Genetic and Functional Modifications Associated with Ovarian Cancer Cell Aggregation and Limited Culture Conditions
Abstract
1. Introduction
2. Results
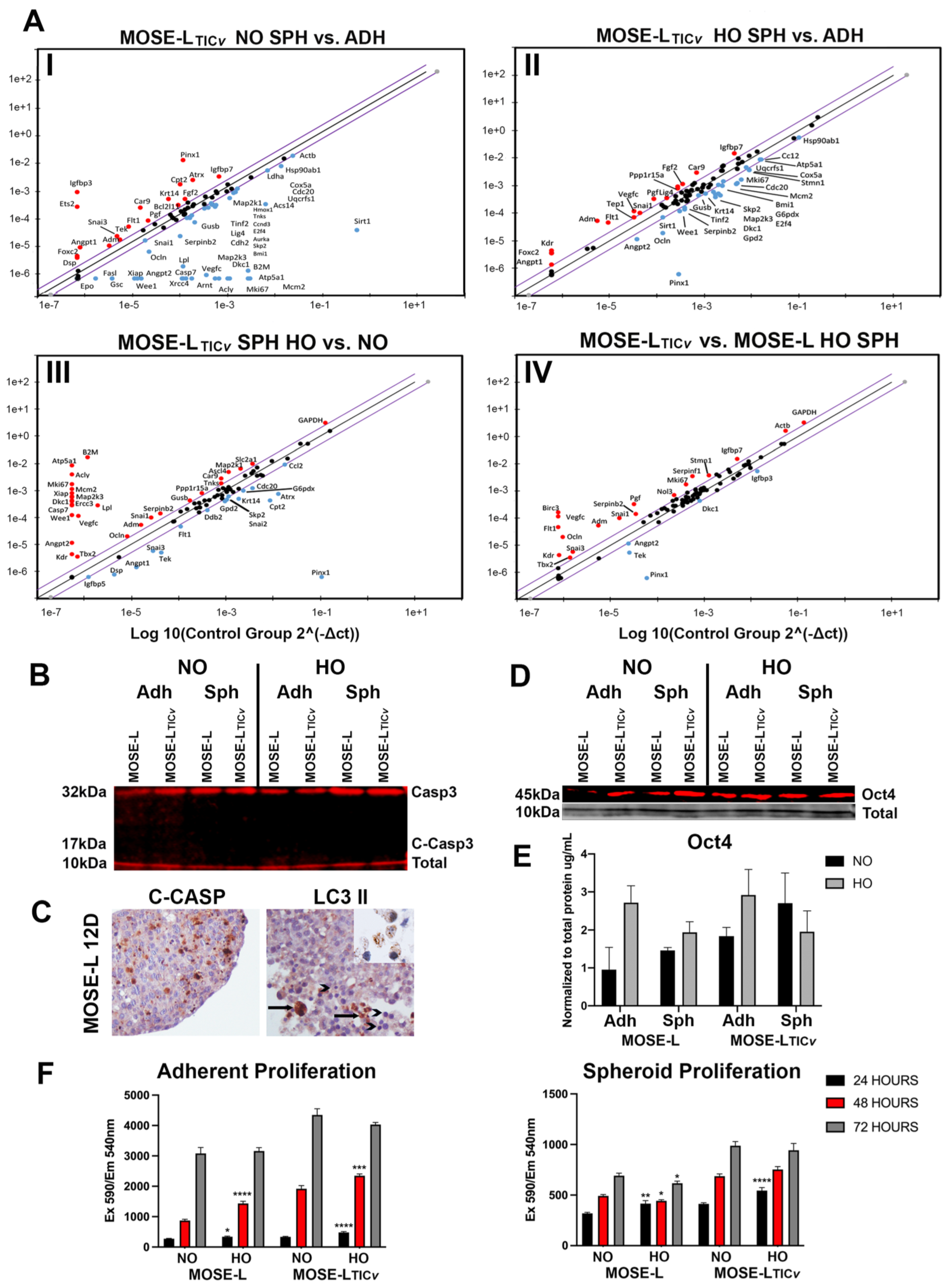
2.1. Expression Levels of Cancer-Related Genes in Response to Aggregation and Hypoxic Media Conditions Correspond with the Aggressive Phenotype of MOSE Cells
2.2. Aggregation Supports Viability and Stemness but Suppresses Proliferative Functions
2.3. Incorporation of Obese SVF into MOSE Spheroids Changes Their Gene Expression
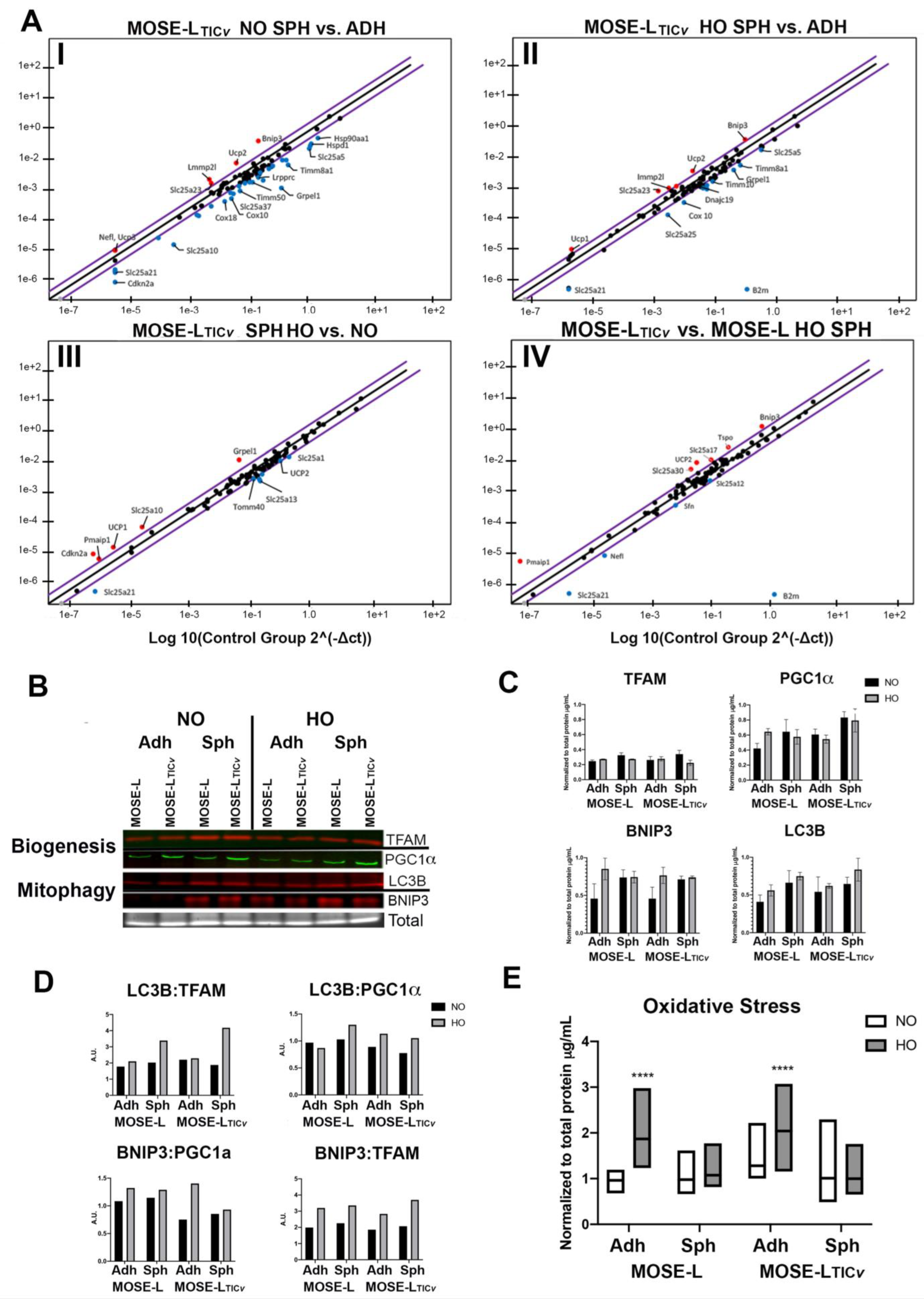
2.4. Changes in Mitochondrial Quality Control Genes Correlate with Increasing Malignancy, Aggregation, and Hypoxia
2.5. Quantification of Mitochondrial Biogenesis and Mitophagy Changes in Response to Aggregation and Hypoxia
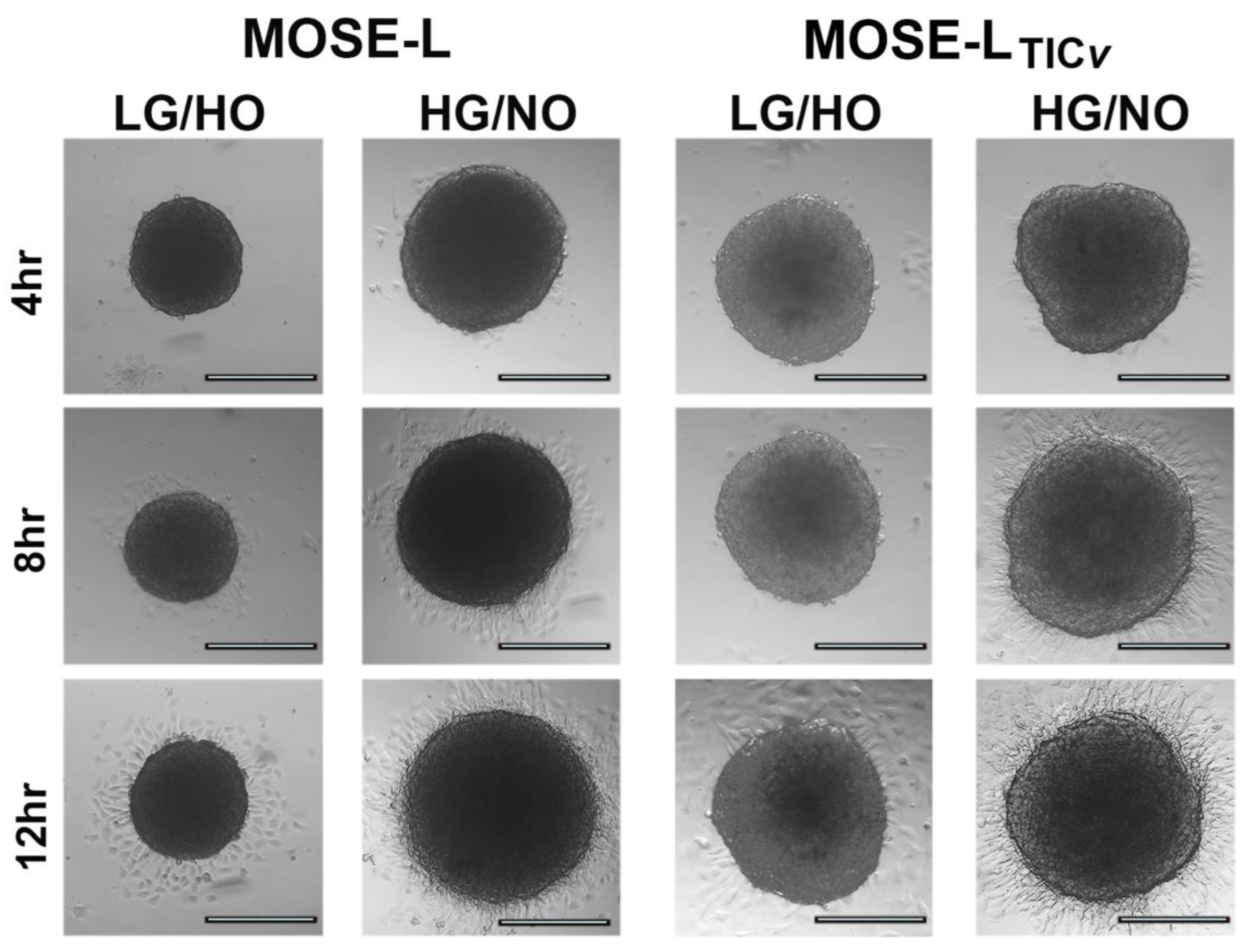
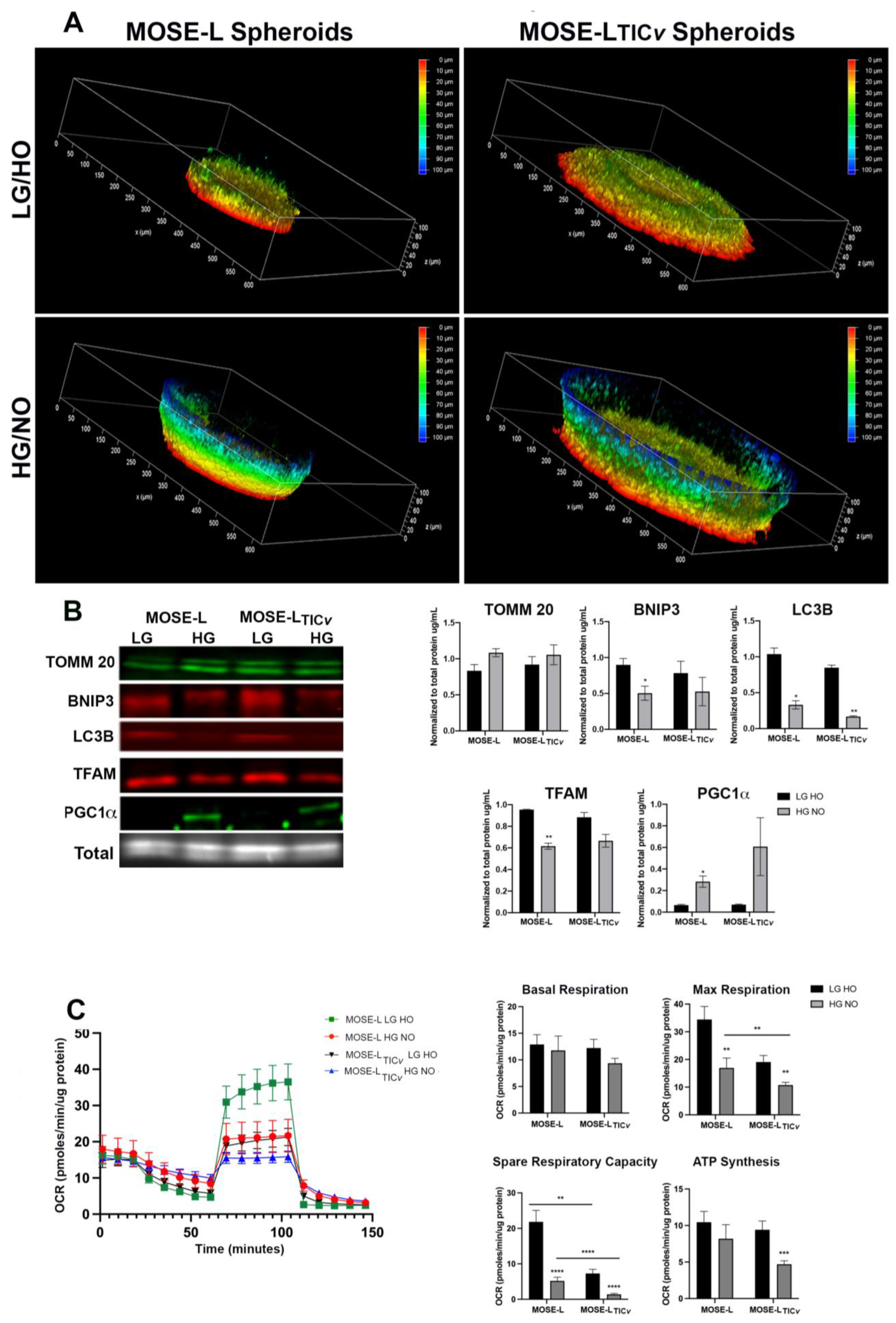
2.6. Determinants of Spheroid Formation and Viability
2.7. Determinants of Adhesion and Outgrowth of Cancer Spheroids
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stromal Vascular Fraction Isolation
4.3. Reverse Transcription Polymerase Chain Reaction
4.4. Monitoring Spheroid Proliferation and Size
4.5. Monitoring of Spheroid Assembly and Outgrowth
4.6. Protein Determination via Western Blotting
4.7. Immunohistochemistry
4.8. Reactive Oxygen Species (ROS) Assay
4.9. Confocal Microscopy
4.10. Seahorse XFe96 Analysis
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2008. [Google Scholar]
- Khalique, L.; Ayhan, A.; Whittaker, J.C.; Singh, N.; Jacobs, I.J.; Gayther, S.A.; Ramus, S.J. The clonal evolution of metastases from primary serous epithelial ovarian cancers. Int. J. Cancer 2009, 124, 1579–1586. [Google Scholar] [CrossRef]
- Bashashati, A.; Ha, G.; Tone, A.; Ding, J.; Prentice, L.M.; Roth, A.; Rosner, J.; Shumansky, K.; Kalloger, S.; Senz, J.; et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J. Pathol. 2013, 231, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-N.; Koo, K.H.; Sung, J.Y.; Yun, U.-J.; Kim, H. Anoikis resistance: An essential prerequisite for tumor metastasis. Int. J. Cell Biol. 2012, 2012, 306879. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.L.E.; Pyne, E.S.; Liu, L.; Guinan, J.; Shea, A.A.; Grieco, J.P.; Frisard, M.I.; Schmelz, E.M. Adaptation of metabolism to multicellular aggregation, hypoxia and obese stromal cell incorporation as potential measure of survival of ovarian metastases. Exp. Cell Res. 2021, 399, 112397. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Bala, L.; Sharma, A.; Yellapa, R.K.; Roy, R.; Choudhuri, G.; Khetrapal, C.L. (1)H NMR spectroscopy of ascitic fluid: Discrimination between malignant and benign ascites and comparison of the results with conventional methods. NMR Biomed. 2008, 21, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Ruff, L.E.; Skubitz, A.P. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Hyler, A.R.; Baudoin, N.C.; Brown, M.S.; Stremler, M.A.; Cimini, D.; Davalos, R.V.; Schmelz, E.M. Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability. PLoS ONE 2018, 13, e0194170. [Google Scholar] [CrossRef]
- Takahashi, N.; Cho, P.; Selfors, L.M.; Kuiken, H.J.; Kaul, R.; Fujiwara, T.; Harris, I.S.; Zhang, T.; Gygi, S.P.; Brugge, J.S. 3D Culture Models with CRISPR Screens Reveal Hyperactive NRF2 as a Prerequisite for Spheroid Formation via Regulation of Proliferation and Ferroptosis. Mol. Cell 2020, 80, 828–844.e826. [Google Scholar] [CrossRef] [PubMed]
- Braunholz, D.; Saki, M.; Niehr, F.; Ozturk, M.; Borras Puertolas, B.; Konschak, R.; Budach, V.; Tinhofer, I. Spheroid Culture of Head and Neck Cancer Cells Reveals an Important Role of EGFR Signalling in Anchorage Independent Survival. PLoS ONE 2016, 11, e0163149. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Sung, J.S.; Park, Y.S.; Chung, S.; Kim, Y.H. Identification of different gene expressions between diffuse- and intestinal-type spheroid-forming gastric cancer cells. Gastric Cancer 2019, 22, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Boylan, K.L.M.; Manion, R.D.; Shah, H.; Skubitz, K.M.; Skubitz, A.P.N. Inhibition of Ovarian Cancer Cell Spheroid Formation by Synthetic Peptides Derived from Nectin-4. Int. J. Mol. Sci. 2020, 21, 4637. [Google Scholar] [CrossRef]
- Ray, A.; Meng, E.; Reed, E.; Shevde, L.A.; Rocconi, R.P. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int. J. Oncol. 2011, 39, 797–804. [Google Scholar] [CrossRef]
- Chen, M.W.; Yang, S.T.; Chien, M.H.; Hua, K.T.; Wu, C.J.; Hsiao, S.M.; Lin, H.; Hsiao, M.; Su, J.L.; Wei, L.H. The STAT3-miRNA-92-Wnt Signaling Pathway Regulates Spheroid Formation and Malignant Progression in Ovarian Cancer. Cancer Res. 2017, 77, 1955–1967. [Google Scholar] [CrossRef]
- Lam, S.S.; Ip, C.K.; Mak, A.S.; Wong, A.S. A novel p70 S6 kinase-microRNA biogenesis axis mediates multicellular spheroid formation in ovarian cancer progression. Oncotarget 2016, 7, 38064–38077. [Google Scholar] [CrossRef]
- Emoto, S.; Kitayama, J.; Yamaguchi, H.; Ishigami, H.; Kaisaki, S.; Nagawa, H. Analysis of pO(2) in Malignant Ascites of Patients with Peritoneal Dissemination of Gastric Cancer. Case Rep. Oncol. 2010, 3, 344–348. [Google Scholar] [CrossRef]
- Creekmore, A.L.; Silkworth, W.T.; Cimini, D.; Jensen, R.V.; Roberts, P.C.; Schmelz, E.M. Changes in gene expression and cellular architecture in an ovarian cancer progression model. PLoS ONE 2011, 6, e17676. [Google Scholar] [CrossRef]
- Roberts, P.C.; Mottillo, E.P.; Baxa, A.C.; Heng, H.H.; Doyon-Reale, N.; Gregoire, L.; Lancaster, W.D.; Rabah, R.; Schmelz, E.M. Sequential molecular and cellular events during neoplastic progression: A mouse syngeneic ovarian cancer model. Neoplasia 2005, 7, 944. [Google Scholar] [CrossRef]
- Grieco, J.P.; Allen, M.E.; Perry, J.B.; Wang, Y.; Song, Y.; Rohani, A.; Compton, S.L.E.; Smyth, J.W.; Swami, N.S.; Brown, D.A.; et al. Progression-Mediated Changes in Mitochondrial Morphology Promotes Adaptation to Hypoxic Peritoneal Conditions in Serous Ovarian Cancer. Front. Oncol. 2021, 10, 600113. [Google Scholar] [CrossRef]
- Compton, S.L.E.; Grieco, J.P.; Gollamudi, B.; Bae, E.; Van Mullkom, J.; Schmelz, E.M. Metabolic reprogramming of ovarian cancer spheroids during adhesion. Cancers 2022, 14, 1399. [Google Scholar] [CrossRef] [PubMed]
- Grieco, J.P.; Compton, S.L.E.; Bano, N.; Brookover, L.; Nichenko, A.S.; Drake, J.C.; Schmelz, E.M. Mitochondrial plasticity supports proliferative outgrowth and invasion of ovarian cancer spheroids during adhesion. Front. Oncol. 2022, 12, 1043670. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Daquinag, A.; Traktuev, D.O.; Amaya-Manzanares, F.; Simmons, P.J.; March, K.L.; Pasqualini, R.; Arap, W.; Kolonin, M.G. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009, 69, 5259–5266. [Google Scholar] [CrossRef]
- Wong, L.H.; McGhie, J.D.; Sim, M.; Anderson, M.A.; Ahn, S.; Hannan, R.D.; George, A.J.; Morgan, K.A.; Mann, J.R.; Choo, K.H. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010, 20, 351–360. [Google Scholar] [CrossRef]
- Juhasz, S.; Elbakry, A.; Mathes, A.; Lobrich, M. ATRX Promotes DNA Repair Synthesis and Sister Chromatid Exchange during Homologous Recombination. Mol. Cell 2018, 71, 11–24.e17. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Y.; Wang, N.; You, N.; Zhu, W.; Zhang, P.; Ren, Q.; Yin, J.; Cheng, T.; Ma, X. GADD45g acts as a novel tumor suppressor, and its activation suggests new combination regimens for the treatment of AML. Blood 2021, 138, 464–479. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 32, 48–55; discussion 47–56. [Google Scholar] [CrossRef]
- Schaner, M.E.; Ross, D.T.; Ciaravino, G.; Sorlie, T.; Troyanskaya, O.; Diehn, M.; Wang, Y.C.; Duran, G.E.; Sikic, T.L.; Caldeira, S.; et al. Gene expression patterns in ovarian carcinomas. Mol. Biol. Cell 2003, 14, 4376–4386. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Roberts, P.C.; Frisard, M.I.; McMillan, R.P.; Brown, T.J.; Lawless, M.H.; Hulver, M.W.; Schmelz, E.M. Metabolic changes during ovarian cancer progression as targets for sphingosine treatment. Exp. Cell Res. 2013, 319, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Balasubramaniam, V.; Srinivas, S.; Sundaram, S.; Venkatraman, G.; Warrier, S.; Dharmarajan, A.; Gandhirajan, R.K. Deregulated Metabolic Pathways in Ovarian Cancer: Cause and Consequence. Metabolites 2023, 13, 560. [Google Scholar] [CrossRef]
- Do, T.V.; Hirst, J.; Hyter, S.; Roby, K.F.; Godwin, A.K. Aurora A kinase regulates non-homologous end-joining and poly(ADP-ribose) polymerase function in ovarian carcinoma cells. Oncotarget 2017, 8, 50376–50392. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal. 2020, 34, 517–530. [Google Scholar] [CrossRef]
- Freedman, R.S.; Deavers, M.; Liu, J.; Wang, E. Peritoneal inflammation—A microenvironment for Epithelial Ovarian Cancer (EOC). J. Transl. Med. 2004, 2, 23. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, Y.; Liu, S.; Wang, C.; Zhang, S.; Zhang, T.; Yang, X. EIF5A2 enhances stemness of epithelial ovarian cancer cells via a E2F1/KLF4 axis. Stem. Cell Res. Ther. 2021, 12, 186. [Google Scholar] [CrossRef]
- Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the maintenance of stemness, viability, and differentiation potential of gingiva-derived stem-cell spheroids. Exp. Ther. Med. 2017, 13, 1757–1764. [Google Scholar] [CrossRef][Green Version]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem. Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Yanes, O.; Ruiz, S.; Kida, Y.S.; Diep, D.; Tautenhahn, R.; Herrerias, A.; Batchelder, E.M.; Plongthongkum, N.; Lutz, M.; et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012, 22, 168–177. [Google Scholar] [CrossRef]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Kheir, T.B.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O. MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Comisso, E.; Scarola, M.; Rosso, M.; Piazza, S.; Marzinotto, S.; Ciani, Y.; Orsaria, M.; Mariuzzi, L.; Schneider, C.; Schoeftner, S.; et al. OCT4 controls mitotic stability and inactivates the RB tumor suppressor pathway to enhance ovarian cancer aggressiveness. Oncogene 2017, 36, 4253–4266. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, H.; Zhang, M.; Ding, L.; Meng, F.; Fan, X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn. Pathol. 2013, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial–mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ware, K.E.; Xu, S.; Gilja, S.; Shetler, S.; Yang, Y.; Wang, X.; Austin, R.G.; Runyambo, D.; Hish, A.J.; et al. E-Cadherin Represses Anchorage-Independent Growth in Sarcomas through Both Signaling and Mechanical Mechanisms. Mol. Cancer Res. 2019, 17, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, R.; Fukuda, T.; Shimomura, M.; Inoue, Y.; Wada, T.; Kawanishi, M.; Yasui, T.; Sumi, T. TBX2 expression is associated with platinum-sensitivity of ovarian serous carcinoma. Oncol. Lett. 2018, 15, 3085–3090. [Google Scholar] [CrossRef]
- Miao, L.; Yang, L.; Li, R.; Rodrigues, D.N.; Crespo, M.; Hsieh, J.T.; Tilley, W.D.; de Bono, J.; Selth, L.A.; Raj, G.V. Disrupting Androgen Receptor Signaling Induces Snail-Mediated Epithelial-Mesenchymal Plasticity in Prostate Cancer. Cancer Res. 2017, 77, 3101–3112. [Google Scholar] [CrossRef]
- Bilandzic, M.; Rainczuk, A.; Green, E.; Fairweather, N.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Keratin-14 (KRT14) Positive Leader Cells Mediate Mesothelial Clearance and Invasion by Ovarian Cancer Cells. Cancers 2019, 11, 1228. [Google Scholar] [CrossRef]
- Davidowitz, R.A.; Selfors, L.M.; Iwanicki, M.P.; Elias, K.M.; Karst, A.; Piao, H.; Ince, T.A.; Drage, M.G.; Dering, J.; Konecny, G.E.; et al. Mesenchymal gene program-expressing ovarian cancer spheroids exhibit enhanced mesothelial clearance. J. Clin. Investig. 2014, 124, 2611–2625. [Google Scholar] [CrossRef]
- Phillips, S.; Prat, A.; Sedic, M.; Proia, T.; Wronski, A.; Mazumdar, S.; Skibinski, A.; Shirley, S.H.; Perou, C.M.; Gill, G.; et al. Cell-state transitions regulated by SLUG are critical for tissue regeneration and tumor initiation. Stem. Cell Rep. 2014, 2, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.M.; Zhou, W.; Breindel, J.L.; Ouyang, J.; Jin, D.X.; Sokol, E.S.; Gupta, P.B.; Huber, K.; Zou, L.; Kuperwasser, C. Loss of Slug Compromises DNA Damage Repair and Accelerates Stem Cell Aging in Mammary Epithelium. Cell Rep. 2019, 28, 394–407 e396. [Google Scholar] [CrossRef] [PubMed]
- Madamba, S.M.; Damri, K.N.; Dejean, L.M.; Peixoto, P.M. Mitochondrial Ion Channels in Cancer Transformation. Front. Oncol. 2015, 5, 120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Liao, W.-T.; Wu, C.-Y.; Chen, G.-S.; Wei, Y.-H.; Yu, H.-S. Aberrant cell proliferation by enhanced mitochondrial biogenesis via mtTFA in arsenical skin cancers. Am. J. Pathol. 2011, 178, 2066–2076. [Google Scholar] [CrossRef]
- Bellot, G.; Cartron, P.; Er, E.; Oliver, L.; Juin, P.; Armstrong, L.; Bornstein, P.; Mihara, K.; Manon, S.; Vallette, F. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2007, 14, 785–794. [Google Scholar] [CrossRef]
- Sowter, H.M.; Ferguson, M.; Pym, C.; Watson, P.; Fox, S.B.; Han, C.; Harris, A.L. Expression of the cell death genes BNip3 and NIX in ductal carcinoma in situ of the breast; correlation of BNip3 levels with necrosis and grade. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 573–580. [Google Scholar]
- Giatromanolaki, A.; Koukourakis, M.I.; Sowter, H.M.; Sivridis, E.; Gibson, S.; Gatter, K.C.; Harris, A.L. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non–small cell lung cancer. Clin. Cancer Res. 2004, 10, 5566–5571. [Google Scholar] [CrossRef]
- Kim, B.; Jung, J.W.; Jung, J.; Han, Y.; Suh, D.H.; Kim, H.S.; Dhanasekaran, D.N.; Song, Y.S. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget 2017, 8, 60299–60311. [Google Scholar] [CrossRef]
- Fisher, K.W.; Das, B.; Kortum, R.L.; Chaika, O.V.; Lewis, R.E. Kinase suppressor of ras 1 (KSR1) regulates PGC1alpha and estrogen-related receptor alpha to promote oncogenic Ras-dependent anchorage-independent growth. Mol. Cell Biol. 2011, 31, 2453–2461. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Jeong, H.G. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic. Biol. Med. 2014, 74, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Delort, L.; Kwiatkowski, F.; Chalabi, N.; Satih, S.; Bignon, Y.J.; Bernard-Gallon, D.J. Central adiposity as a major risk factor of ovarian cancer. Anticancer Res. 2009, 29, 5229–5234. [Google Scholar] [PubMed]
- Paffenholz, S.V.; Salvagno, C.; Ho, Y.J.; Limjoco, M.; Baslan, T.; Tian, S.; Kulick, A.; de Stanchina, E.; Wilkinson, J.E.; Barriga, F.M.; et al. Senescence induction dictates response to chemo- and immunotherapy in preclinical models of ovarian cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2117754119. [Google Scholar] [CrossRef] [PubMed]
- Flasarova, D.; Urban, K.; Strouhal, O.; Klos, D.; Lemstrova, R.; Dvorak, P.; Soucek, P.; Mohelnikova-Duchonova, B. DNA Repair Pathway in Ovarian Cancer Patients Treated with HIPEC. Int. J. Mol. Sci. 2023, 24, 8868. [Google Scholar] [CrossRef]
- van der Meer, R.; Jeuken, J.W.M.; Bosch, S.L.; van Erning, F.N.; Simkens, L.H.J.; de Hingh, I.; Roumen, R.M.H. Biomarker concordance between primary colorectal cancer and ovarian metastases: A Dutch cohort study. J. Cancer Res. Clin. Oncol. 2023, 149, 5677–5685. [Google Scholar] [CrossRef]
- Meng, E.; Taylor, B.; Ray, A.; Shevde, L.A.; Rocconi, R.P. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol. Oncol. 2012, 124, 598–605. [Google Scholar] [CrossRef]
- Guerrieri, A.N.; Zacchini, F.; Onofrillo, C.; Di Viggiano, S.; Penzo, M.; Ansuini, A.; Gandin, I.; Nobe, Y.; Taoka, M.; Isobe, T.; et al. DKC1 Overexpression Induces a More Aggressive Cellular Behavior and Increases Intrinsic Ribosomal Activity in Immortalized Mammary Gland Cells. Cancers 2020, 12, 3512. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Jiang, R.; Hou, P.; Shan, H.; Chen, F.; Jiang, T.; Bai, J.; Zheng, J. DKC1 serves as a potential prognostic biomarker for human clear cell renal cell carcinoma and promotes its proliferation, migration and invasion via the NF-kappaB pathway. Oncol. Rep. 2018, 40, 968–978. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. The parity-associated microenvironmental niche in the omental fat band is refractory to ovarian cancer metastasis. Cancer Prev. Res. 2013, 6, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. Intra-abdominal fat depots represent distinct immunomodulatory microenvironments: A murine model. PLoS ONE 2013, 8, e66477. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. Interleukin-12 Immunomodulation Delays the Onset of Lethal Peritoneal Disease of Ovarian Cancer. J. Interferon. Cytokine Res. 2016, 36, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.M.; Roberts, P.C.; Kustin, E.M.; Lemonnier, L.A.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H., Jr. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001, 61, 6723–6729. [Google Scholar] [PubMed]


| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| MOSE-L + SVF | Lower expression | Higher expression | Lower expression | Higher expression |
| Cox5a, Pfkl | Ccnd2, Errc2, Errc3, Ets2, Ldha, Lig4, Ocl, Snai2 | Atp5a1, Atrx, Ccnd2, Dsp, Errc3, Errc5, Gsc, Map2k1, Mki67, Ocl, Skp2, Snai2, Tnks, Wee1 | ||
| Differential gene expression in MOSE cells and SVF but no change in gene expression in the heterogenous spheroids, suggesting change of expression in either cell type | ||||
| Lower expression | Higher expression | Lower expression | Higher expression | |
| Hmox1 | Aurka, Cdh2, Igfbp3, Sirt2, Skp2, Stmn1, Tep1, Tinf1 | Hmox1 | Acly, Aurka, Bmi, Car9, Casp7, Cdh2, Dkc1, Ets2, Gadd45g, Igfbp3, Tinf2 | |
| MOSE-LTICv + SVF | Normoxia | Hypoxia | ||
| Lower expression | Higher expression | Lower expression | Higher expression | |
| Angpt1, Apaf1, Amt, Atrx, Casp9, Ccnd3, Cpt2, Dsp, Fgf2, Flt1, Ing1, Ldha, Pfkl, Pgf, Pinx1, Tep1 | Acly, Acsl4, Atp5a1, Bmi, Casp2, Casp7, Ccnd2, Dkc1, Errc3, Ets2, Gpd2, Lig4, Mcm2, Ppp1rl15a, Snai2, Tnks, Vegfc | Bmi1, Cox5a, E2f4, Snai1, Tbx2 | Atrx, Bcl2l11, Car9, Ccnd2, Dkc1, Errc5, Gpd2, Igfbp3, Igfbp5, Skp2, Mki67, Snai2 | |
| Differential gene expression in MOSE cells and SVF but no change in gene expression in the heterogenous spheroids, suggesting change of expression in either cell type | ||||
| Lower expression | Higher expression | Lower expression | Higher expression | |
| Gadd45g, Gsc, Igfbp7, Sox10, Tek | Adm, Angpt1, Apaf1, Flt1, Gadd45g, Snai3 | Atp5a1, Aurka, Cdc20, Cdh2, Mcm2, Mki67, Pinx1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieco, J.P.; Compton, S.L.E.; Davis, G.N.; Guinan, J.; Schmelz, E.M. Genetic and Functional Modifications Associated with Ovarian Cancer Cell Aggregation and Limited Culture Conditions. Int. J. Mol. Sci. 2023, 24, 14867. https://doi.org/10.3390/ijms241914867
Grieco JP, Compton SLE, Davis GN, Guinan J, Schmelz EM. Genetic and Functional Modifications Associated with Ovarian Cancer Cell Aggregation and Limited Culture Conditions. International Journal of Molecular Sciences. 2023; 24(19):14867. https://doi.org/10.3390/ijms241914867
Chicago/Turabian StyleGrieco, Joseph P., Stephanie L. E. Compton, Grace N. Davis, Jack Guinan, and Eva M. Schmelz. 2023. "Genetic and Functional Modifications Associated with Ovarian Cancer Cell Aggregation and Limited Culture Conditions" International Journal of Molecular Sciences 24, no. 19: 14867. https://doi.org/10.3390/ijms241914867
APA StyleGrieco, J. P., Compton, S. L. E., Davis, G. N., Guinan, J., & Schmelz, E. M. (2023). Genetic and Functional Modifications Associated with Ovarian Cancer Cell Aggregation and Limited Culture Conditions. International Journal of Molecular Sciences, 24(19), 14867. https://doi.org/10.3390/ijms241914867





