3 m | - | Arthrex ACP double-syringe system, 1× centrifugation | 0.5–2 mL × 2 (3–4 w interval) |
| LLLT | ||||||||
| Brousseau et al., 2005 | RCT, n = 88 (3 CMC patients) | 64.2–65.1 | 69 female | - | 3, 6 w 3, 6 m | Sham LLLT (46) | GaAlAs, Eriel laser; 20 Hz modulation | 3×/w for 6 w (20 min/session) |
| Chondrocyte transplant | ||||||||
| Messina et al., 2020 | Prospective study, n = 10 | 52.4 | 10 female | II–III | 1, 3, 6 m 1, 2, 5 y Last follow-up after 8 y (mean) | - | Cartilage fragments harvested; cells grown on collagenous biphasic matrix (MACI/Novocart) | 3–4 mL of cartilage harvested; scaffold implanted once |
| Authors | Pain Preop (VAS or NRS) | Pain Postop (VAS or NRS) | (Q-)DASH Preop | (Q-)DASH Postop | Pinch and Grip Strength Preop (kg) | Pinch and Grip Strength Postop (kg) | Patient Satisfaction | Adverse Events (AEs) |
|---|---|---|---|---|---|---|---|---|
| Fat grafting | ||||||||
| Erne et al., 2018 | 6.4 | 2.9 | - | 24.0 | - | Pinch: 0.95 (bar) Grip: 33.8 (kg) (comparable between groups) | 8/10 points (satisfaction overall) | None mentioned |
| Froschauer et al., 2021 | 7.0 | 2.0 * | 59.0 | 35.0 * | - | Grip 6.5 kg (n.s.) | 68% of all patients satisfied | No complications observed |
| Haas et al., 2017 | 2.2 at rest, 6.8 under stress | 0.2 at rest *, 3.4 under stress * | 56.0 | 29.0 * | 6.3 23 | 4.8 26 | No adverse events observed | |
| Haas et al., 2019 | 2.1 at rest, 6.6. under stress | 0.8 at rest, 3.7 under stress * | - | - | 5.6 25.8 | 5.8 25.8 | 73% would undergo procedure again | 1 hematoma; 1 case of severe pain requiring analgesics |
| Herold et al., 2014 | 3.8 at rest, 7.4 under stress | 0.8 at rest, 2.4 under stress | 58.0 | 33.0 * | 0.26 0.3 | 0.31 0.42 | 100% of patients satisfied | No complications observed |
| Herold et al., 2017 | At rest: 3.5 (stage II), 7.6 (III), 3.7 (IV) Under stress: 7.7 (II), 7.6 (III), 8.9 (IV) | At rest: 1.0 (II), 1.8 (III), 3.0 (IV) Under stress: 2.4 (II) *, 5.6 (III) *, 6.0 (IV) | 47 (stage II), 50 (III), 57 (IV) | 19 (II), * 40 (III), 51 (IV) | 0.3 bar (II), 0.3 (III), 0.3 (IV) | 0.5 (II) *, 0.4 (III), 0.3 (IV) | 2 cases of temporary paresthesia (superficial radial nerve branches) for ≤2 months | |
| Meyer-Marcotty et al., 2021 | 5.9 | 1.9 ** | 50.8 | 29.6 ** | 3.7 22.2 | 5.1 22.8 | Not mentioned | |
| PRP + FG | ||||||||
| Winter et al., 2023 | NRS at rest: 3.12 (FG) 3.12 (PRP) 3.32 (PRP + FG) NRS under stress: 6.76 (FG) 6.75 (PRP) 6.64 (PRP + FG) | NRS at rest: 1.62 (FG) 2.73 (PRP) 1.7 (PRP + FG) * NRS under stress: 3.67 (FG) 5.18 (PRP) 3.09 (PRP + FG) * | - | Median reduction of Q-DASH: −14.8 (FG) −9.1 (PRP) −15.9 (PRP + FG) | Pinch: 1.28 FG 1.58 PRP 1.46 PRP + FG Grip: 20.76 FG 22.87 PRP 20.78 PRP + FG | Pinch: 1.41 FG 1.78 PRP 1.71 PRP + FG Grip: 20.55 FG 21.32 PRP 22.17 PRP + FG | 1 hematoma, no serious AEs associated with intervention | |
| PRP | ||||||||
| Loibl et al., 2016 | 6.2 | 5.4 * | 32.9 | 26.8 | 6.0 16.4 | 4.9 16.7 | No patient dissatisfied | 1 palmar wrist ganglion 2 |
| Malahias et al., 2018 | 7.5 | 2.0* | 50.4 | 20.4 * | - | - | 69 % satisfied * | Not mentioned |
| Sabah et al., 2020 | 8 | 4 after 4 w 5 after 12 w | - | - | - | Improved after 4 w * | Not mentioned | |
| Swärd et al., 2022 | 2 at rest 8 under stress (NRS) | 1 6 | - | 54 | 5.5 23 | 6.0 23 | - | No AEs |
| LLLT | ||||||||
| Brousseau et al., 2005 | 2.36 (AUSCAN) | 1.94 (AUSCAN) VAS n.s. 1 | - | - | - | Grip strength improved *, 1 | - | 1 erythema 1 other 3 |
| Chondrocyte transplant | ||||||||
| Messina et al., 2020 | 8 | 1 * | 55 | 7.3 * | Pinch 3.76 Grip: 22 | 6.25 * 28.5 | - | No complications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasiba-Pappas, S.; Kamolz, L.-P.; Luze, H.; Nischwitz, S.P.; Lumenta, D.B.; Winter, R. Regenerative Therapies for Basal Thumb Arthritis—A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14909. https://doi.org/10.3390/ijms241914909
Hasiba-Pappas S, Kamolz L-P, Luze H, Nischwitz SP, Lumenta DB, Winter R. Regenerative Therapies for Basal Thumb Arthritis—A Systematic Review. International Journal of Molecular Sciences. 2023; 24(19):14909. https://doi.org/10.3390/ijms241914909
Chicago/Turabian StyleHasiba-Pappas, Sophie, Lars-P. Kamolz, Hanna Luze, Sebastian P. Nischwitz, David B. Lumenta, and Raimund Winter. 2023. "Regenerative Therapies for Basal Thumb Arthritis—A Systematic Review" International Journal of Molecular Sciences 24, no. 19: 14909. https://doi.org/10.3390/ijms241914909
APA StyleHasiba-Pappas, S., Kamolz, L.-P., Luze, H., Nischwitz, S. P., Lumenta, D. B., & Winter, R. (2023). Regenerative Therapies for Basal Thumb Arthritis—A Systematic Review. International Journal of Molecular Sciences, 24(19), 14909. https://doi.org/10.3390/ijms241914909









