Rhizosphere Microbe Affects Soil Available Nitrogen and Its Implication for the Ecological Adaptability and Rapid Growth of Dendrocalamus sinicus, the Strongest Bamboo in the World
Abstract
:1. Introduction
2. Results
2.1. Soil Properties and Biological Characteristics of Various D. sinicus at Different Stages
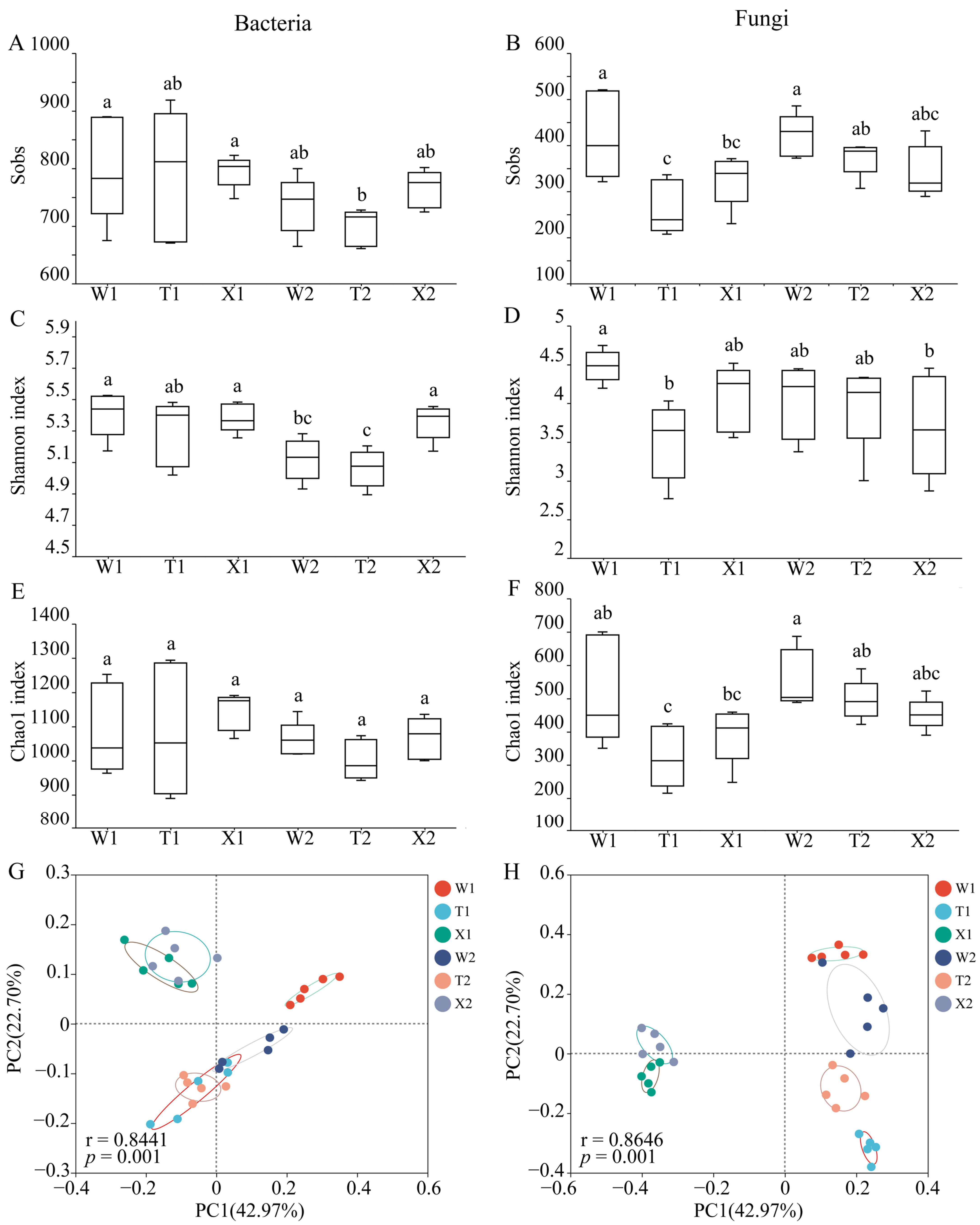
2.2. The α Diversity and β Diversity of Soil Microbial Community
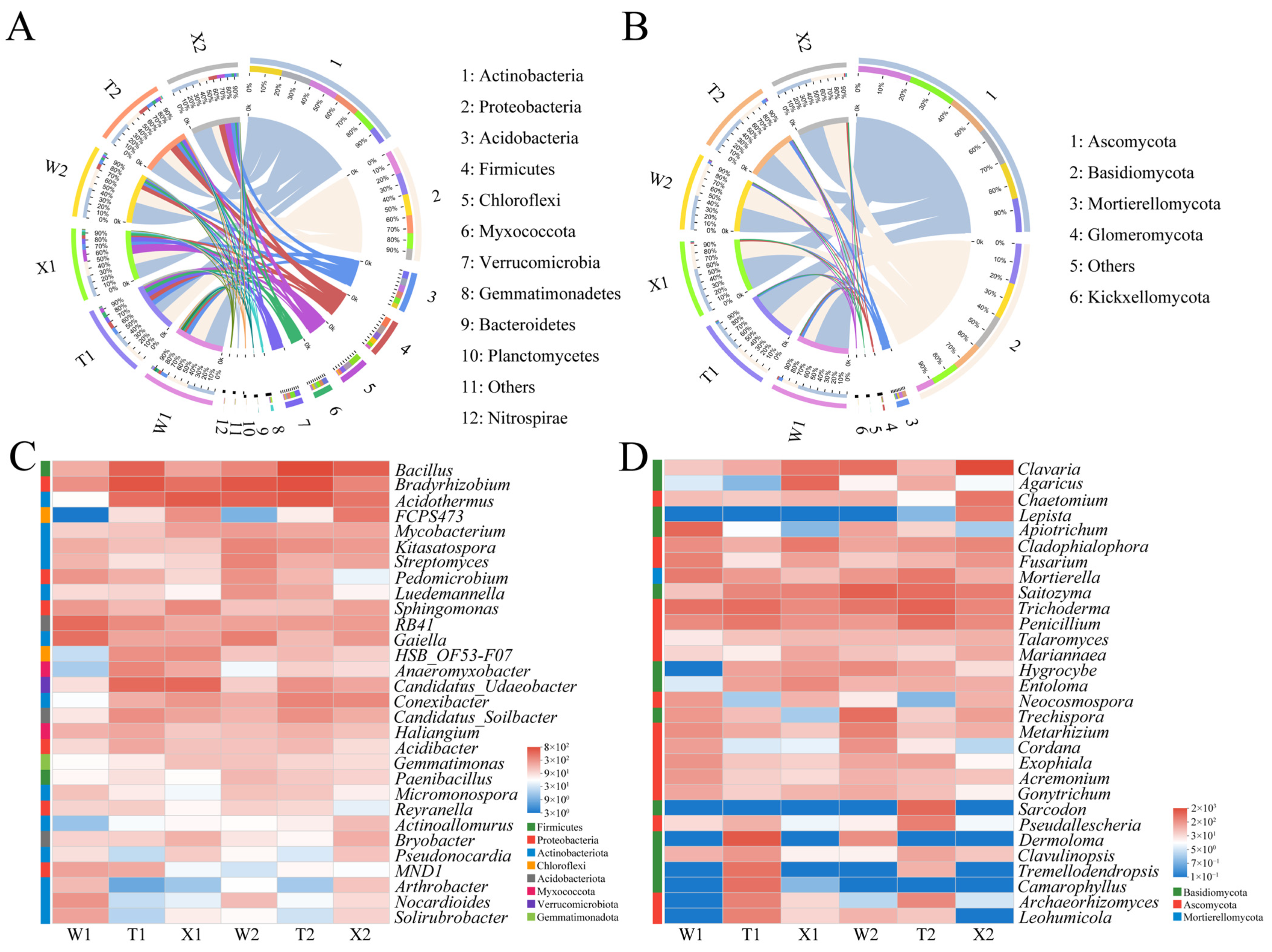
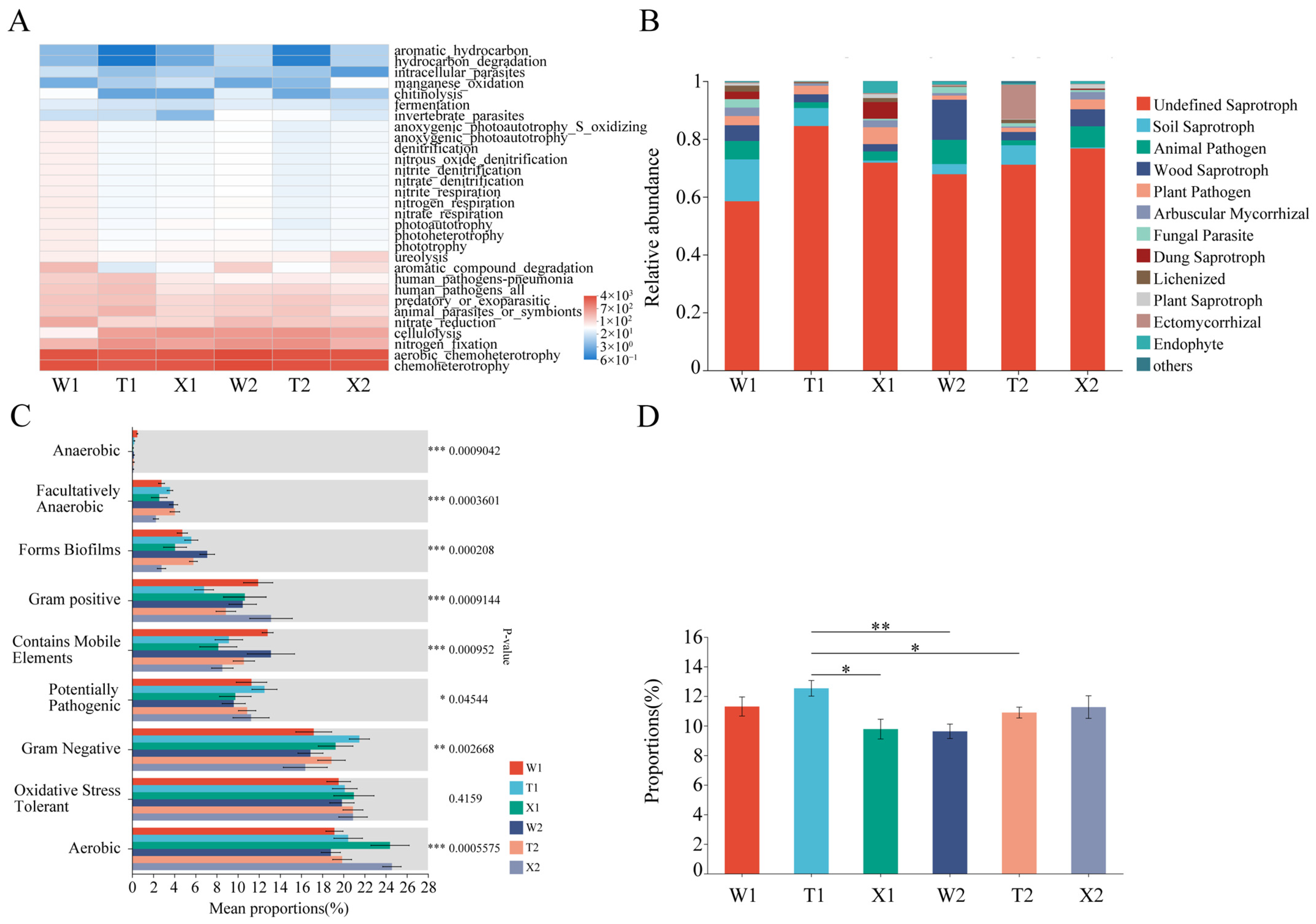
2.3. Soil Microbial Community Composition and Function
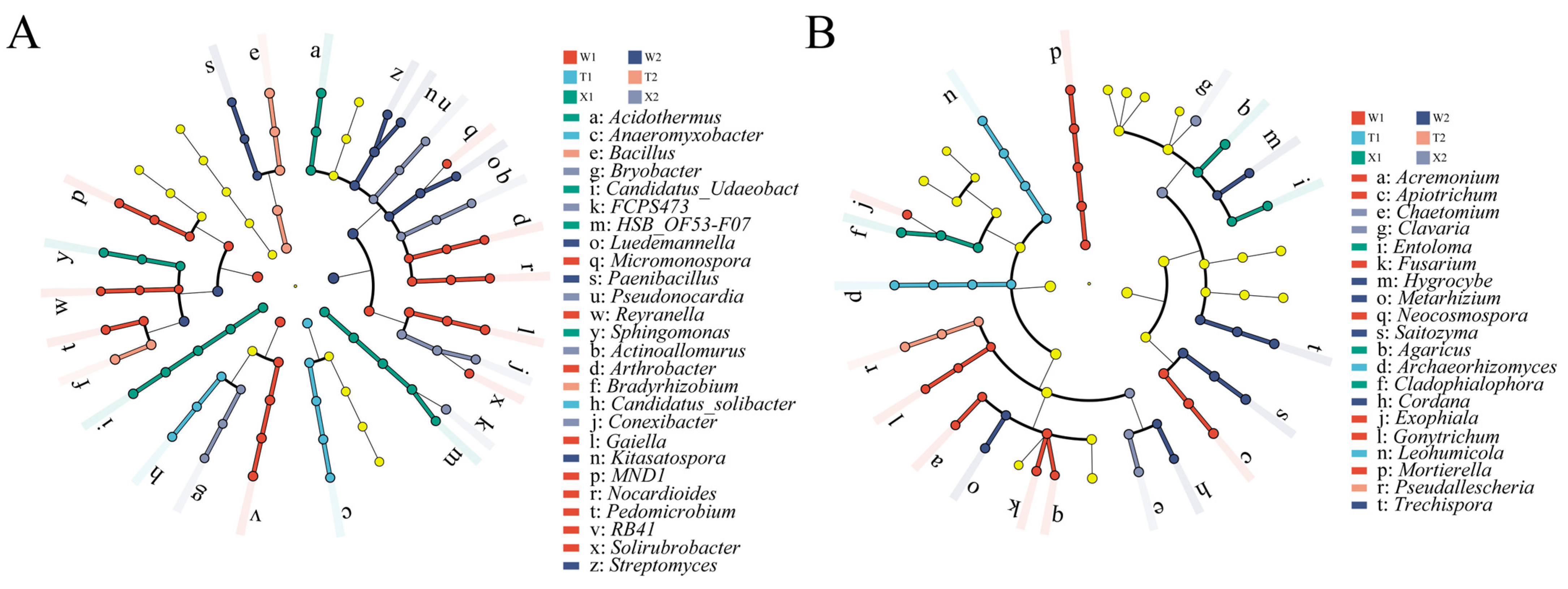
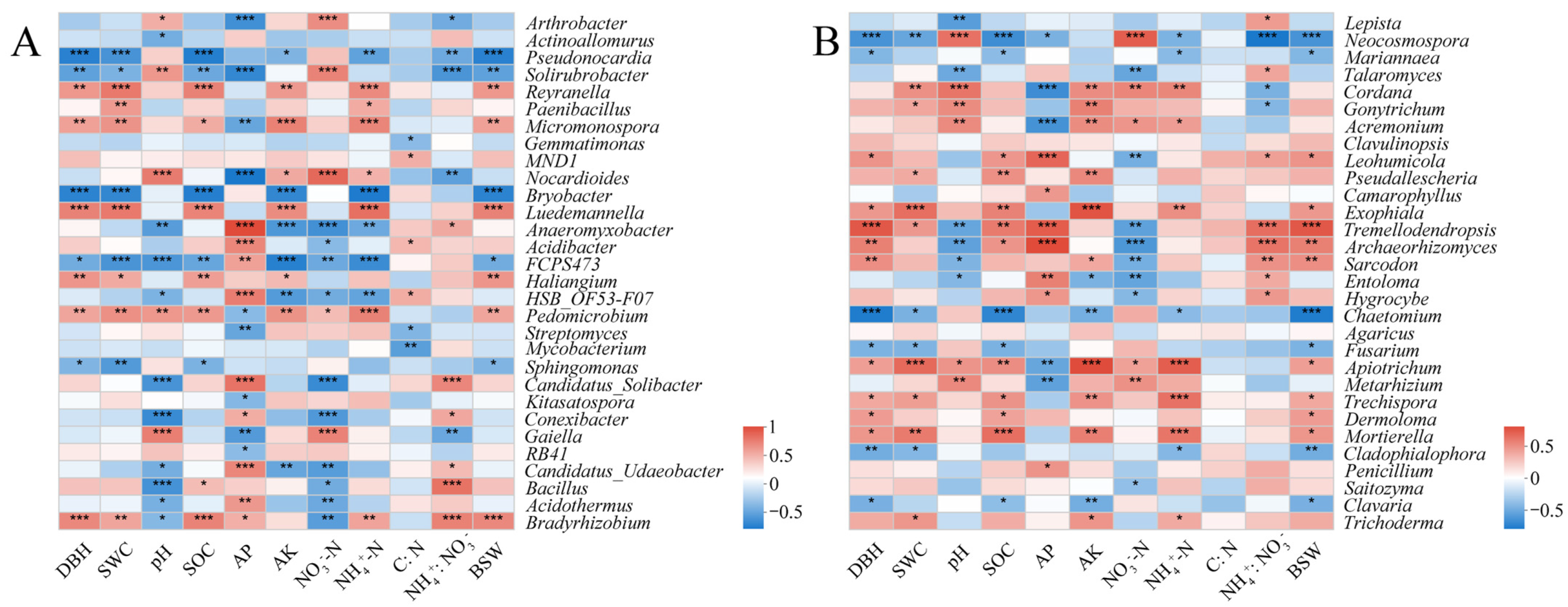
2.4. Soil Microbial Taxa with Significant Differences
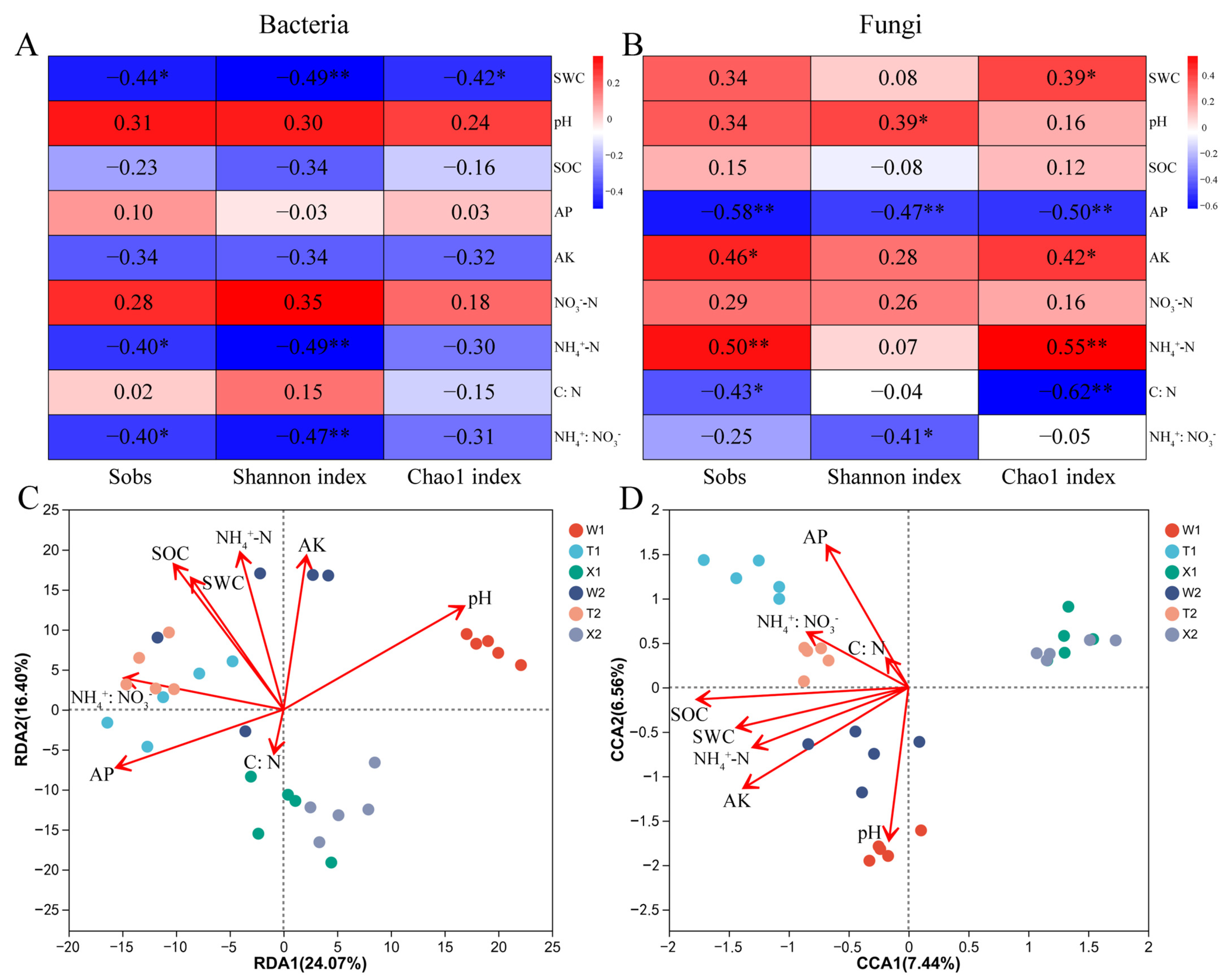
2.5. Soil Properties Affecting the Diversity and Structure of Microbial Community
2.6. Correlations of Microbial Genera with Soil Properties and Biological Characteristics of D. sinicus
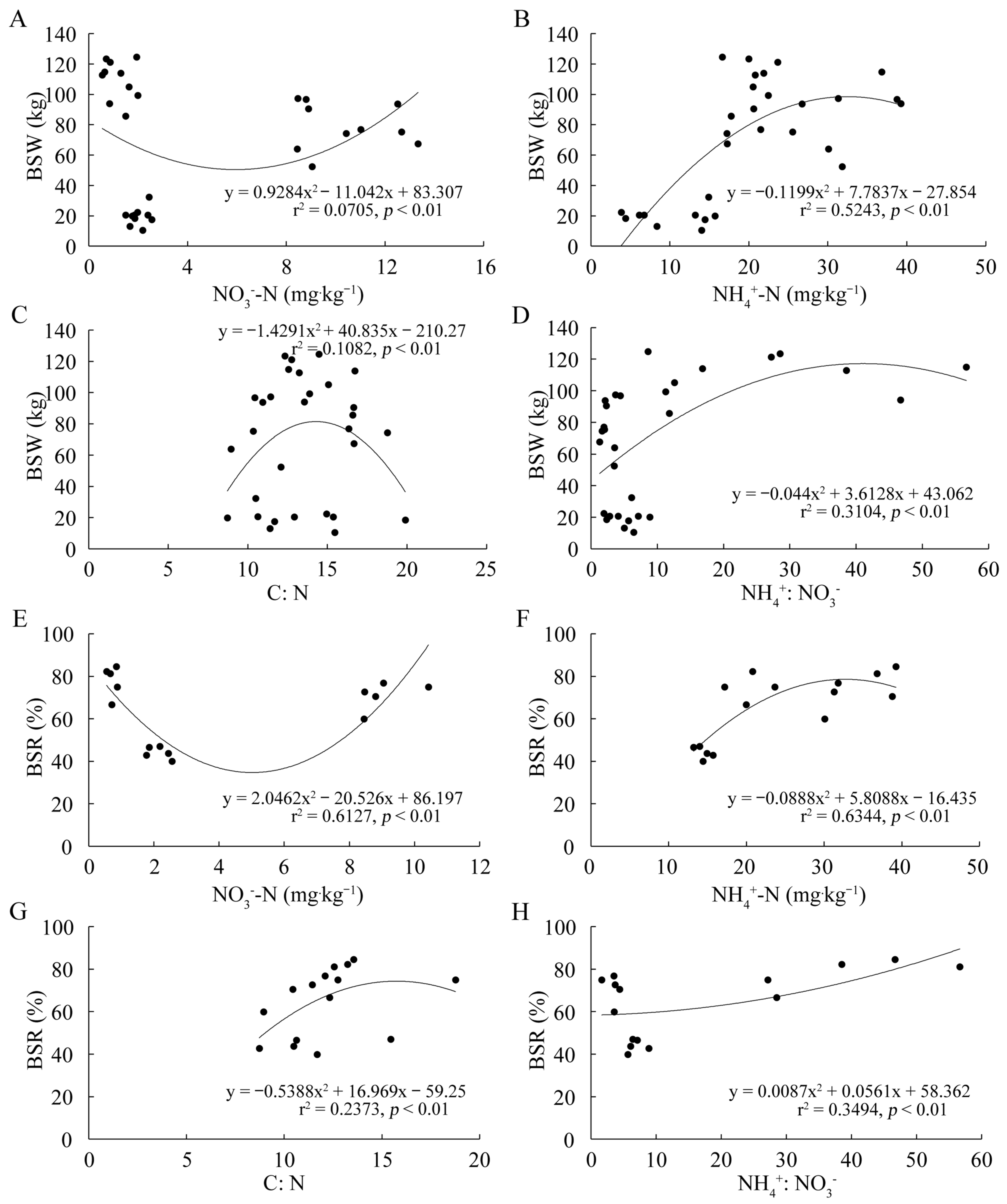
2.7. The Relationship between Selected Soil Properties and the Biological Characteristics of D. sinicus
3. Discussion
3.1. Characteristics of NH4+-N Absorption by D. sinicus
3.2. Influence of Rhizosphere Microbes on NH4+-N Availability in Red Soil
3.3. Influence of Micro-Ecological Factors on the Adaptation of D. sinicus
3.4. Potential Key Microbes Associated with Rapid Growth of D. sinicus
4. Materials and Methods
4.1. Soil Microbes Associated with Rapid Growth of D. sinicus
4.2. Soil Sample Collection and Investigation of Bamboo Biological Characteristics
4.3. The Measurement of Soil Properties
4.4. DNA Extraction, Amplification and Sequencing
4.5. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, D.D.; Li, H.; Song, H.J.; Hu, Z.H.; Ye, H.C.; Li, D.M.; Yu, X.C.; Wang, C.Y.; Liu, K.L. The relationship between chemical structure of organic carbon and stability of aggregates in red soils under long-term fertilization. Chin. J. Soil Sci. 2022, 53, 152–159. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zou, Z.W.; Huang, C.X.; Jin, J. Effect of biochar addition on phosphorus adsorption characteristics of red soil. Front. Environ. Sci. 2022, 10, 893212. [Google Scholar] [CrossRef]
- He, W.S.; Li, S.X.; Li, H.T. Characteristics of ammonium and nitrate uptake in rice. Chin. J. Rice Sci. 1998, 12, 249–252. [Google Scholar] [CrossRef]
- Yi, X.M.; Yuan, J.; Zhu, Y.H.; Yi, X.J.; Zhao, Q.; Fang, K.K.; Cao, L.K. Comparison of the abundance and community structure of N-cycling bacteria in paddy rhizosphere soil under different rice cultivation patterns. Int. J. Mol. Sci. 2018, 19, 3772. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Bledsoe, C.S. Preferences for 15N-ammonium, 15N-nitrate, and 15N-glycine differ among dominant exotic and subordinate native grasses from a California Oak woodland. Environ. Exp Bot. 2009, 65, 205–209. [Google Scholar] [CrossRef]
- Wu, L.k.; Wang, J.Y.; Wu, H.M.; Chen, J.; Xiao, Z.G.; Qin, X.J.; Zhang, Z.Y.; Lin, W.X. Comparative metagenomic analysis of rhizosphere microbial community composition and functional potentials under Rehmannia glutinosa consecutive monoculture. Int. J. Mol. Sci. 2018, 19, 2394. [Google Scholar] [CrossRef]
- Zhang, W.L.; Gao, J.Y.; Shao, A.C.; Li, T.Q. Low specificity but dissimilar mycorrhizal communities associating with roots may contribute to the spatial pattern of four co-occurring Habenaria (Orchidaceae) species. Int. J. Mol. Sci. 2023, 24, 665. [Google Scholar] [CrossRef]
- Phour, M.; Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Interkingdom signaling in plant-rhizomicrobiome interactions for sustainable agriculture. Microbiol. Res. 2020, 241, 126589. [Google Scholar] [CrossRef]
- Stokstad, E. The nitrogen fix. Science 2016, 353, 1225–1227. [Google Scholar] [CrossRef]
- Li, L.S.; Xia, T.Z.; Yang, H.Q. Seasonal patterns of rhizosphere microorganisms suggest carbohydrate-degrading and nitrogen-fixing microbes contribute to the attribute of full-year shooting in woody bamboo Cephalostachyum pingbianense. Front. Microbiol. 2022, 13, 1033293. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, A.; Miller, T. Oxidative status of Medicago truncatula seedlings after inoculation with rhizobacteria of the genus Pseudomonas, Paenibacillus and Sinorhizobium. Int. J. Mol. Sci. 2023, 24, 4781. [Google Scholar] [CrossRef] [PubMed]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K.M. Plant growth promotion using Bacillus cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef] [PubMed]
- Lankau, R.A. Species invasion alters local adaptation to soil communities in a native plant. Ecology 2013, 94, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pickles, B.J.; Twieg, B.D.; O’Neill, G.A.; Mohn, W.W.; Simard, S.W. Local adaptation in migrated interior douglas-fir seedlings is mediated by ectomycorrhizas and other soil factors. New Phytol. 2015, 207, 858–871. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, F.; Chen, H.; Xu, T.; Tang, M. Effects of arbuscular mycorrhizal fungus on sodium and chloride ion channels of Casuarina glauca under salt stress. Int. J. Mol. Sci. 2023, 24, 3680. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- Benning, J.W.; Moeller, D.A. Microbes, mutualism, and range margins: Testing the fitness consequences of soil microbial communities across and beyond a native plant’s range. New Phytol. 2021, 229, 2886–2900. [Google Scholar] [CrossRef]
- Delaux, P.M.; Schornack, S. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 2021, 371, eaba6605. [Google Scholar] [CrossRef]
- Xia, T.Z.; Li, L.S.; Li, B.; Dou, P.T.; Yang, H.Q. Heterotrophic bacteria play an important role in endemism of Cephalostachyum pingbianense (Hsueh & Y.M. Yang ex Yi et al.) D.Z. Li & H.Q. Yang, 2007, a full-year shooting woody bamboo. Forests 2022, 13, 121. [Google Scholar] [CrossRef]
- Xia, T.Z.; Li, L.S.; Yang, H.Q. Soil fungal community characteristics at the upper and lower altitudinal range limits of Cephalostachyum pingbianense. Chin. J. Plant Ecol. 2022, 46, 823–833. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barberá, P.; Gollespie, L.J.; Zuloaga, F.O. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J. Syst. Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef]
- Yi, T.P.; Shi, J.Y.; Ma, L.S.; Wang, H.T.; Yang, L. Iconographia Bambusoidearum sinicarum; Science Press: Beijing, China, 2008. [Google Scholar]
- Dou, P.T.; Dong, Y.R.; Chen, L.N.; Yang, H.Q. Modeling the potential distribution of different types of Dendrocalamus sinicus, the strongest woody bamboo in the world, with MaxEnt model. PeerJ 2022, 10, e13847. [Google Scholar] [CrossRef]
- Chen, L.N.; Dou, P.T.; Li, L.S.; Chen, Y.K.; Yang, H.Q. Transcriptome-wide analysis reveals core transcriptional regulators associated with culm development and variation in Dendrocalamus sinicus, the strongest woody bamboo in the world. Heliyon 2022, 8, e12600. [Google Scholar] [CrossRef]
- Li, L.S.; Xia, T.Z.; Li, B.; Yang, H.Q. Hormone and carbohydrate metabolism associated genes play important roles in rhizome bud full-year germination of Cephalostachyum pingbianense. Physiol. Plant. 2022, 174, e13674. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Liu, F.; Liu, Z.Y.; Huang, Q.L.; Zhang, G.F.; Pan, H. Structural variability and differentiation of niches in the rhizosphere and endosphere bacterial microbiome of Moso Bamboo (Phyllostachys edulis). Sci. Rep. 2021, 11, 1574. [Google Scholar] [CrossRef]
- Singh, L.; Sridharan, S.; Thul, S.T.; Kokate, P.; Kumar, P.; Kumar, S.; Kumar, R. Eco-rejuvenation of degraded land by microbe assisted bamboo plantation. Ind. Crop. Prod. 2020, 155, 112795. [Google Scholar] [CrossRef]
- Hui, C.M.; Yang, Y.M.; Du, F. Study on Valuable and Rare Bamboo Species of Dendrocalamus sinicus; Science and Technology Press: Kunming, China, 2006. [Google Scholar]
- Chen, L.N.; Dou, P.T.; Chen, Y.K.; Yang, H.Q. Mutant IAA21 genes from Dendrocalamus sinicus Chia et J. L. Sun inhibit stem and root growth in transgenic Tobacco by interacting with ARF5. Plant Physiol. Biochem. 2023, 201, 107827. [Google Scholar] [CrossRef]
- Xie, N.; Chen, L.N.; Dong, Y.R.; Yang, H.Q. Mixed mating system and variable mating patterns in tropical woody bamboos. BMC Plant Biol. 2019, 19, 418. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Chang, S.X.; Xu, Q.; Li, Y.; Ma, Z.; Qin, H.; Cai, Y. Linking enhanced soil nitrogen mineralization to increased fungal decomposition capacity with Moso bamboo invasion of broadleaf forests. Sci. Total Environ. 2021, 771, 144779. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar] [CrossRef]
- Larsson, M.; Larsson, C.M.; Whitford, P.N.; Clarkson, D.T. Influence of osmotic stress on nitrate reductase activity in wheat (Triticum aestivum L) and the rule of abscisic acid. J. Exp. Bot. 1989, 40, 222–228. [Google Scholar] [CrossRef]
- Ye, L.S.; Chen, S.L. Effects of nitrogen forms and ratios on photosynthetic characters and enzyme activities in nitrogen metabolism of Phyllostachys violascens. Chin. J. Ecol. 2016, 35, 2355–2360. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, Z.H.; Li, Y.C.; Wu, L.C.; Wu, J.S.; Li, Y.F.; Zhong, B.; Liang, C.F.; Xu, Q.F. Effects of different nitrogen forms and ratios on the growth of Phyllostachys edulis and Quercus glauca seedlings. Chin. J. Ecol. 2019, 38, 2655–2661. [Google Scholar] [CrossRef]
- Luo, Y.L.; Li, Q.Q.; Wang, C.Q.; Zhang, W.; Zhang, H.; Li, L.X.; Chen, J.W.; Ma, Y. Spatial variability of soil C/N ratio and its influence factors at a county scale in hilly area of mid-Sichuan basin, Southwest China. Chin. J. Appl. Ecol. 2015, 26, 177–185. [Google Scholar] [CrossRef]
- Li, Y.C.; Yang, F.J.; Li, Y.Y.; Wang, H.N.; Zhu, H.L.; Yu, F.; Hou, C.C. Contents of carbon, nitrogen and phosphorus in soils in river flat of lower of the yellow river and farmland cultivated from river flat. Wetland Sci. 2021, 19, 479–485. [Google Scholar] [CrossRef]
- Dai, M.D.; Tan, X.F.; Ye, Z.R.; Li, B.J.; Zhang, Y.; Chen, X.T.; Kong, D.D. Soil bacterial community composition and diversity respond to soil environment in rooftop agricultural system. Environ. Technol. Innov. 2023, 30, 103042. [Google Scholar] [CrossRef]
- Tecon, R.; Or, D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol. Rev. 2017, 41, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Campbell, C.A.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, J.; Chang, S.X.; Cai, Z.C.; Müller, C.; Zhang, J.B. Nitrogen deposition affects both net and gross soil nitrogen transformations in forest ecosystems: A review. Environ. Pollut. 2019, 244, 608–616. [Google Scholar] [CrossRef]
- Weverka, J.; Runte, G.C.; Porzig, E.L.; Carey, C.J. Exploring plant and soil microbial communities as indicators of soil organic. Soil Biol. Biochem. 2023, 178, 108952. [Google Scholar] [CrossRef]
- Wekesa, C.; Kiprotich, K.; Okoth, P.; Asudi, G.O.; Muoma, J.O.; Furch, A.C.U.; Oelmüller, R. Molecular characterization of indigenous rhizobia from Kenyan soils nodulating with common beans. Int. J. Mol. Sci. 2023, 24, 9509. [Google Scholar] [CrossRef]
- Clemmensen, K.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.; Lindahl, B.D. Roots and associated fungi drive longterm carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Midgley, M.G.; Phillips, R.P. Spatio-temporal heterogeneity in extracellular enzyme activities tracks variation in saprotrophic fungal biomass in a temperate hardwood forest. Soil Biol. Biochem. 2019, 138, 107600. [Google Scholar] [CrossRef]
- Fang, X.Y.; Yu, T.B.; Yang, L.; Zang, H.D.; Zeng, Z.H.; Yang, Y.D. Progress of bacterial wilt and its soil micro-ecological regulation in peanut. Chin. J. Eco-Agric. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Andrade, L.R.M.; Aquino, F.G.; Echevarria, G.; Oliveira, J.S.; Pereira, C.D.; Malaquias, J.V.; Souza, K.S.; Montargès-Pelletier, E.; Faleiro, F.G.; Junior, F.B.R.; et al. Edaphic factors as genetic selection agents and adaptation drivers of native plant species in harsh environments of the Brazilian savanna. Plant Soil 2022, 479, 301–323. [Google Scholar] [CrossRef]
- Thiergart, T.; Durán, P.; Ellis, T.; Vannier, N.; Garrido-Oter, R.; Kemen, E.; Roux, F.; Alonso-Blanco, C.; Ågren, J.; Schulze-Lefert, P.; et al. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 2020, 4, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, X.; Abbas, T.; Fang, T.; Miao, D.; Li, Y.; Chang, S.X.; Li, Y. Higher ammonium-to-nitrate ratio shapes distinct soil nitrifying community and favors the growth of Moso bamboo in contrast to broadleaf tree species. Biol. Fert. Soils 2021, 57, 1171–1182. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biot. 2019, 103, 4693–4708. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, H.Y.; Shi, P.J.; Wang, Y.R.; Yang, P.L.; Yao, B. Cloning, expression and characterization of an Alkaline Xylanase from Alkali-tolerant fungus Pseudallescheria sp. JSM-2. Curr. Biot. 2011, 1, 61–67. [Google Scholar]
- Dwibedi, V.; Rath, S.K.; Jain, S.; Martínez-Argueta, N.; Prakash, R.; Saxena, S.; Rios-Solis, L. Key insights into secondary metabolites from various Chaetomium species. Appl. Microbiol. Biot. 2023, 107, 1077–1093. [Google Scholar] [CrossRef]
- Soumare, A.; Sarr, D.; Diédhiou, A.G. Potassium sources, microorganisms and plant nutrition: Challenges and future research directions. Pedosphere 2023, 33, 105–115. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, L.; Jiao, C.; Fei, Z.J.; Chen, M.; Cao, J.J.; Ding, Y.L.; Yuan, Q.S. Characterization of the developmental dynamics of the elongation of a bamboo internode during the fast growth stage. Tree Physiol. 2019, 39, 1201–1214. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mohammed, M.; Ibny, F.; Dakora, F. Rhizobia as a source of plant growth-promoting molecules: Potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, S.F.; Lang, X.D.; Huang, X.B.; Su, J.R. Effects of microtopography on soil fungal community diversity, composition, and assembly in a subtropical monsoon evergreen broadleaf forest of southwest China. Catena 2022, 211, 106025. [Google Scholar] [CrossRef]
- Zeng, Q.C.; An, S.S. Identifying the biogeographic patterns of rare and abundant bacterial communities using different primer sets on the Loess Plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]

| W1 | T1 | X1 | W2 | T2 | X2 | |
|---|---|---|---|---|---|---|
| pH | 6.13 ± 0.0231 a | 5.66 ± 0.0182 d | 5.74 ± 0.0130 c | 6.02 ± 0.0114 b | 5.58 ± 0.0180 e | 5.62 ± 0.0100 de |
| SOC (g·kg−1) | 24.88 ± 1.7468 c | 30.40 ± 1.4859 ab | 15.30 ± 1.3172 d | 26.64 ± 1.6675 bc | 32.06 ± 1.5740 a | 10.31 ± 0.7668 e |
| AP (mg·kg−1) | 4.38 ± 0.4409 d | 13.94 ± 1.0245 a | 9.12 ± 0.3652 b | 4.82 ± 0.5472 d | 8.68 ± 0.6967 b | 6.76 ± 0.4336 c |
| TP (g·kg−1) | 1.29 ± 0.1118 a | 1.28 ± 0.2034 a | 0.44 ± 0.0089 b | 1.28 ± 0.1660 a | 1.19 ± 0.1282 a | 0.59 ± 0.0551 b |
| AK (mg·kg−1) | 297.0 ± 6.9498 a | 167.6 ± 7.8333 c | 58.8 ± 3.2465 d | 225.2 ± 16.7404 b | 289.2 ± 26.0776 a | 78.0 ± 7.2732 d |
| TK (g·kg−1) | 5.60 ± 0.2074 c | 11.78 ± 0.2177 a | 7.34 ± 0.1568 b | 5.30 ± 0.3795 c | 11.22 ± 0.2311 a | 7.00 ± 0.3521 b |
| TN (g·kg−1) | 1.85 ± 0.2517 b | 1.99 ± 0.1316 ab | 1.04 ± 0.0592 c | 2.34 ± 0.3391 ab | 2.49 ± 0.1058 a | 0.92 ± 0.0496 c |
| NO3−-N (mg·kg−1) | 11.69 ± 0.7936 a | 1.67 ± 0.1309 cd | 1.88 ± 0.1541 c | 9.04 ± 0.3649 b | 0.72 ± 0.0611 d | 2.16 ± 0.1548 c |
| NH4+-N (mg·kg−1) | 22.32 ± 1.7144 bc | 19.85 ± 1.1401 cd | 5.89 ± 0.8136 e | 29.83 ± 3.5011 a | 28.10 ± 4.1165 ab | 14.46 ± 0.4222 d |
| SWC (%) | 25.79 ± 0.9174 b | 26.29 ± 1.7114 b | 15.55 ± 0.5985 c | 30.30 ± 2.0609 ab | 33.17 ± 2.5209 a | 20.01 ± 0.6092 c |
| C: N | 14.19 ± 1.4521 ab | 15.34 ± 0.5688 a | 14.90 ± 1.4384 ab | 12.33 ± 1.6959 ab | 12.88 ± 0.2239 ab | 11.39 ± 1.1213 b |
| NH4+: NO3− | 1.94 ± 0.1736 c | 12.22 ± 1.3341 b | 3.24 ± 0.5697 c | 3.37 ± 0.4578 c | 39.52 ± 5.5597 a | 6.85 ± 0.5630 bc |
| DBH (cm) | 17.90 ± 0.6058 b | 20.79 ± 0.7266 a | 7.99 ± 0.3923 c | 17.34 ± 1.1352 b | 21.61 ± 0.5640 a | 8.18 ± 0.8046 c |
| BSW (kg) | 80.90 ± 4.9101 b | 105.81 ± 6.5896 a | 19.06 ± 1.6011 c | 77.09 ± 8.9063 b | 113.33 ± 5.1874 a | 20.30 ± 3.5416 c |
| BSR (%) | - | - | - | 71.05 ± 2.9599 a | 77.98 ± 3.2459 a | 44.07 ± 1.3003 b |
| The Type of D. sinicus | Study Site | Altitude/m | Climate Type | Annual Average Temperature/°C | Annual Precipitation/mm | Frost-Free Period/Day | Soil Type |
|---|---|---|---|---|---|---|---|
| Bending type | Menglian County, YN (22.20 N, 99.33 E) | 1040 | south subtropical climate | 19.7 | 1363.6 | 300 | lateritic red soil |
| Straight type | Ximeng County, YN (22.63 N, 99.62 E) | 1122 | subtropical mountain humid monsoon climate | 19.1 | 1629.2 | 362 | lateritic red soil |
| Introduced straight type | Yuxi County, YN (24.12 N, 102.63 E) | 878 | subtropical semi-humid plateau monsoon climate | 19.0 | 950.0 | 315 | red soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, P.; Cheng, Q.; Liang, N.; Bao, C.; Zhang, Z.; Chen, L.; Yang, H. Rhizosphere Microbe Affects Soil Available Nitrogen and Its Implication for the Ecological Adaptability and Rapid Growth of Dendrocalamus sinicus, the Strongest Bamboo in the World. Int. J. Mol. Sci. 2023, 24, 14665. https://doi.org/10.3390/ijms241914665
Dou P, Cheng Q, Liang N, Bao C, Zhang Z, Chen L, Yang H. Rhizosphere Microbe Affects Soil Available Nitrogen and Its Implication for the Ecological Adaptability and Rapid Growth of Dendrocalamus sinicus, the Strongest Bamboo in the World. International Journal of Molecular Sciences. 2023; 24(19):14665. https://doi.org/10.3390/ijms241914665
Chicago/Turabian StyleDou, Peitong, Qian Cheng, Ning Liang, Changyan Bao, Zhiming Zhang, Lingna Chen, and Hanqi Yang. 2023. "Rhizosphere Microbe Affects Soil Available Nitrogen and Its Implication for the Ecological Adaptability and Rapid Growth of Dendrocalamus sinicus, the Strongest Bamboo in the World" International Journal of Molecular Sciences 24, no. 19: 14665. https://doi.org/10.3390/ijms241914665
APA StyleDou, P., Cheng, Q., Liang, N., Bao, C., Zhang, Z., Chen, L., & Yang, H. (2023). Rhizosphere Microbe Affects Soil Available Nitrogen and Its Implication for the Ecological Adaptability and Rapid Growth of Dendrocalamus sinicus, the Strongest Bamboo in the World. International Journal of Molecular Sciences, 24(19), 14665. https://doi.org/10.3390/ijms241914665





